Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Health Benefits of Semaglutide
- Key Takeaways of Semaglutide Guide 2023
- What is Semaglutide?
- How does Semaglutide Work?
- Chemical Structure of Semaglutide
- Research on Semaglutide
- Wegovy, Ozempic, and Rybelus
- Subcutaneous Administration of Wegovy
- Subcutaneous Administration of Ozempic
- Oral Administration of Rybelsus (Semaglutide)
- Oral Semaglutide vs Semaglutide Injection
- Potential Candidates for Semaglutide Treatment
- Semaglutide for Weight Loss in Non-Diabetics
- What is Compounded Semaglutide?
- Semaglutide Side Effects
- Semaglutide Dosage
- Semaglutide Cost
- Semaglutide vs Liraglutide
- Tirzepatide vs Semaglutide
- Tesofensine vs Semaglutide
- Semaglutide Weight Loss Before and After Results
- FAQs
- Blogs
- Reference
Table of Contents
- Potential Health Benefits of Semaglutide
- Key Takeaways of Semaglutide Guide 2023
- What is Semaglutide?
- How does Semaglutide Work?
- Chemical Structure of Semaglutide
- Research on Semaglutide
- Wegovy, Ozempic, and Rybelus
- Subcutaneous Administration of Wegovy
- Subcutaneous Administration of Ozempic
- Oral Administration of Rybelsus (Semaglutide)
- Oral Semaglutide vs Semaglutide Injection
- Potential Candidates for Semaglutide Treatment
- Semaglutide for Weight Loss in Non-Diabetics
- What is Compounded Semaglutide?
- Semaglutide Side Effects
- Semaglutide Dosage
- Semaglutide Cost
- Semaglutide vs Liraglutide
- Tirzepatide vs Semaglutide
- Tesofensine vs Semaglutide
- Semaglutide Weight Loss Before and After Results
- FAQs
- Blogs
- Reference
Potential Health Benefits of Semaglutide
- Promotes weight loss [1-41]
- Fights type 2 diabetes and lowers blood sugar levels [42-69]
- Prevents cognitive decline [70-81]
- Lowers blood pressure [4] [82-88]
- Lowers the risk of cardiovascular disease [89-96]
Key Takeaways of Semaglutide Guide 2023
- Semaglutide is a glucagon-like peptide-1 (GLP-1) receptor agonist used for the treatment of type 2 diabetes and chronic weight management. It works by mimicking the effects of the GLP-1 hormone, leading to reduced appetite, increased feelings of fullness, and slower gastric emptying.
- Semaglutide has demonstrated significant and sustained weight loss in clinical trials, making it a promising option for individuals with obesity. The once-weekly injectable formulation of semaglutide has shown superior weight-loss outcomes compared to placebo and other weight-loss medications.
- Alongside its effectiveness in weight reduction, semaglutide also helps improve glycemic control and cardiovascular risk factors in people with type 2 diabetes.
- Semaglutide is typically used as an adjunct to lifestyle changes, including diet and exercise, to achieve optimal weight loss results. As with any medication, it is crucial for healthcare providers to closely monitor patients on semaglutide therapy and adjust the treatment plan as needed.
What is Semaglutide?
Semaglutide, categorized as a GLP-1 agonist, has obtained approval from the U.S. Food and Drug Administration (FDA) under the brand names Ozempic and Rybelsus for managing type 2 diabetes, and as Wegovy for the treatment of excess weight (overweight) and obesity. At higher doses, semaglutide promotes fat loss by suppressing your appetite. When combined with lifestyle modifications such as a healthy diet and exercise program, semaglutide produces amazing results. For this reason, semaglutide has been employed by health care providers for over 15 years in the management of type 2 diabetes.
How does Semaglutide Work?
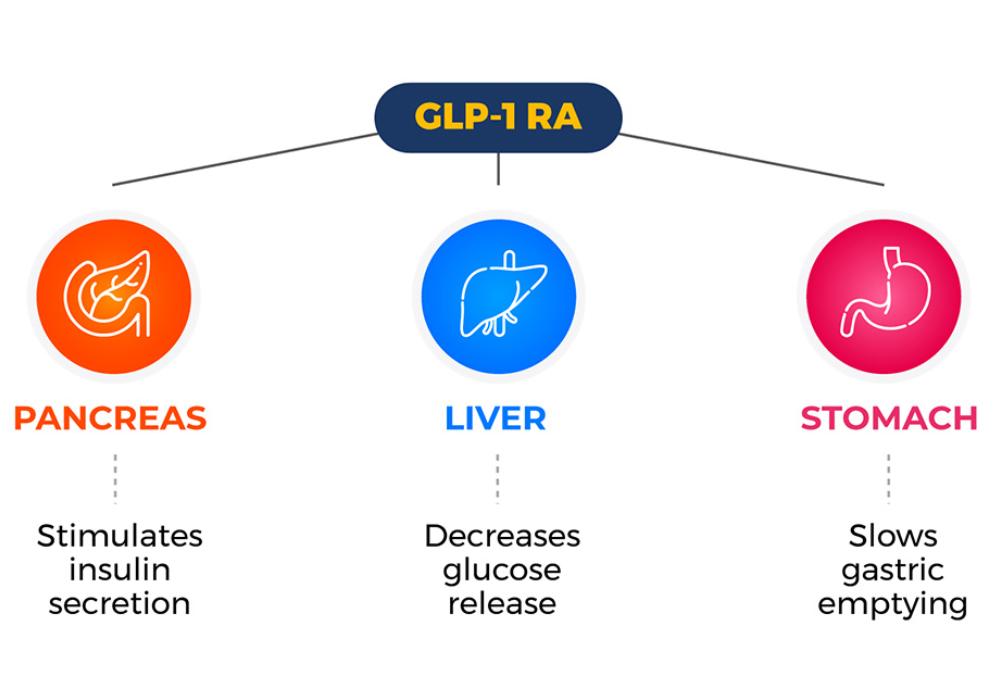
As a glucagon-like peptide-1 receptor agonist (GLP-1 RA), it increases the secretion of the hormone insulin which helps the cells to effectively utilize energy. This process ensures proper fat storage and decreases blood glucose (blood sugar) levels. Semaglutide also suppresses your appetite and slows gastric emptying by blocking certain chemicals in the brain. This in turn helps promote fat loss.
Chemical Structure of Semaglutide
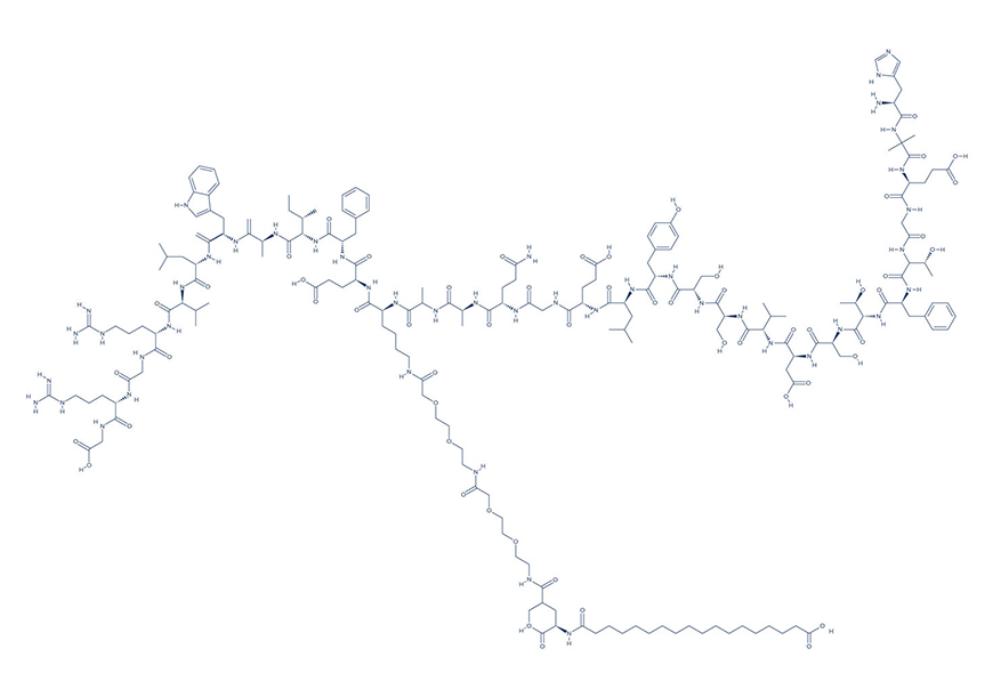
Research on Semaglutide
A. Promotes Weight Loss

Semaglutide is important for long-term weight management due to its efficacy in promoting significant and sustained weight loss in adults with obesity. As a glucagon-like peptide-1 (GLP-1) receptor agonist, semaglutide works by mimicking the effects of the GLP-1 hormone, which regulates appetite and food intake. [1]
The once-weekly injectable formulation of semaglutide has shown remarkable results in clinical trials, demonstrating greater weight loss compared to placebo and other weight-loss medications. By enhancing feelings of fullness, reducing hunger, and slowing down gastric emptying, semaglutide helps individuals achieve meaningful and durable weight reduction, making it a promising and valuable option for managing chronic weight issues and improving overall health outcomes.
Semaglutide’s fat-burning properties are backed by a number of high-quality studies:
- In overweight and obese participants, semaglutide administration once a week resulted in a relevant reduction in body weight. [2]
- In obese people, administration of semaglutide once a week via subcutaneous injections produced superior reductions in weight compared to placebo. [3]
- In obese patients with type 2 diabetes, administration of semaglutide demonstrated huge weight loss efficacy. [4]
- In adults with obesity and excess weight, treatment with 2.4 mg semaglutide once weekly resulted in continued weight loss for the following 48 weeks. [5]
- A study showed that a combination of physical activity and dietary supplementation of semaglutide resulted in greater weight loss. [6]
- In obese, overweight, and type-2 diabetic adults, administration of 2.4 mg semaglutide once a week decreased body weight. [7]
- Among overweight and obese patients, semaglutide used as an intensive behavioral therapy and an initial low-calorie diet resulted in greater weight loss during 68 weeks. [8]
- A study showed that semaglutide induced weight loss in subjects with type 2 diabetes regardless of baseline body mass index (BMI). [9]
- In obese adults, administration of semaglutide 2.4 mg once a week suppressed appetite, improved control of eating, and reduced food cravings, compared to placebo. [10]
- In obese subjects, semaglutide administration once a week reduced appetite and food cravings, and was associated with better control of eating and lower relative preference for fatty, energy‐dense foods. [11]
- A study showed that semaglutide administration once a day produced weight loss by reducing HbA1c, a measure of blood sugar. [12]
- A study showed that intake of semaglutide once a week improved blood sugar control and reduced body weight in patients with type-2 diabetes. [13]
- Studies showed that semaglutide lowers body weight by directly and indirectly affecting the activity of neural pathways involved in food intake, reward, and energy expenditure, without any adverse side effects. [14-16]
- Studies suggest that semaglutide is superior to other weight loss drugs with regard to efficacy and cost-effectiveness. [17-25]
- In overweight and obese adults, once-per-week administration of semaglutide resulted in significant and sustained weight reduction. [26]
- In obese patients, semaglutide was well-tolerated and induced significant weight loss. [27]
- A review of studies showed that once weekly semaglutide was successful in inducing significant weight loss. [28]
- A study showed that oral semaglutide was effective in body weight reduction. [29]
- A review of studies showed that semaglutide demonstrated superior efficacy in obesity compared to other anti-diabetic drugs. [30]
- In obese or overweight adults, semaglutide administration resulted in significant weight loss. [31]
- In obese or overweight adults, semaglutide administration in addition to a weight management program was effective in inducing weight reduction. [32]
- A study showed that semaglutide offered the greatest weight-reducing effect among any obesity medication. [33]
- In obese or overweight adults, once-weekly administration of semaglutide resulted in significant weight loss. [34]
- In adults with overweight or obesity, weekly treatment with subcutaneous semaglutide at a dose of 2.4 mg for 20 weeks resulted in continued weight loss over the following 48 weeks compared with placebo. [35]
- In adults with obesity, with or without type 2 diabetes, the administration of semaglutide at a dose of 2.4 mg once a week for 68 weeks resulted in superior and clinically meaningful reductions in body weight and abdominal visceral fat area compared with placebo. [36-39]
- Once-weekly administration of subcutaneous semaglutide as an adjunct to intensive behavioral therapy and a reduced calorie diet in adults with overweight or obesity produced significant weight loss after 68 weeks. [40]
- In patients with a body mass index (BMI) of 27 or more, weekly 1.7-mg and 2.4-mg doses of semaglutide resulted in a mean weight loss of 6.7 kg at 3 months and 12.3 kg at 6 months. [41]
B. Fights Type 2 Diabetes and Lowers Blood Sugar Levels

Semaglutide may help bring blood sugar to normal levels through the incretin effect. [42] Incretins such as semaglutide and other GLP-1 RA cause a decrease in blood sugar levels once released by the gastrointestinal tract. This in turn alleviates symptoms of diabetes and keeps blood sugar within normal limits.
Studies show that semaglutide, an FDA-approved medication for diabetes, has potent blood-sugar-lowering effects:
- In patients with type 2 diabetes, semaglutide administration once a week resulted in decreased blood sugar levels. [43]
- A study showed that type 2 diabetic patients treated with oral semaglutide had reduced glycosylated hemoglobin, which is chemically linked to sugar. [44]
- In patients with type 2 diabetes, oral semaglutide improved blood sugar control. [45]
- A study showed that oral semaglutide can effectively and safely reduce blood sugar, body weight, and systolic blood pressure. [46]
- In adults with type 2 diabetes, semaglutide reduced blood sugar levels with higher efficacy and tolerability. [47]
- In adult type 2 diabetic patients, once-per-week therapy with semaglutide resulted in significant reductions in blood sugar levels and body weight after 3 to 6 months. [48]
- A review of studies showed that oral semaglutide administration is effective in treating type 2 diabetes without increasing the incidence of major adverse effects. [49]
- In Japanese patients with type 2 diabetes, semaglutide was efficacious in reducing blood sugar levels and was well-tolerated. [50]
- In type 2 diabetic patients, semaglutide was shown to be highly effective in controlling blood sugar levels compared with other diabetic medications. [51-66]
- In patients with type 2 diabetes, semaglutide administration at a dose of 2.0 mg was superior to 1.0 mg in reducing HbA1c (a measure of blood sugar in the past 3 months), with additional body weight loss and a similar safety profile. [67-68]
- Once-daily oral semaglutide (4-week dose escalation from 3 to 7 to 14 mg) administration in type 2 diabetics resulted in reduced body fat mass, increased satiety and fullness after a fat-rich breakfast, and improved eating control compared with placebo. [69]
C. Prevents Cognitive Decline

Semaglutide can help protect brain cells against injury or damage through its anti-inflammatory and antioxidant properties. By reducing inflammation and oxidative stress (free radicals), semaglutide can help improve cognitive function.
Evidence shows that semaglutide has the capacity to prevent age-related cognitive decline and cognitive dysfunction due to brain disorders:
- In mice models of Parkinson’s disease (PD), semaglutide produced significant neuroprotective and cognitive-enhancing effects. [70]
- In PD mouse models, semaglutide attenuated the effects of the disease by protecting the brain neurons against injury. [71]
- In animal models, semaglutide is protected against diabetes and obesity-related cognitive deficits. [72]
- In a mouse model of PD, semaglutide exerted its neuroprotective effects by reducing motor impairments. [73]
- Studies in rodent models of Parkinson’s and Alzheimer’s diseases and mouse models of ischemic stroke have shown that semaglutide prevented brain neuron damage and memory impairment. [74-77]
- In a non-diabetic rat model of acute ischemic stroke, semaglutide reduced infarct size (dead brain tissue) by up to 90%. [78]
- In a Parkinson’s disease mouse model, semaglutide alleviated the inflammation response, reduced lipid peroxidation (oxidative stress), and inhibited programmed cell death. [79-81]
D. Lowers Blood Pressure
Stimulation of the glucagon-like peptide-1 receptor is known to increase blood pressure. [82] As a glucagon-like peptide-1 receptor agonist, semaglutide suppresses the release of glucagon by the liver which in turn lowers blood pressure.
The blood pressure-lowering effects of semaglutide are backed by a number of studies:
- In overweight and obese adults, subcutaneous injection of semaglutide reduced systolic blood pressure after 20 weeks. [4]
- A review of multiple studies found that semaglutide lowered blood pressure compared to placebo. [83]
- In patients with type 2 diabetes, oral semaglutide effectively and safely reduces systolic blood pressure. [84]
- Studies found that glucagon-like peptide-1 receptor agonists such as semaglutide can significantly reduce elevated blood pressure with a low risk of adverse effects. [85-86]
- A study found that both doses of semaglutide (0.5 and 1.0 mg) were associated with reductions in systolic blood pressure. [87-88]
E. Lowers the Risk of Cardiovascular Disease
Semaglutide is thought to lower cardiovascular disease risk through multiple mechanisms of action. As a glucagon-like peptide-1 (GLP-1) receptor agonist, it primarily reduces cardiovascular risk by improving glycemic control in individuals with type 2 diabetes. By stimulating insulin secretion, suppressing glucagon release, and slowing gastric emptying, semaglutide helps lower blood sugar levels, which can positively impact cardiovascular health. Additionally, semaglutide has been shown to promote weight loss, improve blood pressure, and reduce inflammation markers, all of which contribute to its cardioprotective effects.
Evidence shows that semaglutide can help lower the risk of heart disease possibly through its beneficial effects on obesity, blood sugar, and blood pressure:
- In patients with type 2 diabetes, oral semaglutide reduced the rate of cardiovascular death and nonfatal myocardial infarction. [89]
- In subjects with type 2 diabetes and high cardiovascular disease risk, semaglutide (0.5 and 1.0 mg) administration once a week significantly reduced major adverse cardiovascular events. [90-91]
- In patients with type 2 diabetes who are at high risk for cardiovascular disease, the addition of semaglutide to standard treatment decreased the cardiovascular risk by reducing blood sugar and body weight. [92]
- Studies found that semaglutide decreased the risk of atherosclerosis (plaque formation within the heart arteries) and other cardiovascular events in diabetic patients. [93-96]
Semaglutide Brand Names: Wegovy, Ozempic, and Rybelus
Wegovy, Ozempic, and Rybelsus are all medications that contain the active ingredient semaglutide, but they have different approved uses and formulations. Here are the key differences between these medications:
Wegovy
Wegovy is the brand name for a high-dose formulation of semaglutide specifically approved for chronic weight management. This semaglutide brand name is administered as a once-weekly subcutaneous injection. Wegovy is intended for use in adults with obesity (body mass index [BMI] of 30 kg/m² or greater) or overweight individuals (BMI of 27 kg/m² or greater) with at least one weight-related comorbidity, such as type 2 diabetes or high blood pressure. It is a targeted treatment for weight loss and is not indicated for the treatment of type 2 diabetes.
Ozempic
Ozempic is also a brand name for a lower-dose formulation of semaglutide. It is approved for the treatment of type 2 diabetes in adults. Like Wegovy, Ozempic is administered as a once-weekly subcutaneous injection. In addition to its blood sugar-lowering effects, Ozempic has been found to promote weight loss in people with type 2 diabetes. However, its primary indication is for glycemic control in diabetes patients.
Rybelsus
Rybelsus is another brand name for semaglutide, but it is a different formulation than Wegovy and Ozempic. Rybelsus is an oral tablet that is taken once daily, rather than an injectable medication. It is also indicated for the treatment of type 2 diabetes in adults and can be used as a monotherapy or in combination with other antidiabetic medications. While Rybelsus can contribute to weight loss, its primary purpose is to help manage blood sugar levels in diabetes patients.
Subcutaneous Administration of Wegovy (Semaglutide)
Medication Use
Wegovy is a brand name for semaglutide, a medication approved for chronic weight management in adults with obesity or overweight individuals with at least one weight-related comorbidity. It belongs to the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs).
Dosage and Administration
Wegovy is administered through subcutaneous injection, typically once weekly. The recommended dosage is 2.4 mg once a week. The recommended maintenance dose of Wegovy is 2.4 mg, administered via subcutaneous injection once-weekly. In cases where patients experience difficulty tolerating the 2.4 mg once-weekly dose, a temporary reduction to 1.7 mg once-weekly is permissible, but only for a maximum period of 4 weeks. After this 4-week period, the dose should be escalated back to the maintenance dose of 2.4 mg once-weekly.
Injection Technique
Subcutaneous injections are given just below the skin surface, usually in the abdomen, thigh, or upper arm. Healthcare providers will demonstrate the proper injection technique to ensure correct administration.
Combination Therapy
Wegovy is not intended to be used in combination with other GLP-1 RAs or weight-loss medications, as this could lead to an increased risk of adverse effects.
Lifestyle Measures
While using Wegovy, adopting a balanced diet and engaging in regular physical activity remain important components of a comprehensive weight management plan.
Monitoring
Regular follow-up with healthcare providers is essential to monitor progress, adjust dosages if needed, and address any concerns or questions related to treatment.
Pregnancy and Breastfeeding
Wegovy is not recommended during pregnancy or breastfeeding. Women of childbearing potential should use effective contraception while taking the medication.
Patients should consult their healthcare provider before starting Wegovy to discuss its benefits, potential side effects, and individual suitability for treatment. As with any medication, patients should adhere to the prescribed dosing and administration instructions and promptly report any persistent or severe side effects to their healthcare provider.
Subcutaneous Administration of Ozempic (Semaglutide)
Medication Use
Ozempic is a brand name for semaglutide, a medication used for the treatment of type 2 diabetes in adults. It belongs to the class of glucagon-like peptide-1 receptor agonists (GLP-1 RAs).
Dosage and Administration
Ozempic is administered through subcutaneous injection once weekly. The typical starting dosage is 0.5 mg once weekly for the first four weeks. After the initial four weeks, the dosage is increased to 1 mg once weekly. It is important to follow the dosing schedule provided by a healthcare provider and take the injection on the same day each week.
Injection Technique
Subcutaneous injections are given just below the skin surface, usually in the abdomen, thigh, or upper arm. Healthcare providers will demonstrate the proper injection technique to ensure correct administration.
Combination Therapy
Ozempic can be used alone or in combination with other antidiabetic medications, depending on individual treatment needs and healthcare provider’s recommendations.
Monitoring
Regular follow-up with healthcare providers is essential to monitor blood sugar levels, assess the response to treatment, and make any necessary adjustments to achieve optimal glycemic control.
Pregnancy and Breastfeeding
Ozempic is not recommended during pregnancy or breastfeeding. Women of childbearing potential should use effective contraception while taking the medication.
Patients should consult their healthcare provider before starting Ozempic to discuss its benefits, potential side effects, and individual suitability for treatment. As with any medication, patients should adhere to the prescribed dosing and administration instructions and promptly report any persistent or severe side effects to their healthcare provider. It is essential to continue other aspects of diabetes management, such as a balanced diet, regular exercise, and blood sugar monitoring while using Ozempic.
Oral Administration of Rybelsus (Semaglutide)
Medication Use
Rybelsus is a brand name for semaglutide, which is used for the treatment of type 2 diabetes in adults. It belongs to the class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). Rybelsus is available in oral tablet form (semaglutide tablets), making it a convenient option for those who prefer oral administration over injections.
Dosage
The typical starting dosage of Rybelsus is 3 mg once daily. After at least 30 days at this initial dosage, it can be increased to 7 mg once daily if additional glycemic control is required. In some cases, individuals may need further dose escalation to 14 mg once daily to achieve their target blood sugar levels. The dosage should be determined and adjusted by a healthcare provider based on the patient’s response to treatment and individual needs.
Administration
To ensure the medication’s effectiveness, Rybelsus should be taken as directed by a healthcare provider. The tablet should be swallowed whole with a glass of plain water, at least 30 minutes before the first food, beverage, or other oral medications of the day. It is crucial not to crush, chew, or split the tablet. Following the dosing instructions consistently is essential for maintaining steady blood sugar levels.
Monitoring
Regular monitoring of blood sugar levels and other relevant health parameters is essential when using Rybelsus. Healthcare providers will assess the response to treatment and may adjust the dosage as needed to achieve optimal glycemic control.
Combination Therapy
Rybelsus should not be used together with other GLP-1 receptor agonists or medications that belong to the same class, as this could lead to an increased risk of hypoglycemia (low blood sugar).
Lifestyle Measures
While taking Rybelsus, it is essential to continue other aspects of diabetes management, including a balanced diet, regular exercise, and blood sugar monitoring.
Pregnancy and Breastfeeding
Rybelsus is not recommended during pregnancy or breastfeeding. Women of childbearing potential should use effective contraception while taking the medication.
As with any medication, patients should consult their healthcare provider before starting Rybelsus to discuss its benefits, potential side effects, and individual suitability for treatment. It is crucial to follow the prescribed dosage and administration instructions strictly and seek medical advice if any concerns or questions arise during the course of treatment.
Oral Semaglutide vs Semaglutide Injection
Semaglutide is a prescription medication that is used to treat type 2 diabetes and obesity. It is available in two forms: a semaglutide injection and an oral semaglutide.
Semaglutide injection is a once-weekly injection that is given under the skin. It is the most effective form of semaglutide for weight loss. In clinical trials, semaglutide injection was shown to help people lose an average of 15% of their body weight over a period of 68 weeks.
Oral semaglutide is a once-daily pill that is taken by mouth. It is not as effective as semaglutide injection for weight loss. In clinical trials, oral semaglutide was shown to help people lose an average of 7% of their body weight over a period of 56 weeks.
Here are some of the key differences between semaglutide injection and oral semaglutide:
- Form: Semaglutide injection is a once-weekly injection, while oral semaglutide is a once-daily pill.
- Efficacy: Semaglutide injection is more effective for weight loss than oral semaglutide.
- Side effects: Semaglutide injection and oral semaglutide can cause similar side effects, such as nausea, vomiting, diarrhea, and constipation.
- Cost: Semaglutide injection is typically more expensive than oral semaglutide.
Here are some additional things to keep in mind when choosing between semaglutide injection and oral semaglutide:
- Your lifestyle: If you are not comfortable giving yourself injections, then oral semaglutide may be a better option for you.
- Your insurance coverage: Semaglutide injection may be covered by insurance, but oral semaglutide may not be.
- Your personal preferences: Some people prefer the convenience of taking a pill once a day, while others prefer the once-weekly injection.
Ultimately, the best way to choose between semaglutide injection and oral semaglutide is to talk to your doctor. They can help you decide which form is right for you based on your individual needs and preferences.
Potential Candidates for Semaglutide Treatment
Potential candidates for semaglutide treatment may include individuals with the following conditions:
- Type 2 Diabetes: Semaglutide is FDA-approved for the treatment of type 2 diabetes and may be prescribed for individuals who have not achieved adequate glycemic control with other medications.
- Overweight or Obesity: Semaglutide, under the brand name Wegovy, is approved for chronic weight management in adults with obesity or overweight individuals with at least one weight-related comorbidity.
- Those at High Cardiovascular Risk: Semaglutide has demonstrated cardiovascular benefits in clinical trials, making it a consideration for individuals with type 2 diabetes who are at increased risk of cardiovascular events.
- Individuals Seeking Weight Loss Support: Semaglutide may be an option for non-diabetic individuals struggling with weight management.
It is important to keep in mind that this medication is not for everyone. For instance, you are not a good candidate for semaglutide treatment if you have a personal or family history of the following:
- Acute kidney injury
- An allergic reaction to semaglutide-containing products
- Diabetic retinopathy (damage to the retina of the eye)
- Gallbladder disease
- Medullary thyroid carcinoma (also known as thyroid C-cell tumors, is a thyroid cancer characterized by rare neuroendocrine tumors that originate from the C-cells of the thyroid gland)
- Multiple endocrine neoplasia syndrome type 2 (tumors in multiple endocrine glands, commonly affecting the thyroid and adrenal glands)
- Pancreatitis (inflammation of the pancreas)
It is crucial to note that the suitability for semaglutide treatment is determined by healthcare providers based on individual health needs, medical history, and other relevant factors. Only a qualified healthcare professional can assess and recommend semaglutide treatment for each individual case. Always consult with a healthcare provider to discuss potential treatment options and to ensure safe and appropriate usage of semaglutide.
Semaglutide for Weight Loss in Non-Diabetics
Semaglutide has shown promising results for weight loss in non-diabetic individuals. While initially developed as a medication to manage type 2 diabetes, clinical trials have demonstrated its effectiveness in promoting weight loss in people without diabetes who struggle with obesity or overweight.
In recent studies, a higher-dose formulation of semaglutide (2.4 mg once a week) under the brand name Wegovy has been specifically approved for chronic weight management in non-diabetic individuals. It is administered as a subcutaneous injection and has been associated with significant and sustained weight loss when used in combination with lifestyle modifications.
The exact mechanisms by which semaglutide leads to weight loss in non-diabetic individuals are not fully understood, but it is believed to act on the brain’s appetite control centers, leading to reduced food intake and increased feelings of fullness. Additionally, it may affect gastric emptying and improve insulin sensitivity, contributing to weight loss benefits.
Before starting semaglutide for weight loss, individuals should consult their healthcare provider to determine its suitability for their specific health condition and to receive proper guidance on its usage and potential side effects. Weight loss with semaglutide should be part of a comprehensive weight management plan, which includes a balanced diet, regular physical activity, and lifestyle changes to maximize its effectiveness.
What is Compounded Semaglutide?
Compounded semaglutide is a form of semaglutide that is made by a compounding pharmacy. Compounding pharmacies are licensed to create customized medications for individual patients. In the case of compounded semaglutide, the pharmacy may combine semaglutide with other ingredients, such as vitamins or minerals, to create a medication that is tailored to the specific needs of the patient.
Compounded semaglutide is often used as a weight loss medication. Semaglutide is a GLP-1 receptor agonist, which means that it works by binding to the GLP-1 receptor in the gut to help control blood sugar levels. However, semaglutide has also been shown to be effective at promoting weight loss.
There are a few reasons why people might choose to use compounded semaglutide for weight loss. First, compounded semaglutide is often less expensive than brand-name semaglutide. Second, compounded semaglutide can be tailored to the specific needs of the patient. For example, if a patient has trouble swallowing pills, the compounding pharmacy can create a compounded semaglutide that is administered as a liquid or a nasal spray.
However, there are also some risks associated with using compounded semaglutide. First, compounded semaglutide has not been as extensively studied as brand-name semaglutide. This means that there is less information available about the safety and efficacy of compounded semaglutide. Second, there is a risk that compounded semaglutide may not be made correctly. This could lead to the wrong dosage being given, or it could lead to the medication being contaminated.
If you are considering using compounded semaglutide, talk to your doctor about the risks and benefits. Your doctor can help you decide if compounded semaglutide is right for you.
Semaglutide Side Effects

Semaglutide side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on semaglutide. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of semaglutide. Despite this, it was listed as a side effect associated with semaglutide even though these associated side effects are very uncommon.
Side effects associated with semaglutide may include the following:
- Abdominal pain (upper stomach pain)
- Constipation
- Diarrhea
- Loss of appetite
- Low blood sugar
- Nausea
- Vomiting
Semaglutide Dosage
The dosage of semaglutide can vary depending on the specific formulation and approved use of the medication. For chronic weight management, Wegovy is commonly prescribed at a recommended dosage of 2.4 mg once a week. This formulation is administered through a subcutaneous injection and should be taken on the same day each week. It is intended for use in adults with obesity or overweight individuals who have at least one weight-related comorbidity.
For the treatment of type 2 diabetes, Ozempic is another available option. The typical dosage for Ozempic starts at 0.5 mg once weekly for the first four weeks, followed by an increase to 1 mg once weekly. Like Wegovy, Ozempic is also administered via subcutaneous injection and should be taken on the same day each week.
On the other hand, Rybelsus, which is also used for type 2 diabetes management, comes in oral tablet form with varying dosages. The usual starting dosage is 3 mg once daily. After a minimum of 30 days at this initial dosage, it can be increased to 7 mg once daily if additional glycemic control is required. Some individuals may need further dose escalation to 14 mg once daily to achieve their target blood sugar levels.
In some cases, healthcare providers may consider delaying dose escalation to ensure optimal tolerance and minimize potential side effects during the initial phase of treatment. Regardless of the specific formulation, it is crucial to adhere to the dosing instructions provided by the prescribing healthcare professional and carefully review the medication’s labeling and patient information. Doses may be adjusted based on individual response and tolerability. Patients should not modify the dosage or frequency of any medication without consulting a healthcare provider first.
Semaglutide Cost
The cost of Semaglutide can vary depending on the dosage, the form of the medication, and the insurance coverage. You can talk to your doctor about getting a prescription assistance program. These programs can help you get semaglutide at a reduced cost. You can also check with your insurance company to see if they have a copay assistance program. These programs can help you pay for the copays associated with semaglutide.
Semaglutide vs Liraglutide
An overwhelming body of clinical evidence shows that semaglutide is superior to liraglutide (a weight loss medication) in reducing body weight and blood sugar levels:
- In patients with obesity but without type 2 diabetes, semaglutide subcutaneous injection once daily demonstrated superior weight loss efficacy compared with both placebo and once daily 3.0 mg liraglutide. [5]
- In obese patients, semaglutide injections in combination with dietary and physical activity counseling produced clinically relevant weight loss over 52 weeks compared with liraglutide at all doses. [7]
- In adults with type 2 diabetes, subcutaneous semaglutide 1 mg once per week was superior to liraglutide in reducing HbA1c (a measure of blood sugar) and body weight. [8]
- In subjects with inadequately controlled type 2 diabetes, semaglutide-induced weight loss was consistently greater than liraglutide, regardless of baseline BMI. [10]
- A study reported that semaglutide demonstrated superior HbA1c and body weight reductions compared with liraglutide 1.2 mg. [16]
- In diabetic patients who were aged 18 years or older, once-daily oral semaglutide (dose escalated to 14 mg) for 52 weeks was superior to once-daily subcutaneous liraglutide and placebo in reducing blood sugar levels and body weight. [52]
- A study reported that up to 81% of patients achieved reductions in weight and HbA1c with semaglutide 1.6 mg for 12 weeks than those with liraglutide 1.2 and 1.8 mg. [54]
- Several studies showed that both oral and injectable semaglutide demonstrated greater efficacy than liraglutide in reducing body weight and blood sugar levels. [55-66]
- The global SUSTAIN and PIONEER phase III clinical trial programs found that subcutaneous semaglutide 1 mg once a week reduced HbA1c by 1.5-1.8% after 30-56 weeks, which was significantly more than liraglutide. [97]
Tirzepatide vs Semaglutide
Tirzepatide and semaglutide are both GLP-1 receptor agonists, which means they work by binding to the GLP-1 receptor in the gut to help control blood sugar levels. However, there are some key differences between the two medications.
- Tirzepatide is a longer-acting medication than semaglutide. Tirzepatide is given once weekly, while semaglutide is given once weekly or once daily.
- Tirzepatide is more potent than semaglutide. Tirzepatide has a higher affinity for the GLP-1 receptor than semaglutide, which means that it is more effective at lowering blood sugar levels.
- Tirzepatide has a wider range of side effects than semaglutide. Tirzepatide can cause more side effects than semaglutide, including nausea, vomiting, diarrhea, constipation, and headache. However, these side effects are usually mild and go away on their own.
Want to learn more about Tirzepatide? This innovative medication has shown promising results for managing diabetes and obesity. Discover how Tirzepatide can potentially benefit you by exploring its features, benefits, and safety under the guidance of healthcare professionals.
Tesofensine vs Semaglutide
Tesofensine and semaglutide have both shown promise for weight loss in clinical trials, but they vary in their mechanisms of action and approved uses.
In terms of mechanisms, tesofensine inhibits the uptake of serotonin, noradrenaline, and dopamine, leading to appetite suppression and increased feelings of fullness. Conversely, semaglutide is a GLP-1 receptor agonist that imitates the action of the natural hormone GLP-1, which helps regulate blood sugar levels and suppress appetite.
Regarding approved uses, tesofensine is specifically used to treat obesity, while semaglutide is indicated for both type 2 diabetes and obesity management.
Discover the Power of Tesofensine – Know more about tesofensine for weight loss and obesity management! Uncover the potential benefits of this medication and how it can support your weight loss journey.
Semaglutide Weight Loss Before and After Results
About Dr. George Shanlikian
Dr. George Shanlikian, renowned as the world’s best hormone therapy doctor, possesses expertise in various medical domains. These include Bio-Identical Hormone Replacement Therapy, Peptide Replacement Therapy, Anti-Aging Medicine, Regenerative Medicine, Stress Management, Nutrition Consulting, Nutritional Supplement Consulting, and Exercise Consulting.
Read more about him here: https://www.genemedics.com/dr-george-shanlikian-md-best-hormone-therapy-doctor
Read more semaglutide weight loss reviews here:
Men’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/mens-success-stories/
Women’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/womens-success-stories/
FAQ
Where can you buy semaglutide?
Semaglutide is a prescription medication, so you can only buy it from a licensed healthcare professional or a state-licensed pharmacy. You can also buy semaglutide online from some pharmacies, but it is important to make sure that the pharmacy is reputable and that the medication is FDA-approved.
If you are unsure where to buy semaglutide, talk to your doctor. They can help you find a reputable pharmacy that carries the medication.
Discover the Power of Semaglutide Near Me!
Are you looking for an effective solution for weight management or diabetes management? Look no further! Semaglutide, a groundbreaking medication, may be the answer you’ve been searching for. Whether you need help shedding those extra pounds or managing your diabetes, semaglutide near you can make a real difference.
What to avoid when taking semaglutide?
When taking semaglutide, it’s important to avoid excessive alcohol consumption and using other diabetes medications without consulting your doctor. Additionally, steer clear of skipping meals or making significant changes to your diet without proper guidance. Always follow your healthcare provider’s instructions and recommendations while using semaglutide.
What should I monitor when taking semaglutide?
When taking semaglutide, you should monitor your blood sugar levels regularly. Keep an eye out for any changes in your appetite, weight, or any side effects you might experience. It’s important to seek medical help immediately in case of unusual symptoms (e.g. severe pain in your upper stomach spreading to your back).
What are the do's and don'ts of semaglutide?
Do’s:
- Take semaglutide as prescribed by your healthcare provider, without a missed dose.
- Monitor your blood sugar levels regularly.
- Report any unusual symptoms or side effects to your doctor.
- Follow a healthy diet and exercise routine as recommended.
- Be sure to give your healthcare provider a complete list of all the medicines, herbal products, non-prescription drugs (over-the-counter medicines), and dietary supplements you are taking while on semaglutide.
- Inform your healthcare provider if you have a weight-related condition or other medical condition that can affect your treatment.
Don’ts:
- Don’t skip doses of semaglutide without consulting your doctor.
- Avoid using other diabetes medications or making significant changes to your treatment plan without medical guidance.
- Don’t consume excessive alcohol while on semaglutide.
- Avoid skipping meals or making drastic changes to your diet without discussing it with your healthcare provider.
- Don’t take semaglutide if you have a history of medullary thyroid carcinoma (medullary thyroid cancer), gallbladder disease, pancreatitis, multiple endocrine neoplasia syndrome type 2 (MEN2), or allergic reactions to the medication or other semaglutide containing products.
- Semaglutide should not be placed near the refrigerator cooling element because exposure to extremely cold temperatures can affect its stability and potency.
What happens when you stop semaglutide?
Semaglutide is used to treat diabetes, but can also cause weight loss. When you stop taking semaglutide, your body will no longer receive the effects of the medication. As a result, your blood sugar levels may change, and you may not experience the same benefits in managing your diabetes. It’s essential to discuss any plans to stop semaglutide or other weight loss drugs with your healthcare provider to ensure a smooth transition and appropriate management of your condition.
How do you know if semaglutide is working?
You can tell if semaglutide is working by monitoring your blood sugar levels regularly. If your blood sugar levels are becoming more stable and closer to your target range, it indicates that semaglutide is helping you manage your diabetes effectively. Additionally, you might notice improvements in your overall well-being, such as increased energy levels, weight changes, or a reduction in diabetes-related symptoms. However, always discuss your progress with your healthcare provider to ensure the treatment is working optimally for you.
What to do while on semaglutide?
While on semaglutide, following your healthcare provider’s instructions and taking the medication as prescribed is essential. Monitor your blood sugar levels regularly and report any unusual symptoms or side effects to your doctor. Stick to a healthy diet and exercise program. Also, attend regular check-ups with your healthcare provider to discuss your progress and make any necessary adjustments to your treatment plan.
Make sure to give your healthcare provider a comprehensive list of all the medicines, herbal products, non-prescription drugs (over-the-counter medicines), and dietary supplements you are currently using while on semaglutide. Additionally, disclose your smoking, alcohol consumption, or illegal drug usage to your healthcare provider. Certain substances may interact with your prescribed medicine, and this information is crucial for ensuring your safety and optimizing the effectiveness of your treatment. Transparency in sharing your complete health history will assist your healthcare provider in making informed decisions for your care.
How long does it take your body to adjust to semaglutide?
The time it takes for your body to adjust to semaglutide can vary from person to person. In general, it may take a few weeks to a couple of months for your body to adapt to the medication and for you to notice its effects on managing your diabetes. It’s important to be patient and consistent with taking semaglutide as prescribed by your healthcare provider to give your body enough time to adjust and achieve the desired results. Always communicate any concerns or questions with your healthcare team during this adjustment period.
How can I reduce the side effects of semaglutide?
To reduce the side effects of semaglutide, you can follow these simple steps:
- Take semaglutide as directed by your healthcare provider, without a missed dose.
- Follow a healthy diet and exercise regularly as recommended.
- Stay well-hydrated by drinking plenty of water throughout the day.
- Avoid consuming large amounts of alcohol.
If you experience any side effects, discuss them with your healthcare provider. They may suggest adjusting the dosage or provide additional guidance to manage the side effects effectively.
What is the best day to take semaglutide?
The best day to take semaglutide is the day that your healthcare provider prescribes it for you. They will determine the appropriate dosage and frequency that best suits your individual needs. Follow your doctor’s instructions carefully, and if you have any questions or concerns, don’t hesitate to ask them for guidance. Consistency and adherence to your prescribed treatment plan are essential for the best results with semaglutide.
What should I do if I miss a dose of semaglutide?
In the event of a missed dose, promptly take it as soon as possible within 5 days of the scheduled dose. Afterward, continue with your regular weekly dosing schedule. If more than 5 days have elapsed since the missed dose, do not take the missed dose and proceed with your next dose at the usual time. Avoid taking double or additional doses to compensate for the missed dose.
To prevent missed medication doses, set a daily routine or use medication reminders, such as alarms or smartphone apps, to ensure you take your medications consistently. If you accidentally miss a dose, take it as soon as you remember, and continue with the next dose at your regular scheduled time to maintain the effectiveness of the treatment. Using these strategies can help you stay on track with your medication regimen and avoid missing the next dose.
Does semaglutide damage kidneys?
Semaglutide has not been shown to cause kidney damage or kidney disease in clinical studies. However, like any medication, it’s essential to use semaglutide as prescribed by your healthcare provider and to attend regular check-ups. Your doctor will monitor your kidney function and overall health to ensure that semaglutide is safe and suitable for you. If you have any concerns about your kidney health or the use of semaglutide, talk to your healthcare provider for personalized advice and reassurance.
What is the protocol for semaglutide?
The protocol for semaglutide is determined by your healthcare provider based on your individual needs and medical condition. Typically, semaglutide is prescribed as an injection that you administer once a week. The dosage and frequency will be tailored to your specific requirements. It’s essential to follow your doctor’s instructions carefully and attend regular check-ups to monitor your progress and response to the medication. If you have any questions or concerns about the protocol for semaglutide, don’t hesitate to discuss them with your healthcare provider.
How long does it take to start losing weight on semaglutide?
The time it takes to start losing weight on semaglutide can vary from person to person. Some individuals may notice weight loss within a few weeks of starting the medication, while others may take a few months to see significant changes. It’s important to remember that weight loss results can be influenced by various factors, including your diet, exercise routine, metabolism, and overall health.
Do you have to change your diet on semaglutide?
Yes, you may need to make some changes to your diet while on semaglutide. Your healthcare provider may recommend following a healthy eating plan to help manage your diabetes and support your weight loss goals. This may include eating balanced meals with a focus on whole foods, fruits, vegetables, lean proteins, and whole grains. Avoiding high-calorie and sugary foods can also be beneficial. It’s essential to discuss any dietary changes with your healthcare provider to ensure they are suitable for your specific needs and health condition.
How do I stop weight gain after semaglutide?
To prevent weight gain after using semaglutide, it’s essential to continue following a healthy diet and exercise regularly. Focus on eating balanced meals with nutrient-rich foods, such as fruits, vegetables, lean proteins, and whole grains. Be mindful of portion sizes and avoid excessive consumption of high-calorie or sugary foods.
Incorporating regular physical activity into your routine can also help maintain weight loss achieved with semaglutide. Engage in activities you enjoy, such as walking, jogging, cycling, or swimming. Staying active can help burn calories and support weight management.
Additionally, attending regular check-ups with your healthcare provider and discussing any concerns or challenges you face can provide valuable guidance and support to maintain a healthy weight. Remember that adopting a sustainable and balanced lifestyle is key to long-term weight management.
How long does it take for semaglutide to suppress appetite?
Semaglutide can start to suppress appetite shortly after you begin taking it, usually within a few days to a week. However, the full appetite-suppressing effects may take a few weeks to become noticeable. It’s important to be patient and consistent with taking semaglutide as prescribed by your healthcare provider to allow your body enough time to adjust and experience the desired appetite-suppressing benefits. If you have any concerns or questions about the effects of semaglutide on your appetite, don’t hesitate to discuss them with your healthcare provider.
Is semaglutide safe to take long-term?
Semaglutide has been found to be safe for long-term use in managing type 2 diabetes for many individuals. However, like any medication, it may cause side effects in some people. It’s essential to attend regular check-ups with your healthcare provider to monitor your response to the medication and ensure its continued safety and effectiveness for your specific health condition.
Does semaglutide affect hunger?
Yes, semaglutide can have an effect on hunger. It is known to help suppress appetite, which means it can make you feel less hungry. This appetite-suppressing effect can be helpful for managing weight and improving blood sugar levels in people with type 2 diabetes. However, the extent of appetite suppression can vary from person to person. If you have any concerns or questions about how semaglutide may affect your hunger, it’s best to discuss them with your healthcare provider.
Can you maintain weight loss after semaglutide?
Yes, it is possible to maintain weight loss achieved with semaglutide. To do so, you should continue following a healthy diet and staying physically active. Adopting a balanced lifestyle with regular exercise and mindful eating habits can help you sustain the weight loss benefits of semaglutide over the long term. It’s important to work with your healthcare provider to create a personalized plan that suits your needs and supports your weight management goals even after you stop taking semaglutide.
Do you lose weight every week on semaglutide?
While some individuals may experience weight loss every week while taking semaglutide, weight loss progress can vary from person to person. The rate of weight loss depends on various factors, including diet, exercise, metabolism, and individual response to the medication. Some people may see consistent weight loss each week, while others may have fluctuations or experience weight loss in different patterns. It’s essential to be patient and consistent with your treatment plan, follow a healthy lifestyle, and discuss your weight loss progress with your healthcare provider to ensure you are on track to achieve your goals.
How does semaglutide control appetite?
Semaglutide controls appetite by affecting certain hormones in your body. It belongs to a class of medications called GLP-1 receptor agonists. When you take semaglutide, it mimics the action of a natural hormone called GLP-1 in your body. This hormone helps regulate blood sugar levels and also has an appetite-suppressing effect.
By activating GLP-1 receptors in your brain, semaglutide signals to your body that you are full, which can lead to a reduced feeling of hunger and decreased appetite. This can be helpful for managing weight and improving blood sugar levels, especially in people with type 2 diabetes. The appetite-suppressing effect of semaglutide can contribute to weight loss and support overall diabetes management.
Is semaglutide weight loss permanent?
The weight loss achieved with semaglutide can be maintained as long as you continue to follow a healthy lifestyle with proper diet and regular exercise. Semaglutide can help you lose weight, but its effects are not permanent on their own. If you stop taking semaglutide and return to unhealthy eating habits or a sedentary lifestyle, you may regain the weight you lost.
To make weight loss more lasting, it’s essential to adopt sustainable lifestyle changes. Continue to eat a balanced diet, stay physically active, and develop healthy habits that you can maintain over the long term. If you have concerns about weight maintenance after using semaglutide, discuss them with your healthcare provider for personalized advice and support.
How does semaglutide promote weight loss?
Semaglutide promotes weight loss by affecting hormones in your body that control appetite and blood sugar levels. When you take semaglutide, it activates certain receptors in your brain that signal you are full and reduces your appetite. This can lead to eating less and consuming fewer calories, which can help with weight loss.
Additionally, semaglutide helps regulate blood sugar levels by increasing insulin production and reducing the amount of sugar released by the liver. When blood sugar levels are stable, it can prevent excessive hunger and cravings, further supporting weight loss.
By combining these effects, semaglutide can help you manage your weight more effectively, especially when combined with a healthy diet and regular exercise. It is essential to use semaglutide as your healthcare provider prescribes to get the best weight loss results.
Does semaglutide affect creatinine?
Semaglutide does not have a direct effect on creatinine levels in the body. Creatinine is a waste product produced by the muscles and excreted by the kidneys. It is commonly used as a marker to assess kidney function.
While semaglutide has been shown to be safe and effective in managing type 2 diabetes, it’s essential to monitor kidney function regularly, especially in individuals with pre-existing kidney disease (e.g. kidney failure). If you have any concerns about your kidney health or creatinine levels while taking semaglutide, discuss them with your healthcare provider. They can provide personalized guidance and ensure that semaglutide is safe for your individual health needs.
Does semaglutide affect immune system?
Semaglutide does not directly affect the immune system. It is a medication used to manage type 2 diabetes and promote weight loss by regulating blood sugar levels and suppressing appetite. While it does not target the immune system, it’s essential to be cautious if you have any pre-existing medical conditions or are taking other medications that may impact your immune system.
Can semaglutide affect hormones?
Yes, semaglutide can affect hormones in your body. It belongs to a class of medications called GLP-1 receptor agonists, which work by mimicking the action of a hormone called GLP-1. This hormone helps regulate blood sugar levels and has other effects on the body, including appetite regulation.
When you take semaglutide, it activates certain receptors in your body that respond to GLP-1, leading to increased insulin production, reduced sugar release from the liver, and suppressed appetite. These hormonal effects can be beneficial for managing type 2 diabetes and promoting weight loss.
Does semaglutide melt fat?
Semaglutide does not directly “melt” fat. However, it can help with weight loss by suppressing appetite, which means you may eat less and consume fewer calories. By regulating blood sugar levels and reducing hunger, semaglutide can support weight loss efforts in people with type 2 diabetes.
The weight loss achieved with semaglutide is a result of its effects on appetite and blood sugar levels, along with a healthy diet and regular exercise. It’s important to remember that weight loss takes time and requires a comprehensive approach to maintaining a healthy lifestyle.
Does semaglutide lose muscle?
Semaglutide itself does not cause muscle loss. However, when using semaglutide for weight loss, some people may experience a reduction in overall body weight, which can include a combination of fat and muscle loss.
To help preserve muscle while taking semaglutide, it’s important to engage in regular exercise that includes strength training or resistance exercises. This can help maintain muscle mass while promoting healthy weight loss.
Does semaglutide affect cholesterol?
Yes, semaglutide can reduce high cholesterol levels. Studies have shown that semaglutide can have a positive impact on cholesterol by reducing levels of total cholesterol and low-density lipoprotein (LDL) cholesterol, often referred to as “bad” cholesterol. Additionally, semaglutide may increase levels of high-density lipoprotein (HDL) cholesterol, which is considered “good” cholesterol.
By improving cholesterol levels, semaglutide can contribute to better heart health and reduce the risk of cardiovascular complications, especially in individuals with type 2 diabetes.
Does semaglutide cause high blood sugar?
No, semaglutide does not cause high blood sugar. In fact, semaglutide is used to help lower blood sugar levels in people with type 2 diabetes. It belongs to a class of medications called GLP-1 receptor agonists, which work by stimulating the release of insulin and reducing the production of glucose in the liver. This helps to control blood sugar levels and improve glycemic control in individuals with type 2 diabetes.
How does semaglutide affect fatty liver?
Semaglutide can have a positive effect on fatty liver. It has been shown to reduce liver fat content in people with non-alcoholic fatty liver disease (NAFLD). When you take semaglutide, it helps regulate blood sugar levels and can also promote weight loss.
By improving blood sugar control and aiding in weight loss, semaglutide can help reduce the accumulation of fat in the liver. This can be beneficial for people with NAFLD, as excess fat in the liver can lead to liver inflammation and damage over time.
Does semaglutide affect the brain?
Yes, semaglutide can affect the brain, but in a beneficial way. Semaglutide belongs to a class of medications called GLP-1 receptor agonists, and when it is taken, it can activate certain receptors in the brain. These receptors help regulate appetite and feelings of fullness, which can lead to reduced hunger and decreased food intake.
By affecting these brain receptors, semaglutide can help control appetite and promote weight loss, especially in people with type 2 diabetes. It can also positively affect blood sugar levels and overall diabetes management.
While semaglutide affects the brain in this way, it is generally considered safe and well-tolerated when used as prescribed by a healthcare provider. If you have any concerns or notice any unusual effects while taking semaglutide, it’s essential to discuss them with your healthcare provider for personalized guidance and support.
Does semaglutide cause tumors?
Semaglutide does not directly cause tumors. However, like any medication, there is always a possibility of side effects or adverse reactions in some individuals. In clinical trials and real-world use, there has been no evidence to suggest that semaglutide can increase the risk of tumors.
Does semaglutide affect metabolism?
Semaglutide is a medication that belongs to the class of GLP-1 receptor agonists. This drug has been shown to have a positive impact on metabolism by inducing various metabolic changes. Its consumption can lead to a decrease in appetite, an increase in insulin secretion, and a reduction in the production of glucose by the liver. These metabolic changes can lead to weight loss and improved glucose control, making semaglutide a promising treatment option for individuals with type 2 diabetes and obesity.
Does semaglutide increase triglycerides?
No, semaglutide does not typically increase triglycerides. In fact, it has been shown to have a positive effect on triglyceride levels in some people. Triglycerides are a type of fat found in the blood, and high levels of triglycerides can increase the risk of heart disease.
Semaglutide belongs to a class of medications called GLP-1 receptor agonists, which can help improve blood sugar control and promote weight loss. These effects may lead to a decrease in triglyceride levels for some individuals.
However, as with any medication, individual responses can vary, and some people may experience different effects on their triglyceride levels. If you have any concerns about your triglyceride levels or any other aspect of your health while taking semaglutide, discuss it with your healthcare provider. They can monitor your progress and provide personalized advice to support your health and well-being.
What are the long-term effects of semaglutide?
The long-term effects of semaglutide are generally positive for people with type 2 diabetes and obesity who use it as prescribed by their healthcare provider. Some potential long-term benefits of semaglutide include improved blood sugar levels, weight loss, normal blood pressure, and a reduced risk of heart disease.
Before undergoing semaglutide treatment, it is essential to undergo a thorough assessment by a qualified healthcare provider to identify any potential contraindications (e.g. thyroid cancer or thyroid tumors). Your healthcare provider will carefully evaluate your medical history, current health status, and any existing health conditions to ensure that semaglutide is safe and appropriate for you. This professional assessment aims to prioritize your well-being and optimize the effectiveness of the treatment, providing you with the best possible care throughout your journey with semaglutide.
Does semaglutide dehydrate you?
Semaglutide itself does not directly cause dehydration. However, like any medication, there can be side effects or individual responses that may lead to dehydration in some people.
One possible reason for dehydration could be related to side effects like nausea, vomiting, or diarrhea that some individuals may experience when taking semaglutide. These symptoms can lead to fluid loss and potentially result in dehydration if not adequately managed.
To prevent dehydration while taking semaglutide, it’s important to stay hydrated by drinking plenty of fluids throughout the day. If you experience any side effects or notice signs of dehydration, such as feeling very thirsty, having dark yellow urine, or feeling lightheaded, contact your healthcare provider. They can provide guidance on managing side effects and ensuring your overall health and well-being while using semaglutide.
What is the success rate of semaglutide?
The success rate of semaglutide can vary from person to person. In clinical trials, semaglutide has been shown to be effective in helping people with type 2 diabetes and obesity achieve significant weight loss and improve blood sugar control. Many individuals experience positive results with semaglutide, including weight loss, reduced appetite, and better diabetes management.
The FDA conducted a thorough drug evaluation of semaglutide before approving its use for the treatment of type 2 diabetes and chronic weight management. However, the success rate depends on various factors, including individual health conditions, lifestyle choices, and adherence to treatment. Some people may see more significant results with semaglutide, while others may experience more modest changes.
How do I inject semaglutide?
To inject semaglutide, follow these steps:
- Choose a clean injection site on your abdomen, thigh, or upper arm.
- Clean the area with an alcohol swab and let it dry.
- Remove the cap from the pre-filled pen and attach a new needle.
- Dial the prescribed dose on the pen’s dose selector.
- Pinch the skin at the injection site and insert the needle at a 90-degree angle.
- Press the injection button to deliver the medication and hold for 6 seconds.
- Remove the needle and dispose of it safely.
- Store the pen in a refrigerator and avoid freezing. Rotate injection sites to prevent skin reactions. Always follow your healthcare provider’s instructions for safe and proper injection techniques.
Blog

The Science of Semaglutide: How It Works in the Body
As research continues to unfold, semaglutide holds promise for improving the lives of millions of people worldwide who are affected by these conditions. However, it is crucial to consult with healthcare professionals to determine if semaglutide is appropriate and safe for individual circumstances..
Introduction:
Semaglutide is a medication that has gained significant attention in recent years due to its remarkable efficacy in the treatment of type 2 diabetes and obesity. With its unique mechanism of action, semaglutide offers a promising solution for individuals struggling with these conditions. In this blog post, we will delve into the science behind semaglutide and explore how it works within the body to achieve its therapeutic effects.
The Basics of Semaglutide:
Semaglutide belongs to a class of medications called glucagon-like peptide-1 receptor agonists (GLP-1 RAs). It is a synthetic analog of a naturally occurring hormone called glucagon-like peptide-1 (GLP-1), which is secreted by the intestines in response to food intake. GLP-1 plays a crucial role in regulating blood sugar levels and appetite.
Enhancing Glucose Control:
One of the primary actions of semaglutide is to improve glucose control. It does this by binding to GLP-1 receptors in the pancreas, which stimulates the release of insulin, a hormone that helps lower blood sugar levels. Insulin promotes the uptake of glucose by cells, reducing its concentration in the bloodstream. Semaglutide also suppresses the release of glucagon, another hormone that increases blood sugar levels, further contributing to improved glucose control.
Slowing Gastric Emptying:
Another important aspect of semaglutide’s mechanism of action is its ability to slow down the rate at which the stomach empties. By doing so, semaglutide helps to prolong the feeling of fullness and reduces the urge to eat. This effect is particularly beneficial for individuals with obesity, as it can aid in weight loss by reducing calorie intake and promoting a sense of satiety.
Impact on Body Weight:
Semaglutide has been extensively studied for its role in weight management. The medication has consistently demonstrated significant weight loss effects in clinical trials. The exact mechanism by which semaglutide promotes weight loss is not fully understood, but it is believed to involve a combination of reduced appetite, increased satiety, and improved energy expenditure.
Effect on Cardiovascular Health:
In addition to its beneficial effects on glucose control and weight management, semaglutide has shown promising results in improving cardiovascular health. Clinical studies have demonstrated a reduced risk of major adverse cardiovascular events, such as heart attack and stroke, in individuals treated with semaglutide. The exact mechanisms underlying these cardiovascular benefits are still being investigated but may involve a combination of improved blood sugar control, weight loss, and direct effects on blood vessels and the heart.
Administration and Dosage:
Semaglutide is administered as a subcutaneous injection, typically once a week. The dosage may vary depending on the condition being treated, and it is essential to follow the prescribed instructions provided by healthcare professionals.
Side Effects:
Like any medication, semaglutide can cause side effects, although they are generally mild and well-tolerated. The most commonly reported side effects include nausea, vomiting, and diarrhea. These symptoms usually subside over time as the body adjusts to the medication. It is important to discuss any concerns or side effects with a healthcare provider.
Conclusion:
Semaglutide represents a significant advancement in the treatment of type 2 diabetes and obesity. Through its complex mechanisms of action, it effectively improves glucose control, promotes weight loss, and shows potential cardiovascular benefits. As research continues to unfold, semaglutide holds promise for improving the lives of millions of people worldwide who are affected by these conditions. However, it is crucial to consult with healthcare professionals to determine if semaglutide is appropriate and safe for individual circumstances.

Lowering Blood Pressure with Semaglutide: A New Approach to Hypertension
However, it is crucial to consult with healthcare professionals to determine if semaglutide is appropriate and safe for individual circumstances. As research continues, semaglutide may pave the way for more effective and personalized treatment strategies for individuals living with hypertension..
Introduction:
Hypertension, commonly known as high blood pressure, is a prevalent condition that affects millions of people worldwide. If left uncontrolled, it can lead to serious health complications. In recent years, a medication called semaglutide, primarily used for the treatment of type 2 diabetes and obesity, has shown promising results in lowering blood pressure. In this blog post, we will explore the potential of semaglutide as a new approach to managing hypertension.
Understanding Hypertension:
Before delving into the role of semaglutide in lowering blood pressure, it is essential to understand hypertension itself. Hypertension occurs when the force of blood against the walls of the arteries is consistently too high. It is often referred to as the “silent killer” because it typically presents no noticeable symptoms, yet it increases the risk of heart disease, stroke, and other serious conditions.
Semaglutide: A Glucagon-like Peptide-1 Receptor Agonist (GLP-1 RA):
Semaglutide belongs to a class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs). Originally developed for the management of type 2 diabetes, semaglutide has shown additional benefits beyond glycemic control.
The Mechanism of Action:
Semaglutide acts by binding to GLP-1 receptors in various tissues throughout the body, including the pancreas, brain, and blood vessels. This binding triggers a cascade of physiological responses that contribute to the medication’s effects on blood pressure regulation.
Vasodilation:
One of the key mechanisms by which semaglutide lowers blood pressure is through vasodilation, the widening of blood vessels. When semaglutide activates GLP-1 receptors in blood vessels, it promotes the relaxation and expansion of the vessel walls, allowing blood to flow more freely. This dilation reduces the resistance that the heart must work against, thus lowering blood pressure.
Renal Effects:
Semaglutide has also been found to have beneficial effects on the kidneys, which play a crucial role in blood pressure regulation. The medication promotes natriuresis, the excretion of sodium through the urine, which helps to reduce fluid volume and subsequently lower blood pressure. Semaglutide also improves the function of the glomerular filtration rate, which is a measure of kidney function.
Weight Loss and Blood Pressure:
Obesity is a significant risk factor for hypertension, and weight loss has been shown to have a positive impact on blood pressure levels. Semaglutide has been associated with substantial weight loss in clinical trials, making it a potentially valuable option for individuals with both obesity and hypertension. By promoting weight loss, semaglutide may indirectly contribute to blood pressure reduction.
Cardiovascular Benefits:
In addition to its blood pressure-lowering effects, semaglutide has demonstrated cardiovascular benefits in clinical trials. Research has shown a reduced risk of major adverse cardiovascular events, such as heart attack and stroke, in individuals treated with semaglutide. These benefits may be attributed to improved glycemic control, weight loss, reduced inflammation, and direct effects on blood vessels and the heart.
Combination Therapy:
Semaglutide can be used as an adjunct to other antihypertensive medications to further enhance blood pressure control. Its unique mechanism of action complements existing treatments, providing an additional avenue for managing hypertension.
Conclusion:
Semaglutide, initially developed for the treatment of type 2 diabetes and obesity, has emerged as a potential new approach to managing hypertension. Through its vasodilatory effects, renal benefits, weight loss promotion, and cardiovascular advantages, semaglutide offers a multi-faceted approach to blood pressure control.

The Amazing Health Benefits of Semaglutide: A Comprehensive Guide
However, it is essential to consult with healthcare professionals to assess individual suitability and safety considerations. As research continues, semaglutide holds great promise for transforming the lives of individuals by addressing various aspects of their overall health and well-being.
Introduction:
Semaglutide, a medication known for its efficacy in treating type 2 diabetes and obesity, offers a range of remarkable health benefits beyond its primary indications. As a glucagon-like peptide-1 receptor agonist (GLP-1 RA), semaglutide exerts its effects through various mechanisms within the body. In this comprehensive guide, we will explore the diverse health benefits of semaglutide and its potential impact on multiple aspects of well-being.
Glycemic Control and Diabetes Management:
Semaglutide is highly effective in improving glycemic control in individuals with type 2 diabetes. By stimulating insulin secretion and suppressing glucagon release, semaglutide helps regulate blood sugar levels, reducing the risk of hyperglycemia and its associated complications. Additionally, semaglutide promotes beta-cell preservation and function, which is crucial for long-term diabetes management.
Weight Management:
Semaglutide has gained attention for its significant impact on weight loss. Clinical trials have consistently shown that semaglutide leads to substantial reductions in body weight, making it an invaluable tool for individuals struggling with obesity. Through appetite suppression, increased satiety, and reduced caloric intake, semaglutide aids in achieving sustainable weight loss goals and improving overall metabolic health.
Cardiovascular Benefits:
Emerging research suggests that semaglutide offers cardiovascular benefits beyond glycemic control. Clinical trials have demonstrated a reduced risk of major adverse cardiovascular events, such as heart attack and stroke, in individuals treated with semaglutide. These cardioprotective effects may be attributed to improved blood sugar control, weight loss, reduced inflammation, and direct effects on blood vessels and the heart.
Blood Pressure Regulation:
Semaglutide has shown promising results in lowering blood pressure levels. By promoting vasodilation and reducing fluid volume through natriuresis, semaglutide helps regulate blood pressure, making it a potentially valuable treatment option for individuals with hypertension. Additionally, the medication’s weight loss effects contribute to improved blood pressure control.
Renal Benefits:
Semaglutide has been associated with positive effects on kidney function. The medication promotes natriuresis, reducing fluid volume and potentially benefiting individuals with conditions such as chronic kidney disease. Semaglutide also improves the glomerular filtration rate, supporting overall renal health.
Potential Impact on Non-Alcoholic Fatty Liver Disease (NAFLD):
Non-alcoholic fatty liver disease is a prevalent condition closely linked to obesity and metabolic disorders. Preliminary studies suggest that semaglutide may improve liver health by reducing hepatic fat accumulation and inflammation. These findings provide hope for individuals with NAFLD who are seeking effective treatment options.
Neuroprotective Effects:
Emerging evidence suggests that semaglutide may have neuroprotective effects, potentially benefiting individuals with neurodegenerative diseases such as Alzheimer’s and Parkinson’s. These effects may involve reduced inflammation, improved insulin sensitivity in the brain, and enhanced neuronal survival.
Gastrointestinal Benefits:
Semaglutide can alleviate gastrointestinal symptoms commonly experienced by individuals with diabetes, such as gastroparesis and constipation. By improving gastric emptying and motility, semaglutide enhances digestive function and offers relief from these troublesome symptoms.
Conclusion:
Semaglutide, initially developed for the management of type 2 diabetes and obesity, offers a wide range of health benefits beyond its primary indications. From improved glycemic control and weight management to cardiovascular benefits, blood pressure regulation, and potential impacts on liver health, neuroprotection, and gastrointestinal function, semaglutide represents a multifaceted treatment option.

Reducing the Risk of Cardiovascular Disease with Semaglutide
As further research continues to unfold, semaglutide holds great promise for individuals at risk of cardiovascular disease, offering a potential paradigm shift in the prevention and management of this prevalent condition. However, it is essential to consult with healthcare professionals to assess individual suitability and safety considerations for semaglutide treatment..
Introduction:
Cardiovascular disease (CVD) remains a leading cause of mortality worldwide, emphasizing the need for effective prevention and management strategies. In recent years, semaglutide, a medication primarily used for the treatment of type 2 diabetes and obesity, has demonstrated significant potential in reducing the risk of cardiovascular events. In this blog post, we will explore the exciting research surrounding semaglutide and its ability to lower the risk of cardiovascular disease.
Understanding Cardiovascular Disease:
Cardiovascular disease encompasses a range of conditions affecting the heart and blood vessels, including coronary artery disease, heart failure, and stroke. Risk factors such as high blood pressure, high cholesterol levels, obesity, and diabetes significantly contribute to the development and progression of CVD.
Semaglutide as a Cardiovascular Disease Modifier:
Semaglutide belongs to the class of medications known as glucagon-like peptide-1 receptor agonists (GLP-1 RAs), which have been found to offer cardioprotective effects beyond glycemic control.
Clinical Trials:
Semaglutide has undergone extensive clinical trials to evaluate its impact on cardiovascular outcomes. In landmark trials such as SUSTAIN-6 and PIONEER-6, semaglutide demonstrated a significant reduction in major adverse cardiovascular events, including cardiovascular death, non-fatal heart attacks, and strokes, compared to placebo or standard care.
Glycemic Control and Cardiovascular Health:
Improved glycemic control is crucial for individuals with diabetes to reduce the risk of cardiovascular complications. Semaglutide effectively lowers blood sugar levels by stimulating insulin secretion and suppressing glucagon release. By achieving optimal glycemic control, semaglutide helps mitigate the detrimental effects of hyperglycemia on the cardiovascular system.
Weight Loss and Metabolic Benefits:
Obesity is a major risk factor for cardiovascular disease. Semaglutide has consistently demonstrated significant weight loss effects in clinical trials. By promoting weight reduction, semaglutide improves metabolic parameters, including blood pressure, cholesterol levels, and insulin sensitivity, thereby reducing the risk of cardiovascular events.
Anti-inflammatory Effects:
Chronic inflammation plays a pivotal role in the development and progression of cardiovascular disease. Semaglutide has been shown to have anti-inflammatory effects by reducing inflammatory markers in the body. These anti-inflammatory properties may contribute to the medication’s cardioprotective benefits.
Blood Pressure Regulation:
Hypertension, or high blood pressure, is a major risk factor for cardiovascular disease. Semaglutide has been observed to lower blood pressure levels in individuals with and without diabetes. By promoting vasodilation and reducing fluid volume through natriuresis, semaglutide helps regulate blood pressure and reduces the strain on the cardiovascular system.
Lipid Profile Improvements:
Elevated cholesterol levels, specifically LDL cholesterol, contribute to the development of atherosclerosis and cardiovascular disease. Semaglutide has been shown to improve lipid profiles by reducing LDL cholesterol levels and increasing HDL cholesterol levels, thereby positively impacting cardiovascular health.
Endothelial Function:
The endothelium, a layer of cells lining blood vessels, plays a vital role in maintaining vascular health. Impaired endothelial function is associated with an increased risk of cardiovascular events. Semaglutide has been found to improve endothelial function, promoting proper blood vessel dilation and reducing the risk of cardiovascular disease.
Conclusion:
Semaglutide, originally developed for the treatment of type 2 diabetes and obesity, offers a significant breakthrough in reducing the risk of cardiovascular disease. Through its multifaceted mechanisms of action, including improved glycemic control, weight loss, anti-inflammatory effects, blood pressure regulation, lipid profile improvements, and enhancement of endothelial function, semaglutide provides a comprehensive approach to cardiovascular risk reduction.
References:
- Drucker D. J. (2018). Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1. Cell metabolism, 27(4), 740–756. https://doi.org/10.1016/j.cmet.2018.03.001.
Mechanisms of Action and Therapeutic Application of Glucagon-like Peptide-1
By raising insulin production and reducing glucagon secretion, the hormone glucagon-like peptide-1 (GLP-1) regulates blood sugar levels before and after meals. It is secreted from the gut’s endocrine cells. Additionally, it slows down the emptying of the stomach and reduces appetite, allowing for more effective nutrition absorption. This process also helps with weight gain.
A review focused on the mechanisms involved in the actions of natural and synthetic GLP-1, highlighting the cell types that express the GLP-1 receptor (GLP-1 R) and the signaling pathways that mediate the hormone’s metabolic and non-glycemic effects. The review also discussed the role of GLP-1 in the context of bariatric surgery, as well as its impact on inflammation, cardiovascular health, appetite control, and pancreatic function. The discovery of novel GLP-1 R agonists, which include orally bioavailable agonists, allosteric modulators, and multi-agonists, is noted in this review. It was found that in the first 10 years of GLP-1 therapeutics, the use of these medications in patients with type 2 diabetes mellitus and obesity is growing. In contrast to insulin, GLP-1 R agonists were associated with a lower risk of cardiovascular events such as myocardial infarction, cardiovascular death, and stroke.
The development of next-generation improved GLP-1 therapies need a thorough understanding of their mechanisms of action. Advances in understanding how certain factors such as bacteria, microbes, and nutrients control enteroendocrine cells (EECs) GLP-1 secretion may provide an opportunity for the development of more potent GLP-1 secretagogues.
You can read the full article at https://www.cell.com/cell-metabolism/fulltext/S1550-4131(18)30179-7?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1550413118301797%3Fshowall%3Dtrue. - Wilding, J., Batterham, R. L., Calanna, S., Davies, M., Van Gaal, L. F., Lingvay, I., McGowan, B. M., Rosenstock, J., Tran, M., Wadden, T. A., Wharton, S., Yokote, K., Zeuthen, N., Kushner, R. F., & STEP 1 Study Group (2021). Once-Weekly Semaglutide in Adults with Overweight or Obesity. The New England journal of medicine, 384(11), 989. https://doi.org/10.1056/NEJMoa2032183.
Once-Weekly Semaglutide in Adults with Overweight or Obesity
With few medicinal solutions available, obesity is a severe global health issue. This condition can lead to health problems such as insulin resistance, hypertension, and dyslipidemia (abnormal lipid levels). More recently, obesity has been linked to a significant increase in the rate of hospitalizations and deaths. Although lifestyle intervention that includes proper diet and exercise remains the cornerstone of managing obesity, achieving long-term weight loss poses a significant challenge.
A study determined whether once-weekly administration of semaglutide at a dose of 2.4 mg along with a lifestyle intervention may help adults with obesity lose weight. A total of 1961 adults with a body-mass index (BMI) of 30 or above (or ≥27 in individuals with at least one weight-related medical condition) and without diabetes were enrolled in the study. Participants were randomly assigned to receive either a once-weekly subcutaneous semaglutide or a placebo for 68 weeks in combination with lifestyle intervention. The coprimary endpoints were the percentage change in body weight and weight reduction of at least 5%.
Results showed that participants who received semaglutide experienced greater improvements in cardiometabolic risk factors and physical functioning than those who received a placebo. Although nausea and diarrhea were the most frequent side effects associated with semaglutide, they were often mild to moderate in intensity and eventually went away. In comparison to the placebo group, more participants in the semaglutide group stopped taking the medication due to gastrointestinal problems.
In conclusion, the study found that semaglutide, in combination with lifestyle intervention, was associated with a sustained and clinically relevant reduction in body weight in individuals with overweight or obesity.
Read the full article on: https://www.nejm.org/doi/full/10.1056/NEJMoa2032183 - Kushner, R. F., Calanna, S., Davies, M., Dicker, D., Garvey, W. T., Goldman, B., Lingvay, I., Thomsen, M., Wadden, T. A., Wharton, S., Wilding, J., &Rubino, D. (2020). Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity (Silver Spring, Md.), 28(6), 1050–1061. https://doi.org/10.1002/oby.22794.
Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5
Public health officials are becoming increasingly concerned about the obesity pandemic. To manage this situation, more research into pharmacological approaches to supporting weight management in conjunction with lifestyle changes is required.
In order to find out how semaglutide compares to placebo in terms of weight loss, safety, and tolerability in individuals who are overweight or obese, the Semaglutide Treatment Effect in People with Obesity (STEP) program has been established. The STEP program encompasses five phase 3 trials that focus on the different aspects of weight management, including weight management in type 2 diabetes, weight management with intensive behavioral therapy, sustained weight management, and long-term weight management. Roughly 5,000 participants are being randomly assigned to receive a once-weekly subcutaneous injection of semaglutide 2.4 mg or a placebo. The participants range in age from 46.2 to 55.3 years, are mostly female, and have a mean BMI of 35.7 to 38.5 kg/m2 and a mean waist circumference of 113.0 to 115.7 cm.
In a broad population, the STEP program aims to evaluate the efficacy and safety of semaglutide 2.4 mg once weekly administered subcutaneously. The trials could lead to new and more effective ways to address the obesity epidemic by shedding light on the possible advantages and disadvantages of this weight-management therapy option. As a researcher, it is critical to keep looking into and assessing fresh approaches to help people who are obese or overweight healthily maintain their weight.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7318657/..
- Christou, G. A., Katsiki, N., Blundell, J., Fruhbeck, G., &Kiortsis, D. N. (2019). Semaglutide as a promising antiobesity drug. Obesity reviews : an official journal of the International Association for the Study of Obesity, 20(6), 805–815. https://doi.org/10.1111/obr.12839.
Semaglutide as a promising antiobesity drug
An agonist for the glucagon-like peptide-1 receptor (GLP-1 RA), semaglutide is a medication that can be administered subcutaneously once every week. Recently, once-weekly subcutaneous semaglutide for the treatment of type 2 diabetes mellitus (T2DM) received approval from both the Food and Drug Administration (FDA) and the European Medicines Agency (EMA).
Researchers have shown that once-weekly subcutaneous semaglutide appears to be more effective than other once-weekly GLP-1 RAs in patients with T2DM for weight loss. In a phase II dose-finding trial, semaglutide was evaluated as an anti-obesity medication, demonstrating superior weight loss efficacy when administered once daily subcutaneously compared to both placebo and once-daily 3.0 mg liraglutide in patients with obesity but without T2DM. The degree of semaglutide-induced weight loss in this study exceeded the EMA and FDA’s criteria for anti-obesity drugs and there were no safety concerns, indicating the potential of once-daily subcutaneous semaglutide as an anti-obesity drug in the future.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1111/obr.12839..
- Rubino, D., Abrahamsson, N., Davies, M., Hesse, D., Greenway, F. L., Jensen, C., Lingvay, I., Mosenzon, O., Rosenstock, J., Rubio, M. A., Rudofsky, G., Tadayon, S., Wadden, T. A., Dicker, D., & STEP 4 Investigators (2021). Effect of Continued Weekly Subcutaneous Semaglutidevs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity: The STEP 4 Randomized Clinical Trial. JAMA, 325(14), 1414–1425. https://doi.org/10.1001/jama.2021.3224.
Effect of Continued Weekly Subcutaneous Semaglutide vs Placebo on Weight Loss Maintenance in Adults With Overweight or Obesity
The study’s goal was to evaluate the effectiveness of continuing treatment with subcutaneous semaglutide 2.4 mg versus switching to a placebo for people who had completed a 20-week run-in phase with the drug. A total of 902 patients received once-weekly subcutaneous semaglutide during the run-in phase of the research. Out of these, 803 participants who reached the semaglutide maintenance dose were randomized to 48 weeks of continued subcutaneous semaglutide or switched to placebo, both with lifestyle intervention.
The researchers found that among adults who were overweight or obese who completed the 20-week run-in period with subcutaneous semaglutide, maintaining treatment with semaglutide resulted in continued weight loss over the following 48 weeks compared to switching to placebo. The study emphasized the significance of continuing subcutaneous semaglutide therapy for weight loss in people who are overweight or obese. The findings of the study revealed that semaglutide-treated participants continued to lose weight whereas those who switched to placebo did not see any discernible weight loss. Waist circumference, systolic blood pressure, and physical functioning score also improved in participants who continuously used semaglutide than in the placebo-treated group. The need for lifestyle changes in addition to medicine is also emphasized in the study as a key component of effective weight management.
In conclusion, the study suggests that continued treatment with subcutaneous semaglutide 2.4 mg along with lifestyle intervention is effective for weight maintenance in adults who are overweight or obese. The researchers recommend the use of subcutaneous semaglutide as a part of a comprehensive weight loss management program for individuals who are overweight or obese.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7988425/..
- O’Neil, P. M., Birkenfeld, A. L., McGowan, B., Mosenzon, O., Pedersen, S. D., Wharton, S., Carson, C. G., Jepsen, C. H., Kabisch, M., & Wilding, J. (2018). Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet (London, England), 392(10148), 637–649. https://doi.org/10.1016/S0140-6736(18)31773-2.
Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial
Obesity is a major public health issue worldwide. In this case, new medications to address this condition are needed. Therefore, a study evaluated the safety and efficacy of semaglutide, a glucagon-like peptide-1 (GLP-1) analogue in comparison with another GLP-1 analogue liraglutide and placebo in promoting weight loss.
The study, a phase 2 multicenter, dose-ranging trial including 71 clinical sites across eight nations, was conducted. Adults without diabetes and those with a BMI of 30 kg/m2 or higher qualified as participants. The participants were randomly assigned to each active treatment group, semaglutide, liraglutide, or a corresponding placebo group using a block size of 56 in the study’s randomized, double-blind, placebo-controlled design. Subcutaneous injections were used to administer all therapy doses once a day. The primary endpoint of the study was percentage weight loss at week 52. The primary analysis was done using intention-to-treat ANCOVA estimation with missing data derived from the placebo pool. The researchers found that all semaglutide doses were generally well tolerated with no new safety concerns. The most common adverse events were dose-related gastrointestinal symptoms, primarily nausea, which were previously seen with GLP-1 receptor agonists.
The researchers concluded that semaglutide was well tolerated over 52 weeks when used in conjunction with dietary and exercise counseling and demonstrated clinically meaningful weight loss as compared to placebo at all doses. Semaglutide may be a useful new pharmacological alternative for weight management, addressing the public health problem of obesity, according to the study’s findings.
You can read the abstract at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31773-2/fulltext..
- Davies, M., Færch, L., Jeppesen, O. K., Pakseresht, A., Pedersen, S. D., Perreault, L., Rosenstock, J., Shimomura, I., Viljoen, A., Wadden, T. A., Lingvay, I., & STEP 2 Study Group (2021). Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial. Lancet (London, England), 397(10278), 971–984. https://doi.org/10.1016/S0140-6736(21)00213-0.
Semaglutide 2·4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double-blind, double-dummy, placebo-controlled, phase 3 trial
A group of researchers conducted a phase 3 clinical trial to examine the efficacy and safety of subcutaneous semaglutide 2.4 mg once per week, semaglutide 1.0 mg (licensed for the treatment of diabetes), and placebo in managing weight in an adult population who were overweight or obese and have type 2 diabetes. Adults with a body mass index of at least 27 kg/m2 and glycated hemoglobin levels between 7 and 10% who had been diagnosed with type 2 diabetes for at least 180 days prior to screening were included in the double-blind, double-dummy study.
In 149 outpatient clinics spread across 12 nations in Europe, North America, South America, the Middle East, South Africa, and Asia, the trial was carried out. Patients were randomly allocated to receive subcutaneous injections of semaglutide 2.4 mg, semaglutide 1.0 mg, or placebo once a week for 68 weeks, along with lifestyle intervention. The coprimary endpoints of the trial were the percentage change in body weight and achieving a weight reduction of at least 5% at 68 weeks for semaglutide 2.4 mg versus placebo, assessed by intention to treat. Safety was assessed in all patients who received at least one dose of the study drug.
According to the trial’s findings, semaglutide 2.4 mg once a week was considerably more effective than a placebo at helping adults with type 2 diabetes who were overweight or obese lose body weight. The decrease in body weight was regarded as clinically significant. Semaglutide 2.4 mg had a similar safety profile to semaglutide 1.0 mg and placebo. At week 68, patients who received semaglutide achieved weight reductions of at least 5% compared to the placebo-treated group. In addition, mild to moderate gastrointestinal side effects were more frequent in the semaglutide-treated group compared to the placebo-treated group.
In conclusion, the study demonstrated that semaglutide 2.4 mg once a week, in combination with lifestyle intervention, can effectively manage weight in adults with obesity and type 2 diabetes. The findings have important clinical implications and suggest that semaglutide 2.4 mg could be an effective option for managing weight in this patient population.
You can read the abstract at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(21)00213-0/fulltext..
- Wadden, T. A., Bailey, T. S., Billings, L. K., Davies, M., Frias, J. P., Koroleva, A., Lingvay, I., O’Neil, P. M., Rubino, D. M., Skovgaard, D., Wallenstein, S., Garvey, W. T., & STEP 3 Investigators (2021). Effect of Subcutaneous Semaglutidevs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA, 325(14), 1403–1413. https://doi.org/10.1001/jama.2021.1831.
Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial
Among people who are overweight or obese, it is well established that weight loss can reduce cardiometabolic risk factors. Pharmacotherapy and rigorous lifestyle modification are the two most efficient noninvasive methods for weight loss.
A randomized controlled trial was carried out to examine the effectiveness of once-weekly subcutaneous semaglutide 2.4 mg as an addition to intensive behavioral therapy and initial low-calorie diet in individuals with overweight or obesity. The goal was to compare semaglutide’s weight-loss results over 68 weeks with those of a placebo. Participants were randomized to receive either semaglutide or placebo in a 2:1 ratio, combined with a low-calorie diet for the first 8 weeks, followed by intensive behavioral therapy for 68 weeks, including 30 counseling visits. The study found that adults with overweight or obesity who received semaglutide, compared to those who received placebo, experienced a significant reduction in weight over the 68-week period. According to the findings of the study, a once-weekly subcutaneous semaglutide regimen combined with intense behavioral treatment and an initial low-calorie diet can help people who are overweight or obese lose weight. To ascertain whether these findings will be valid in the long run, more study is required.
In conclusion, the researchers found that semaglutide is a promising weight loss treatment option for individuals with overweight or obesity. When combined with intensive behavioral therapy and a low-calorie diet, it can result in greater weight loss. These findings have important implications for the management of overweight and obesity, as well as the prevention of associated cardiometabolic risk factors. However, additional research is needed to determine the long-term effects and durability of this treatment approach.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7905697/..
- Ahrén, B., Atkin, S. L., Charpentier, G., Warren, M. L., Wilding, J., Birch, S., Holst, A. G., &Leiter, L. A. (2018). Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials. Diabetes, obesity & metabolism, 20(9), 2210–2219. https://doi.org/10.1111/dom.13353.
Semaglutide induces weight loss in subjects with type 2 diabetes regardless of baseline BMI or gastrointestinal adverse events in the SUSTAIN 1 to 5 trials
In the SUSTAIN 1 to 5 studies, semaglutide was found to cause more weight loss and HbA1c level reductions than other drugs, although the underlying mechanisms causing this weight loss were not previously known. To determine the weight loss effects of semaglutide, a once-weekly glucagon-like peptide 1 analogue used to treat type 2 diabetes, a group of researchers included subjects with poorly controlled type 2 diabetes who were either drug-naïve or on background treatment in a study.
The subjects were randomly assigned to receive subcutaneous semaglutide at doses of 0.5 mg or comparator medications (placebo, sitagliptin, exenatide extended-release, or insulin glargine). The subjects were categorized based on their baseline BMI and whether they experienced nausea and/or vomiting. The researchers assessed the change in body weight from baseline within each trial and subgroup and they also carried out a mediation analysis to differentiate between direct and indirect effects of semaglutide on weight loss, the latter of which was mediated by nausea or vomiting. The results of the study showed that semaglutide consistently induced greater weight loss compared to comparators across all trials, irrespective of baseline BMI. The role of nausea or vomiting in this weight loss was found to be minor, indicating that the weight loss effects of semaglutide were primarily due to direct mechanisms unrelated to nausea or vomiting.
In conclusion, the researchers found that semaglutide was effective in promoting weight loss in subjects with poorly controlled type 2 diabetes, regardless of their baseline BMI. While nausea and vomiting were observed in some subjects, these symptoms were not significant mediators of the weight loss effects of semaglutide.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6099440/..
- Friedrichsen, M., Breitschaft, A., Tadayon, S., Wizert, A., &Skovgaard, D. (2021). The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating,and gastric emptying in adults with obesity. Diabetes, obesity & metabolism, 23(3), 754–762. https://doi.org/10.1111/dom.14280.
The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity
A study investigated the effects of once-weekly subcutaneous (s.c.) semaglutide 2.4 mg on gastric emptying, appetite, and energy intake in people with obesity. In a double-blind, parallel-group trial, the researchers randomly assigned 72 obese people to receive semaglutide or placebo.
For 20 weeks, the subjects were given semaglutide (dose-escalated to 2.4 mg) or a placebo. To measure stomach emptying after a typical breakfast, the researchers utilized paracetamol absorption. They examined caloric intake during an open-ended meal as well as participant-reported appetite ratings and Control of Eating Questionnaire (CoEQ) responses. The results of the study showed that once-weekly s.c. semaglutide 2.4 mg had a significant impact on the appetite and eating habits of adults with obesity. The researchers found that the medication suppressed appetite, improved control of eating, and reduced food cravings, ad libitum energy intake, and body weight compared to the placebo. Interestingly, there was no evidence of delayed gastric emptying at week 20, assessed indirectly via paracetamol absorption. The study concluded that once-weekly s.c. semaglutide 2.4 mg may be a useful therapy choice for obese people who have trouble controlling their appetites and eating patterns. Food cravings appeared to be significantly reduced by semaglutide, which may make it easier for people to stick to healthier eating habits and lose weight. Additionally, the absence of evidence of delayed stomach emptying shows that the drug has no negative effects on the digestive system, which is crucial to take into account when assessing the efficacy and safety of weight-loss drugs.
Overall, the study provides valuable insights into the potential benefits of once-weekly s.c. semaglutide 2.4 mg as a treatment option for adults with obesity. The findings highlight the importance of addressing appetite control and eating habits in the management of obesity and suggest that medications like s.c. semaglutide may be a useful tool in this regard. Further research is needed to fully understand the long-term effects and safety profile of this medication but the results of this study provide a promising foundation for future investigations.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7898914/..
- Blundell, J., Finlayson, G., Axelsen, M., Flint, A., Gibbons, C., Kvist, T., &Hjerpsted, J. B. (2017). Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity. Diabetes, obesity & metabolism, 19(9), 1242–1251. https://doi.org/10.1111/dom.12932.
Effects of once-weekly semaglutide on appetite, energy intake, control of eating, food preference and body weight in subjects with obesity
The goal of the study was to find out how semaglutide encourages weight loss. The researchers included 30 obese patients in a randomized, double-blind, placebo-controlled, two-period crossover experiment over a 12-week period to accomplish this. Semaglutide was administered subcutaneously to the subjects once per week at doses that were eventually increased to 1.0 mg.
Throughout the trial, the researchers measured several variables related to appetite, eating behavior, and metabolism. These included ad libitum energy intake, ratings of appetite, thirst, nausea, well-being, control of eating, food preference, resting metabolic rate, body weight, and body composition. The results of the trial showed that semaglutide was associated with less hunger and food cravings, better control of eating, and a lower preference for high-fat foods. However, resting metabolic rate, adjusted for lean body mass, did not differ between treatments. The study’s most important finding was the average 5.0 kg drop in body weight that was seen with semaglutide, mostly due to a reduction in body fat mass. This weight loss was probably caused by a decrease in energy intake but the researchers also discovered a number of additional processes that may have helped, such as a decrease in hunger and food cravings, improved behavior control for eating, and a decreased relative preference for fatty, energy-dense foods.
Overall, the study provided important insights into the mechanism of action for semaglutide-induced weight loss. The findings suggest that semaglutide may be a useful tool in the management of obesity, not only due to its effects on energy intake but also due to its impact on appetite and eating behavior.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5573908/. .
- Available from https://care.diabetesjournals.org/content/41/9/1926.figures-only..
A 26-Week Randomized Controlled Trial of Semaglutide Once Daily Versus Liraglutide and Placebo in Patients With Type 2 Diabetes Suboptimally Controlled on Diet and Exercise With or Without Metformin
Glucagon-like peptide 1 (GLP-1) is a powerful hormone that significantly decreases blood glucose (sugar) levels. GLP-1 is derived from the gut and lowers the risk of hypoglycemia (low blood sugar levels). GLP-1 also decreases body weight and calorie consumption by preventing stomach emptying and producing feelings of fullness.
The hypothalamus, a part of the brain that controls satiety and appetite, is considered to express GLP-1 receptors, which are thought to play a role in weight loss. These characteristics have made GLP-1 medicines a crucial part of managing type 2 diabetes, along with indications that some GLP-1 receptor agonists can enhance cardiovascular outcomes.
A 26-week, multicenter, double-blind trial assessed the efficacy and safety of once-daily semaglutide compared to once-daily liraglutide and placebo in patients with type 2 diabetes. The study included patients with HbA1c levels ranging from 7.0-10.0% (53-86 mmol/mol) who were being treated with diet and exercise with or without metformin. The researchers randomized the patients into three groups: once-daily semaglutide, liraglutide, or placebo in one of four volume-matched doses (semaglutide 0.05, 0.1, 0.2, or 0.3 mg and liraglutide 0.3, 0.6, 1.2, or 1.8 mg, with both compared within each volume-matched dose group). The HbA1c change between baseline and week 26 was the main goal. The findings demonstrated that when compared to liraglutide or placebo, once-daily semaglutide at doses up to 0.3 mg/day caused larger reductions in HbA1c. Patients who received semaglutide, however, reported more gastrointestinal adverse events (AEs).
The study’s findings show that semaglutide may be a better treatment option for lowering HbA1c levels in type 2 diabetic patients than liraglutide or a placebo. However, when selecting a course of treatment, it is important to take into account the frequency of gastrointestinal adverse events (AEs).
You can read the full article atYou can read the full article at https://diabetesjournals.org/care/article/41/9/1926/40726/A-26-Week-Randomized-Controlled-Trial-of..
- Ji, L., Dong, X., Li, Y., Li, Y., Lim, S., Liu, M., Ning, Z., Rasmussen, S., Skjøth, T. V., Yuan, G., &Eliaschewitz, F. G. (2021). Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week, double-blind, phase 3a, randomized trial. Diabetes, obesity & metabolism, 23(2), 404–414. https://doi.org/10.1111/dom.14232.
Efficacy and safety of once-weekly semaglutide versus once-daily sitagliptin as add-on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30-week, double-blind, phase 3a, randomized trial
Due to increased obesity and inactivity rates, type 2 diabetes (T2D) is a complex, multifaceted disease with a rising prevalence rate. T2D was present in 116.4 million people (10.9%) in China in 2019 and it is predicted that this figure will rise to 140.5 million by 2030, placing a considerable load on the country’s healthcare system.
Optimizing glycemic control to reduce the risks of microvascular and macrovascular consequences is the main goal of T2D care. Semaglutide is a long-acting GLP-1 RA medication that is administered once weekly and is authorized for the treatment of T2D under the brand name Ozempic®. The GLP-1 portion of semaglutide has been modified by adding a fatty diacid chain and making two amino acid substitutions. These modifications enhance its half-life by increasing albumin binding and inhibiting degradation by DPP-4, allowing for once-weekly dosing. The efficacy and safety of semaglutide have been established in over 10,000 patients in the SUSTAIN clinical trial program.
In a multiregional clinical trial, researchers compared the safety and efficacy of once-weekly subcutaneous injections of semaglutide, an analog of glucagon-like peptide-1 (GLP-1), to once-daily injections of sitagliptin in patients with type 2 diabetes (T2D). Semaglutide has consistently demonstrated better reductions in HbA1c and body weight compared to placebo and a variety of active comparators, including sitagliptin, exenatide extended release, insulin glargine, dulaglutide, canagliflozin, and liraglutide. In addition, semaglutide was associated with a significant 26% reduction in major adverse cardiovascular events compared to placebo in a preapproval cardiovascular outcomes trial (CVOT).
In conclusion, the researchers discovered that once-weekly semaglutide was more effective than sitagliptin in improving glycemic control and reducing body weight in T2D patients who were inadequately controlled on metformin. The safety and tolerability profiles of semaglutide were comparable to those of other GLP-1 RAs. Semaglutide is an efficient once-weekly treatment alternative for the Chinese population with T2D.
Read the full article on:Efficacy and safety of once‐weekly semaglutide versus once‐daily sitagliptin as add‐on to metformin in patients with type 2 diabetes in SUSTAIN China: A 30‐week, double‐blind, phase 3a, randomized trial – Ji – 2021 – Diabetes, Obesity and Metabolism – Wiley Online Library. - Available from https://insight.jci.org/articles/view/133429..
Semaglutide lowers body weight in rodents via distributed neural pathways
Six GLP-1 receptor agonists, including four long-acting drugs, have been discovered to be approved for the treatment of type 2 diabetes, according to research. But only liraglutide has been given the go-ahead to treat obesity while semaglutide is currently being studied. Since both liraglutide and semaglutide are fatty acid acylated analogs of human GLP-1, their structures are identical. Semaglutide has a much longer half-life and full dipeptidyl peptidase-4 stability compared to ligandutide, which largely lowers calorie intake.
In comparison to other GLP-1RAs, semaglutide was found to produce up to three times higher weight loss in type 2 diabetic patients and up to twice as much weight loss in obese patients, with some patients losing up to 15 kg. Clinical trials have also shown that semaglutide reduced energy intake by 25% over three meals while liraglutide reduced energy intake by 15% over one meal. Additionally, semaglutide lowered cardiovascular risk in patients with diabetes. Semaglutide is currently being investigated in two large phase III clinical trial programs for treating obesity: the STEP program, which aims to obtain regulatory approval for semaglutide as an anti-obesity drug and is evaluating cardiovascular outcomes in 17,500 patients. The researchers discovered that semaglutide causes weight loss without affecting energy expenditure through altering food preferences and dietary consumption.
Semaglutide does not cross the blood-brain barrier, yet it directly targets the brainstem, septal nucleus, and hypothalamus. Instead, it communicates with the brain by means of the structures that surround the ventricles and a few specific locations nearby. In addition, semaglutide causes central c-Fos activation in eleven brain regions, including secondary regions devoid of direct GLP-1R interaction and portions of the hindbrain that are directly targeted by the drug.
Based on the findings, the researchers suggest that semaglutide lowers body weight by directly interacting with diverse GLP-1R populations and directly and indirectly affecting the activity of neural pathways involved in food intake, reward, and energy expenditure. Transcriptomic analysis of microdissected brain areas from semaglutide-treated rats also showed upregulation of prolactin-releasing hormone and tyrosine hydroxylase in the area postrema (part of the brainstem).
You can read the full article at https://insight.jci.org/articles/view/133429.
- Lingvay, I., Hansen, T., Macura, S., Marre, M., Nauck, M. A., de la Rosa, R., Woo, V., Yildirim, E., & Wilding, J. (2020). Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events. BMJ open diabetes research & care, 8(2), e001706. https://doi.org/10.1136/bmjdrc-2020-001706
Superior weight loss with once-weekly semaglutide versus other glucagon-like peptide-1 receptor agonists is independent of gastrointestinal adverse events
The usage of glucagon-like peptide-1 receptor agonists (GLP-1RAs) has been linked to adverse events (AEs). Researchers discovered that gastrointestinal AEs are the most often reported AEs associated with these medications. People who use GLP-1RAs and have GI AEs typically lose a little bit more weight than those who do not.
When compared to other GLP-1RA treatments, once-weekly semaglutide caused a larger weight loss, according to a previous study of the SUSTAIN 1-5 trials. In the SUSTAIN 3, 7, and 10 trials, semaglutide was found to be superior to other GLP-1RAs at lowering glycated hemoglobin and body weight. The goal of the study was to ascertain whether GI adverse events (GI AEs), such as nausea/vomiting, diarrhea, constipation, and dyspepsia (indigestion), during the dose escalation phase (baseline to week 12) and from baseline to the end of treatment, are responsible for the greater weight loss seen with semaglutide compared to other GLP-1RAs. The individuals in each trial were split into groups based on whether they reported having nausea, vomiting, or any other GI AE. The change in body weight was evaluated by the researchers, who then used mediation analysis to distinguish between the direct and indirect effects of weight reduction (mediated by GI AEs).
The results showed that in the SUSTAIN 3, 7, and 10 trials, nausea/vomiting during dose escalation or throughout treatment contributed minimally (<0.1 kg) to the superior weight loss seen with semaglutide compared to GLP-1RA comparators at the end of treatment. Therefore, the researchers concluded that the greater weight loss seen with semaglutide compared to other GLP-1RAs is not primarily mediated by GI AEs, including nausea/vomiting, but rather by other direct effects of the drug. You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7594204/.
- Ard, J., Fitch, A., Fruh, S., & Herman, L. (2021). Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists. Advances in therapy, 38(6), 2821–2839. https://doi.org/10.1007/s12325-021-01710-0.
Weight Loss and Maintenance Related to the Mechanism of Action of Glucagon-Like Peptide 1 Receptor Agonists
Obesity is a chronic condition that has a number of problems. A weight decrease of 5–15% of body weight can help reduce several obesity-related problems. Despite these benefits, it might be difficult to lose weight and keep it off for an extended period of time. Pharmacotherapy can help obese people lose the weight they want and keep it off, which can reduce the risk of obesity-related problems.
In the United States, the prevalence of obesity has risen in recent decades, and while approved anti-obesity medications (AOMs) are available, the usage rate of these treatments may not match the disease’s prevalence. Researchers have identified several reasons for the low initiation and long-term use of AOMs, such as reluctance from public health and medical organizations to acknowledge obesity as a disease, a lack of reimbursement, provider inexperience, and misunderstandings about the effectiveness and safety of available treatments.
A review of studies educated primary care providers about the mechanism of action of GLP-1 receptor agonists (GLP-1RAs), a type of AOM, in weight loss and the long-term maintenance of weight loss, as well as the effectiveness and safety of this medication class. GLP-1RA therapy was originally developed to treat type 2 diabetes but it has proven effective in reducing body weight, leading to the approval of liraglutide 3.0 mg for once-daily subcutaneous administration and the investigation of semaglutide 2.4 mg for once-weekly subcutaneous administration in phase III trials for obesity management. The review found that GLP-1RA-mediated weight loss can be achieved through several pathways including effects on the central nervous system such as increased feelings of satiety, reduced energy intake, and impaired food preferences. In addition, it was also found that the risk of adverse events associated with GLP-1RAs was higher but mild-to-moderate and transient. These adverse events are not the main reason for observed weight loss during treatment with GLP-1RAs.
Overall, GLP-1RAs have higher safety, efficacy, and tolerability in obese patients and this treatment is associated with maintenance of body weight reductions of 5–10%. Therefore, the treatment can improve complications associated with obesity in addition to its weight-loss effects.
full artical read https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8189979/.
- Johansen, P., Chubb, B., Hunt, B., Malkin, S., Sandberg, A., &Capehorn, M. (2020). Evaluating the Long-Term Cost-Effectiveness of Once-Weekly Semaglutide Versus Once-Daily Liraglutide for the Treatment of Type 2 Diabetes in the UK. Advances in therapy, 37(5), 2427–2441.https://doi.org/10.1007/s12325-020-01337-7.
Evaluating the Long-Term Cost-Effectiveness of Once-Weekly Semaglutide Versus Once-Daily Liraglutide for the Treatment of Type 2 Diabetes in the UK
In the SUSTAIN 10 study, once-weekly semaglutide 1 mg, a novel GLP-1 RA, was shown to be more effective than once-daily liraglutide 1.2 mg at lowering HbA1c and body weight, according to the researchers. This study’s objective was to examine the long-term cost-effectiveness of once-weekly semaglutide 1 mg with once-daily liraglutide 1.2 mg from the viewpoint of a UK healthcare payer.
In the base-case analysis, the researchers found that once-weekly semaglutide 1 mg led to a higher discounted life expectancy of 0.21 years and a higher discounted quality-adjusted life expectancy of 0.30 quality-adjusted life years compared to once-daily liraglutide 1.2 mg. The study showed that once-weekly semaglutide 1 mg was associated with cost savings of GBP 140 per patient, owing to a reduction in diabetes-related complications, particularly cardiovascular disease (with an average cost saving of GBP 279 per patient). Therefore, once-weekly semaglutide 1 mg was deemed superior to once-daily liraglutide 1.2 mg, as it provided clinical benefits at a lower cost.
The sensitivity analyses conducted by the researchers showed similar results, indicating the robustness of the base-case analysis. The researchers concluded that once-weekly semaglutide 1 mg was a cost-effective treatment option compared to once-daily liraglutide 1.2 mg, based on the SUSTAIN 10 trial, from the perspective of a UK healthcare payer.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7467468/. - Johansen, P., Håkan-Bloch, J., Liu, A. R., Bech, P. G., Persson, S., &Leiter, L. A. (2019). Cost Effectiveness of Once-Weekly Semaglutide Versus Once-Weekly Dulaglutide in the Treatment of Type 2 Diabetes in Canada. PharmacoEconomics – open, 3(4), 537–550.https://doi.org/10.1007/s41669-019-0131-6.
Cost Effectiveness of Once-Weekly Semaglutide Versus Once-Weekly Dulaglutide in the Treatment of Type 2 Diabetes in Canada
The researchers evaluated the cost-effectiveness of semaglutide in comparison to dulaglutide for the treatment of type 2 diabetes (T2D) when added to metformin monotherapy from a Canadian societal perspective. To achieve this, they employed the Swedish Institute for Health Economics Cohort Model of T2D. This model was used to analyze the cost-effectiveness of once-weekly semaglutide (0.5 or 1.0 mg) versus once-weekly dulaglutide (0.75 or 1.5 mg) over a 40-year period.
In this study, the researchers used data from the SUSTAIN 7 trial to conduct a deterministic base-case analysis and scenario simulation. The results showed that semaglutide was more effective than dulaglutide at lowering systolic blood pressure, body mass index, and glycated hemoglobin (HbA1c). They also performed 15 deterministic sensitivity analyses and 15 probabilistic sensitivity analyses to assess the robustness of their findings. The results of the study showed that, in the base-case analysis, semaglutide was a more prevalent treatment option than dulaglutide. In comparison to dulaglutide, semaglutide was likewise linked to decreased overall expenses for both low-dose and high-dose therapies. Semaglutide was therefore determined to be a cost-effective therapeutic choice for T2D patients whose condition was not sufficiently controlled by metformin alone from a Canadian social standpoint.
In conclusion, the researchers found that semaglutide was a more cost-effective treatment option for T2D patients compared with dulaglutide when added to metformin monotherapy, as it was associated with lower costs and superior clinical outcomes. The findings of this study could have implications for healthcare providers, policymakers, and patients with T2D who are seeking cost-effective treatment options.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6861407/. - Capehorn, M., Hallén, N., Baker-Knight, J., Glah, D., & Hunt, B. (2021). Evaluating the Cost-Effectiveness of Once-Weekly Semaglutide 1 mg VersusEmpagliflozin 25 mg for Treatment of Patients with Type 2 Diabetes in the UK Setting. Diabetes therapy : research, treatment and education of diabetes and related disorders, 12(2), 537–555. https://doi.org/10.1007/s13300-020-00989-6
Evaluating the Cost-Effectiveness of Once-Weekly Semaglutide 1 mg Versus Empagliflozin 25 mg for Treatment of Patients with Type 2 Diabetes in the UK Setting
In the past, researchers evaluated the cost-effectiveness of once-weekly semaglutide 1 mg versus empagliflozin 25 mg for treating type 2 diabetes mellitus patients who had insufficient glycemic (blood sugar) control on metformin monotherapy in the UK. Using the IQVIA CORE Diabetes Model, the researchers predicted outcomes throughout the patients’ lifetimes. Since there was no direct clinical study comparing the two treatments head-to-head, the baseline cohort characteristics and treatment outcomes of once-weekly semaglutide 1 mg and empagliflozin 25 mg were based on an indirect comparison utilizing patient-level data.
The modeled patients received treatment until glycated hemoglobin exceeded 7.5% (58 mmol/mol), at which point they initiated basal insulin. The analysis captured pharmacy costs and the costs of diabetes-related complications expressed in 2019 pounds sterling (GBP). The researchers discounted projected outcomes at 3.5% annually and prepared scenario analyses to assess the uncertainty around projected outcomes. The analysis’s findings demonstrated that when compared to empagliflozin 25 mg, once-weekly semaglutide 1 mg was linked with increases in life expectancy and quality-adjusted life expectancy of 0.12 years and 0.23 QALYs, respectively. Reduced cumulative incidence and postponed onset of diabetes-related problems are what led to the anticipated increases in life quality and longevity. Once-weekly semaglutide was linked to higher pharmacy expenditures, however, this was somewhat compensated by the avoidance of complications-related medical expenses.
The study’s findings revealed that once-weekly semaglutide was linked to a GBP 1017 rise in expenses per patient, resulting in an additional cost-effectiveness ratio of GBP 4439 for every QALY gained. Based on their analysis, the researchers concluded that once-weekly semaglutide 1 mg was a cost-effective treatment option from a healthcare payer perspective compared to empagliflozin 25 mg for treating patients with type 2 diabetes in the UK setting.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7846640/. - Ericsson, Å.,&Fridhammar, A. (2019). Cost-effectiveness of once-weekly semaglutide versus dulaglutide and lixisenatide in patients with type 2 diabetes with inadequate glycemic control in Sweden. Journal of medical economics, 22(10), 997–1005. https://doi.org/10.1080/13696998.2019.1614009
Cost-effectiveness of once-weekly semaglutide versus dulaglutide and lixisenatide in patients with type 2 diabetes with inadequate glycemic control in Sweden
Researchers in Sweden evaluated the cost-effectiveness of once-weekly semaglutide against glucagon-like peptide-1 receptor agonists (GLP-1 RAs) in patients with type 2 diabetes (T2D) who were not responsive to either metformin or basal insulin. This cost-effectiveness analysis (CEA) made use of the Diabetes Cohort Model from the Swedish Institute of Health Economics (IHE). Over a 40-year period, the examination was done from the standpoint of Swedish society.
For patients uncontrolled on metformin, the researchers used dulaglutide as the comparator and obtained data from the SUSTAIN 7 clinical trial. For patients uncontrolled on basal insulin, lixisenatide was selected as the comparator, and data were obtained from a network meta-analysis. The researchers found that in patients with inadequate control of metformin, semaglutide 1.0 mg provided greater clinical benefit and was less expensive than dulaglutide 1.5 mg, which means it dominated dulaglutide. Similarly, in patients with inadequate control of basal insulin, semaglutide 1.0 mg dominated lixisenatide. The researchers noted that the reduction in costs was mainly due to the reduction in complications seen with once-weekly semaglutide. They also pointed out that the analysis may have underestimated the cardiovascular (CV) cost benefits associated with treatment with once-weekly semaglutide. Due to the lack of a head-to-head clinical study for this comparison, evaluations versus lixisenatide for patients with poorly regulated basal insulin were based on NMA findings.
The researchers concluded that once-weekly semaglutide is a GLP-1 RA medication that is cost-effective for treating T2D in patients who are insufficiently managed by metformin or basal insulin. The treatment can help address many of the unmet demands of Swedish clinicians, patients, and payers at the moment.
You can read the full article at https://www.tandfonline.com/doi/full/10.1080/13696998.2019.1614009. - Viljoen, A., Hoxer, C. S., Johansen, P., Malkin, S., Hunt, B., & Bain, S. C. (2019). Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK. Diabetes, obesity & metabolism, 21(3), 611–621. https://doi.org/10.1111/dom.13564.
Evaluation of the long-term cost-effectiveness of once-weekly semaglutide versus dulaglutide for treatment of type 2 diabetes mellitus in the UK
A group of researchers evaluated the long-term cost-effectiveness of two once-weekly glucagon-like peptide-1 (GLP-1) receptor agonists, semaglutide 0.5 and 1 mg, in comparison to dulaglutide 1.5 mg in people with type 2 diabetes mellitus (T2DM) in the UK healthcare system. The research was based on the SUSTAIN 7 trial, a head-to-head comparison meant to aid in healthcare decision-making.
The study found that both doses of once-weekly semaglutide improved quality-adjusted life expectancy compared to dulaglutide. Semaglutide 0.5 mg and 1 mg were associated with improvements in quality-adjusted life expectancy of 0.04 and 0.10 quality-adjusted life years, respectively. Moreover, the clinical benefits of semaglutide were achieved at a lower cost, resulting in lifetime cost savings of GBP 35 for semaglutide 0.5 mg and GBP 106 for semaglutide 1 mg, due to fewer diabetes-related complications resulting from better glycemic control. Because they produced better results and were less expensive than dulaglutide 1.5 mg, the once-weekly semaglutide doses were deemed dominant. Since once-weekly semaglutide is a cost-effective alternative for T2DM patients in the UK who are not controlling their blood sugar with metformin, it is expected to enhance clinical results and lower costs.
In conclusion, the researchers found that once-weekly semaglutide was a cost-effective treatment option for individuals with T2DM in the UK healthcare system. Semaglutide not only improved quality-adjusted life expectancy but also reduced costs due to better glycemic control and fewer diabetes-related complications. This study provides important information for healthcare decision-makers in the UK regarding the optimal treatment for individuals with T2DM who are not achieving glycemic control with metformin.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6587509/. - Gorgojo-Martínez, J. J., Malkin, S., Martín, V., Hallén, N., & Hunt, B. (2020). Assessing the cost-effectiveness of a once-weekly GLP-1 analogue versus an SGLT-2 inhibitor in the Spanish setting: Once-weekly semaglutide versus empagliflozin. Journal of medical economics, 23(2), 193–203. https://doi.org/10.1080/13696998.2019.1681436
Assessing the cost-effectiveness of a once-weekly GLP-1 analogue versus an SGLT-2 inhibitor in the Spanish setting: Once-weekly semaglutide versus empagliflozin
By evaluating the cost-effectiveness of two different drugs for the treatment of type 2 diabetes (T2D) patients in the Spanish setting who had insufficient glycemic control on oral anti-hyperglycemic medications, a group of researchers addressed the challenge of balancing healthcare gains with costs. They compared the cost-effectiveness of subcutaneous once-weekly semaglutide (0.5 mg and 1 mg) to empagliflozin (10 mg and 25 mg) over the long run.
To do this, the researchers used the IQVIA CORE Diabetes Model to project outcomes over patient lifetimes with once-weekly semaglutide versus empagliflozin. They based their treatment effects on a network meta-analysis and captured treatment costs, costs of diabetes-related complications, and the impact of complications on quality of life using published sources. They discounted outcomes at 3.0% per annum. The results showed that both once-weekly semaglutide 0.5 mg and 1 mg were associated with improvements in quality-adjusted life expectancy compared to empagliflozin 10 mg and 25 mg. Specifically, once-weekly semaglutide 0.5 mg and 1 mg were associated with improvements in discounted quality-adjusted life expectancy of 0.12 and 0.15 quality-adjusted life years (QALYs), respectively, versus empagliflozin 10 mg, and improvements of 0.11 and 0.14 QALYs, respectively, versus empagliflozin 25 mg. When compared to empagliflozin, once-weekly semaglutide had greater treatment costs but the researchers found that this was somewhat offset by cost savings from avoiding complications associated with diabetes. In particular, compared to empagliflozin 10 mg, once-weekly semaglutide 0.5 mg and 1 mg were associated with incremental cost-effectiveness ratios of EUR 2,285 and EUR 161 per QALY gained, respectively, and EUR 3,090 and EUR 625 per QALY gained, respectively.
In conclusion, based on a willingness-to-pay threshold of EUR 30,000 per QALY gained, the researchers projected that once-weekly semaglutide 0.5 mg and 1 mg were cost-effective compared to empagliflozin 10 mg and 25 mg for the treatment of patients with T2D with inadequate glycemic control on oral anti-hyperglycemic medications in the Spanish setting, regardless of patients’ BMI at baseline.
You can read the full article at https://www.tandfonline.com/doi/full/10.1080/13696998.2019.1681436. - Ruan, Z., Yang, L., Shi, H., Yue, X., Wang, Y., Liang, M., &Hu, H. (2021). The cost-effectiveness of once-weekly semaglutide compared with other GLP-1 receptor agonists in type 2 Diabetes: a systematic literature review. Expert review of pharmacoeconomics& outcomes research, 21(2), 221–233. https://doi.org/10.1080/14737167.2021.1860022
The cost-effectiveness of once-weekly semaglutide compared with other GLP-1 receptor agonists in type 2 Diabetes: a systematic literature review
Semaglutide, a once-weekly glucagon-like peptide-1 receptor agonist (GLP-1 RA) and innovative treatment for type 2 diabetes (T2D), has been assessed for its economic value in a number of nations. In order to shed light on future research, researchers set out to thoroughly review the most recent pharmacoeconomic literature on the cost-effectiveness of once-weekly semaglutide in comparison to other GLP-1 RAs.
To accomplish this, a systematic literature review of cost-effectiveness analyses (CEA) comparing once-weekly semaglutide to other GLP-1 RAs in T2D was conducted. The study searched PubMed, Web of Science, and the ISPOR presentation database for articles published up to 25 July 2020. Nineteen studies were identified, including eight short-term and 11 long-term studies, and their general characteristics and main results were summarized. The review provided references for other countries to understand the value of once-weekly semaglutide compared to other GLP-1 RAs in T2D in the healthcare decision-making process and to conduct their own CEA studies related to once-weekly semaglutide. The authors found that current studies underestimated the cardiovascular (CV) benefits of once-weekly semaglutide and suggested that methods for economic evaluations of novel anti-diabetic drugs with CV benefits should be improved in future research.
In conclusion, once-weekly semaglutide’s economic worth in comparison to other GLP-1 RAs has been evaluated in a number of nations and this systematic evaluation adds to the understanding of the drug’s cost-effectiveness. The results of the analysis could be used to inform future studies on the economic benefits of once-weekly semaglutide as well as healthcare decision-making procedures. The necessity for continuous research in this area is highlighted by the authors’ advice to enhance procedures for economic analyses of novel anti-diabetic medications with CV advantages.
You can read the full article at https://www.tandfonline.com/doi/abs/10.1080/14737167.2021.1860022?journalCode=ierp20. - Vidal, J., Malkin, S., Hunt, B., Martín, V., Hallén, N., & Javier Ortega, F. (2020). The Short-Term Cost-Effectiveness of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin and Once-Weekly Dulaglutide for the Treatment of Patients with Type 2 Diabetes: A Cost of Control Analysis in Spain. Diabetes therapy : research, treatment and education of diabetes and related disorders, 11(2), 509–521. https://doi.org/10.1007/s13300-019-00751-7
The Short-Term Cost-Effectiveness of Once-Weekly Semaglutide Versus Once-Daily Sitagliptin and Once-Weekly Dulaglutide for the Treatment of Patients with Type 2 Diabetes: A Cost of Control Analysis in Spain
The researchers conducted a study aimed at optimizing care for patients with type 2 diabetes by achieving glycemic control targets, preventing weight gain, and avoiding hypoglycemic events using modern interventions like GLP-1 receptor agonists and DPP4 inhibitors. They recognized that healthcare payers are concerned about the increasing prevalence of type 2 diabetes and need interventions that achieve these aims in a cost-effective manner.
In a trial of individuals with type 2 diabetes in Spain, the researchers compared the cost-effectiveness of semaglutide, sitagliptin, and dulaglutide. They assessed the effectiveness of the treatments in terms of lowering glycated hemoglobin (HbA1c), body weight, and the percentage of patients meeting treatment goals using data from the SUSTAIN 2 and 7 clinical trials. The study found that both doses of semaglutide were associated with lower costs of control for all three endpoints compared to sitagliptin and dulaglutide 1.5 mg. When endpoints incorporating hypoglycemia (low blood sugar) and weight loss alongside glycemic control were considered, semaglutide had comparable or lower costs of control than sitagliptin. Additionally, semaglutide had lower costs of control than dulaglutide 1.5 mg for all endpoints.
In conclusion, the study raises the possibility that semaglutide may be a more affordable treatment choice for individuals with type 2 diabetes in Spain than sitagliptin and dulaglutide, especially when taking into account outcomes like hypoglycemia and weight loss in addition to glycemic control.
Read the full article on: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6995797/. - Hunt, B., Malkin, S., Moes, R., Huisman, E. L., Vandebrouck, T., &Wolffenbuttel, B. (2019). Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands. BMJ open diabetes research & care, 7(1), e000705. https://doi.org/10.1136/bmjdrc-2019-000705.
Once-weekly semaglutide for patients with type 2 diabetes: a cost-effectiveness analysis in the Netherlands
Choosing safe and effective treatments for type 2 diabetes is vital in order to maximize the health of affected individuals. In order to help healthcare systems around the world reach this goal, researchers sought to identify type 2 diabetes treatments that are both efficient and affordable.
In the Netherlands, they carried out an analysis to compare the once-weekly GLP-1 receptor agonist semaglutide with dulaglutide, a different once-weekly GLP-1 receptor agonist, and insulin glargine U100, the most widely used basal insulin, in terms of cost-effectiveness. To project outcomes for once-weekly semaglutide 0.5 mg and 1 mg versus insulin glargine U100, once-weekly semaglutide 0.5 mg versus dulaglutide 0.75 mg, and once-weekly semaglutide 1 mg versus dulaglutide 1.5 mg, the researchers utilized the IQVIA CORE Diabetes Model. They sourced clinical data from the SUSTAIN 4 and SUSTAIN 7 clinical trials. The analysis captured direct and indirect costs, mortality, and the impact of diabetes-related complications on the quality of life. The researchers’ projections of outcomes over patient lifetimes suggest that once-weekly semaglutide 0.5 mg and 1 mg are likely to improve clinical outcomes for patients with type 2 diabetes compared to insulin glargine U100 and dulaglutide. Improvements in clinical outcomes compared to insulin glargine U100 were observed at an increased cost but once-weekly semaglutide was still deemed cost-effective even at the lowest willingness-to-pay threshold identified in the Netherlands.
From a societal perspective, improvements were observed at a reduced cost versus dulaglutide, and therefore once-weekly semaglutide was considered dominant. Semaglutide used once a week is more affordable than insulin glargine U100 and superior to dulaglutide 0.75 and 1.5 mg for the treatment of type 2 diabetes, according to the study’s findings. The usage of once-weekly semaglutide was cited as an effective way for the Netherlands to use its healthcare resources.
You can read the full article at https://drc.bmj.com/content/7/1/e000705. - Wilding JPH, Batterham RL, Calanna S, Davies M, Van Gaal LF, Lingvay I, McGowan BM, Rosenstock J, Tran MTD, Wadden TA, Wharton S, Yokote K, Zeuthen N, Kushner RF; STEP 1 Study Group. Once-Weekly Semaglutide in Adults with Overweight or Obesity. N Engl J Med. 2021 Mar 18;384(11):989. doi: 10.1056/NEJMoa2032183. Epub 2021 Feb 10. PMID: 33567185.
Once-Weekly Semaglutide in Adults with Overweight or Obesity
The goal of the study was to determine whether once-weekly administration of semaglutide at a dose of 2.4 mg as a supplement to lifestyle changes could help adults with obesity lose weight. In a double-blind trial, they enrolled 1961 adults with a body mass index (BMI) of 30 or higher who were free of diabetes. Along with a lifestyle intervention, the participants were randomly assigned to receive either a placebo or once-weekly subcutaneous semaglutide (at a dose of 2.4 mg) for 68 weeks.
The researchers used two primary endpoints to assess the effectiveness of the treatment: the percentage change in body weight and weight reduction of at least 5%. The primary estimand evaluated the treatment effects regardless of treatment discontinuation or rescue interventions. The researchers found that participants who received semaglutide had a greater improvement in cardiometabolic risk factors and a greater increase in participant-reported physical functioning from baseline than those who received a placebo. The most common adverse events associated with semaglutide were nausea and diarrhea, which were typically transient and mild-to-moderate in severity and subsided with time. However, more participants in the semaglutide group than in the placebo group discontinued treatment owing to gastrointestinal events.
In conclusion, the researchers discovered that in patients with overweight or obesity, 2.4 mg of semaglutide once weekly combined with lifestyle modification was associated with persistent, clinically significant weight loss. These discoveries offer fresh pharmaceutical possibilities for the global health problem of obesity.
You can read the full article at https://www.nejm.org/doi/10.1056/NEJMoa2032183?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed. - O’Neil PM, Birkenfeld AL, McGowan B, Mosenzon O, Pedersen SD, Wharton S, Carson CG, Jepsen CH, Kabisch M, Wilding JPH. Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial. Lancet. 2018 Aug 25;392(10148):637-649. doi: 10.1016/S0140-6736(18)31773-2. Epub 2018 Aug 16. PMID: 30122305.
Efficacy and safety of semaglutide compared with liraglutide and placebo for weight loss in patients with obesity: a randomised, double-blind, placebo and active controlled, dose-ranging, phase 2 trial
Obesity was recognized as a significant public health issue. Because of this, more new treatment options are needed to achieve and sustain a healthy weight in affected individuals.
A group of researchers investigated the effectiveness and safety of the glucagon-like peptide-1 (GLP-1) analogue semaglutide for weight management. To accomplish this, they conducted a phase 2 trial that was randomized, double-blind, placebo and active- controlled, multicentre, and dose-ranging. The study was conducted in eight countries, and 71 clinical sites were involved. Adults without diabetes who met the eligibility requirements participated in the trial. The semaglutide, liraglutide, or corresponding placebo groups were the active therapy groups that the researchers randomly assigned the subjects to. To assure randomness, a block size of 56 was employed. With the exception of the target dose, all doses of the medication were given once daily by subcutaneous injection, and both the participants and the researchers were unaware of the assigned study treatment. The primary endpoint of the study was the percentage of weight loss at week 52. The researchers analyzed the data using intention-to-treat ANCOVA estimation and the missing data was derived from the placebo pool. All semaglutide doses were generally well tolerated, and there were no new safety concerns. The most common adverse events were dose-related gastrointestinal symptoms, primarily nausea, as previously seen with GLP-1 receptor agonists.
The researchers concluded that semaglutide, in combination with dietary and physical activity counseling, was well tolerated over 52 weeks and showed clinically relevant weight loss compared with placebo at all doses. The results of this study suggest that semaglutide may be a promising pharmaceutical treatment option for weight management.
You can read the full article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(18)31773-2/fulltext.. - Andreadis P, Karagiannis T, Malandris K, Avgerinos I, Liakos A, Manolopoulos A, Bekiari E, Matthews DR, Tsapas A. Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes ObesMetab. 2018 Sep;20(9):2255-2263. doi: 10.1111/dom.13361. Epub 2018 Jun 10. PMID: 29756388.
Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis
In this study, researchers assessed the effectiveness and safety of semaglutide, a glucagon-like peptide 1 receptor agonist (GLP-1 RA), for the treatment of type 2 diabetes. They conducted a comprehensive search in electronic databases and grey literature sources to identify randomized controlled trials comparing semaglutide with placebo or other antidiabetic medications. The primary objective was to measure the change in HbA1c from baseline, while secondary endpoints included changes in body weight, blood pressure, heart rate, incidence of hypoglycemia (low blood sugar levels), gastrointestinal side effects, pancreatitis, and diabetic retinopathy (an eye complication of diabetes).
The researchers included a total of 6 placebo-controlled studies and 7 studies comparing semaglutide with other antidiabetic drugs administered subcutaneously. They found only one trial that investigated the use of oral semaglutide. Both doses of subcutaneous semaglutide demonstrated superior efficacy in lowering blood glucose (sugar) levels compared to other antidiabetic agents such as sitagliptin, exenatide, liraglutide, dulaglutide, and insulin glargine. Semaglutide also had a positive impact on body weight (compared to placebo for semaglutide 1 mg) and systolic blood pressure. The researchers did not observe an increased risk of hypoglycemia with semaglutide, but they noted a higher incidence of nausea, vomiting, and diarrhea. Pancreatitis cases were rare, and the odds ratio for diabetic retinopathy compared to placebo was 1.32.
Based on their findings, the authors concluded that semaglutide is an effective weekly GLP-1 RA that significantly reduces systolic blood pressure, body weight, and HbA1c levels. However, it is associated with a higher occurrence of gastrointestinal adverse effects. They recommend further evaluation of the pancreatitis and retinopathy findings through post-approval pharmacovigilance studies.
You can read the full article at https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/dom.13361.. - Andersen A, Knop FK, Vilsbøll T. A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes. Drugs. 2021 Jun;81(9):1003-1030. doi: 10.1007/s40265-021-01499-w. Epub 2021 May 8. PMID: 33964002; PMCID: PMC8217049.
A Pharmacological and Clinical Overview of Oral Semaglutide for the Treatment of Type 2 Diabetes
The researchers of the study wanted to find better ways to manage type 2 diabetes and prevent complications. They aimed to control blood sugar levels, help patients lose weight, and manage their cardiovascular health. To do this, they studied different medications that could lower blood sugar levels effectively. One commonly used medication called metformin was often used as the first-line treatment, but many patients needed additional medications to control their blood sugar levels well. The choice of medication depended on factors like cost and the presence of other health problems.
The researchers studied a new medication called oral semaglutide (Rybelsus®), which belongs to a class of drugs called GLP-1 receptor agonists. It is taken as a pill and is designed to help control blood sugar levels. Previous GLP-1 receptor agonists were given as injections, but this new one could be taken orally. The results of the study showed that oral semaglutide, either alone or in combination with other medications, effectively controlled blood sugar levels, helped with weight loss, and reduced high blood pressure. It had similar safety and side effects to other medications in its class. It rarely caused low blood sugar levels, and the most common side effects were nausea and diarrhea. The study also showed that oral semaglutide was safe for the heart, similar to taking a placebo (a fake pill), and its effects on the heart were likely similar to the injected form of semaglutide.
GLP-1 receptor agonists should be more widely accepted by patients and healthcare professionals. When used as an early treatment for diabetes, it can produce beneficial effects on blood sugar levels, body weight, and blood pressure.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8217049/. - Singh G, Krauthamer M, Bjalme-Evans M. Wegovy (semaglutide): a new weight loss drug for chronic weight management. J Investig Med. 2022 Jan;70(1):5-13. doi: 10.1136/jim-2021-001952. Epub 2021 Oct 27. PMID: 34706925; PMCID: PMC8717485.
Wegovy (semaglutide): a new weight loss drug for chronic weight management.
The provided reference is an article by Singh et al. titled “Wegovy (semaglutide): a new weight loss drug for chronic weight management.” The study was published in the Journal of Investigative Medicine in January 2022. The authors discuss Wegovy (semaglutide), a medication indicated for chronic weight management. The article provides an overview of semaglutide’s mechanism of action, clinical trials, efficacy in promoting weight loss, safety profile, and potential side effects. For more in-depth information, the publication can be accessed using the PMID: 34706925 and PMCID: PMC8717485.
In all three clinical trials, semaglutide treatment led to significant weight loss, which was greater than with placebo or other antidiabetic drugs, according to the study’s findings. Patients who received semaglutide also had a lower probability of getting diabetes or other comorbidities. The trials showed that semaglutide was generally well-tolerated despite some adverse effects, such as gastrointestinal discomfort, during therapy. Semaglutide may be a viable non-invasive treatment approach for obesity, according to the findings of these clinical trials and other relevant articles.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8717485/. - Rubino DM, Greenway FL, Khalid U, O’Neil PM, Rosenstock J, Sørrig R, Wadden TA, Wizert A, Garvey WT; STEP 8 Investigators. Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial. JAMA. 2022 Jan 11;327(2):138-150. doi: 10.1001/jama.2021.23619. PMID: 35015037; PMCID: PMC8753508.
Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes
The provided reference is a study conducted by Rubino et al. titled “Effect of Weekly Subcutaneous Semaglutide vs Daily Liraglutide on Body Weight in Adults With Overweight or Obesity Without Diabetes: The STEP 8 Randomized Clinical Trial.” The study was published in JAMA (Journal of the American Medical Association) in January 2022. The authors aimed to compare the effects of weekly subcutaneous semaglutide with daily liraglutide on body weight in adults with overweight or obesity but without diabetes. The randomized clinical trial investigated the efficacy of these medications in promoting weight loss. The publication can be accessed for more detailed information using the PMID: 35015037 and PMCID: PMC8753508.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8753508/. - Mares AC, Chatterjee S, Mukherjee D. Semaglutide for weight loss and cardiometabolic risk reduction in overweight/obesity. CurrOpinCardiol. 2022 Feb 16. doi: 10.1097/HCO.0000000000000955. Epub ahead of print. PMID: 35175229.
Semaglutide for weight loss and cardiometabolic risk reduction in overweight/obesity.
The provided reference is an article by Mares et al. titled “Semaglutide for weight loss and cardiometabolic risk reduction in overweight/obesity.” The article was published ahead of print in the journal Current Opinion in Cardiology on February 16, 2022. The authors discuss the use of semaglutide for weight loss and its potential in reducing cardiometabolic risk in individuals with overweight or obesity. The publication explores the current understanding of semaglutide’s efficacy in weight management and its impact on cardiometabolic parameters. For more detailed information, the article can be accessed using the PMID: 35175229.
You can read the full article at https://journals.lww.com/cocardiology/Abstract/2022/07000/Semaglutide_for_weight_loss_and_cardiometabolic.8.aspx. - Chao AM, Tronieri JS, Amaro A, Wadden TA. Semaglutide for the treatment of obesity. Trends Cardiovasc Med. 2021 Dec 21:S1050-1738(21)00158-4. doi: 10.1016/j.tcm.2021.12.008. Epub ahead of print. PMID: 34942372.
Semaglutide for the treatment of obesity
The provided reference is an article by Chao et al. titled “Semaglutide for the treatment of obesity.” The article was published ahead of print in the journal Trends in Cardiovascular Medicine on December 21, 2021. The authors discuss the use of semaglutide as a treatment for obesity. The publication explores the current trends and developments in utilizing semaglutide for weight management, its efficacy, safety profile, and potential cardiovascular benefits. For more detailed information, the article can be accessed using the PMID: 34942372.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1050173821001584?via%3Dihub. - Zhong P, Zeng H, Huang M, Fu W, Chen Z. Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: a meta-analysis. Endocrine. 2022 Mar;75(3):718-724. doi: 10.1007/s12020-021-02945-1. Epub 2022 Jan 4. PMID: 34981419.
Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: a meta-analysis
The provided reference is a meta-analysis conducted by Zhong et al. titled “Efficacy and safety of once-weekly semaglutide in adults with overweight or obesity: a meta-analysis.” The study was published in the journal Endocrine in March 2022. The authors aimed to assess the efficacy and safety of once-weekly semaglutide in adults with overweight or obesity. By analyzing relevant studies, the meta-analysis provided insights into the effectiveness of semaglutide in promoting weight loss and its safety profile. For more detailed information, the publication can be accessed using the PMID: 34981419.
You can read the full article at https://link.springer.com/article/10.1007/s12020-021-02945-1. - Andreadis, P., Karagiannis, T., Malandris, K., Avgerinos, I., Liakos, A., Manolopoulos, A., Bekiari, E., Matthews, D. R., &Tsapas, A. (2018). Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes, obesity & metabolism, 20(9), 2255-2263.https://doi.org/10.1111/dom.13361
Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis.
Subcutaneous semaglutide 0.5 and 1 mg, compared with placebo, reduced HbA1c by 1.01% (95% CI, 0.56-1.47) and 1.38%, respectively. Both doses were more effective than other antidiabetic agents, such as sitagliptin, exenatide, liraglutide, dulaglutide, and insulin glargine, in terms of glycaemic efficacy. Semaglutide also had a positive effect on body weight (mean difference vs placebo -4.11 kg, 95% CI -4.85 to -3.37 for semaglutide 1 mg) and systolic blood pressure. The researchers did not observe an increase in hypoglycaemia rates with semaglutide; however, they noted a higher incidence of nausea, vomiting, and diarrhoea. Pancreatitis cases were rare, and the odds ratio for diabetic retinopathy compared with placebo was 1.32.
The researchers concluded that semaglutide is a potent once-weekly GLP-1 RA that significantly reduces HbA1c, body weight, and systolic blood pressure. However, they noted an increased incidence of gastrointestinal adverse events associated with semaglutide. Further studies are required to assess the results for pancreatitis and retinopathy in post-approval pharmacovigilance studies.
the article can be accessed using the provided DOI: 10.1111/dom.13361.
You can read the full article at https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/dom.13361. - Kadowaki, T., Isendahl, J., Khalid, U., Lee, S. Y., Nishida, T., Ogawa, W., Tobe, K., Yamauchi, T., Lim, S., & STEP 6 investigators (2022). Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial. The lancet. Diabetes & endocrinology, 10(3), 193–206. https://doi.org/10.1016/S2213-8587(22)00008-0
Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double-blind, double-dummy, placebo-controlled, phase 3a trial.
Researchers examined semaglutide 24 mg for weight management in a variety of groups around the world. The definitions of obesity and disparities in body composition between Asian and non-Asian populations, however, were noted.
The Semaglutide Treatment Effect in People with Obesity (STEP) 6 study examined the effectiveness of semaglutide against placebo for weight management in individuals from East Asia with obesity. This phase 3a superiority trial was a randomized, double-blind, double-dummy, placebo-controlled trial carried out at 28 outpatient clinics in Japan and South Korea. The study aimed to investigate the effect of semaglutide 2•4 mg or 1.7 mg versus placebo for 68 weeks, along with lifestyle recommendations. Eligible participants were adults with a BMI of at least 27•0 kg/m2 with two or more weight-related comorbidities or a BMI of 35•0 kg/m2 or more with one or more weight-related comorbidity. One of the comorbidities had to be either hypertension, dyslipidemia, or, in Japan only, type 2 diabetes. Participants had to report at least one unsuccessful dietary attempt to lose bodyweight. The percentage of participants who had successfully lost at least 5% of their initial bodyweight by week 68 and the change in bodyweight from baseline as a whole served as the study’s primary objectives. Additionally, a subset of participants had their abdominal visceral fat area changed using computed tomography scanning as a supportive secondary endpoint. All individuals who received at least one dose of the study medication had their safety evaluated.
The full analysis set, which included all randomly assigned participants according to the intention-to-treat principle, was used to evaluate the efficacy outcomes. The results showed that adults from East Asia with obesity, with or without type 2 diabetes, who were given semaglutide 2•4 mg once a week had superior and clinically meaningful reductions in bodyweight, as well as greater reductions in abdominal visceral fat area compared to placebo. These findings suggest that semaglutide 2•4 mg once a week could be a promising treatment option for weight management in this population.
You can read the full article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00008-0/fulltext.
You can read the full article at https://www.thelancet.com/journals/landia/article/PIIS2213-8587(22)00008-0/fulltext. - Gao, X., Hua, X., Wang, X., Xu, W., Zhang, Y., Shi, C., & Gu, M. (2022). Efficacy and safety of semaglutide on weight loss in obese or overweight patients without diabetes: A systematic review and meta-analysis of randomized controlled trials. Frontiers in pharmacology, 13, 935823. https://doi.org/10.3389/fphar.2022.935823
Efficacy and safety of semaglutide on weight loss in obese or overweight patients without diabetes: A systematic review and meta-analysis of randomized controlled trials.
The researchers aimed to investigate the weight loss effects and safety of semaglutide as an anti-obesity drug in overweight and obese patients without diabetes. Obesity and overweight are chronic diseases and major public health issues associated with an increased risk of various complications, including diabetes, hypertension, hyperlipidemia, stroke, and malignant tumors. Sustained weight loss is crucial in preventing the progression of these complications, and diet and exercise interventions are generally considered the most effective ways to achieve weight loss. However, long-term adherence to these interventions is challenging.
Anti-obesity medications’ safety and tolerability are subpar, which restricts their clinical application. Cost and safety issues with bariatric surgery are further drawbacks. Therefore, the researchers sought to systematically investigate the safety and weight loss effects of semaglutide. The researchers conducted a systematic review of randomized controlled trials (RCTs) of semaglutide in overweight and obese patients without diabetes. They retrieved data from various databases, including PubMed, Cochrane Library, EMBASE, and ClinicalTrials.gov. Semaglutide significantly reduced body weight for patients who were overweight or obese without diabetes, according to the study’s findings. After data extraction and quality assessment, the researchers conducted statistical analysis using Review Manager 5.3 and Stata 14.
The results indicated that semaglutide exhibited a positive effect on blood pressure, C-reactive protein, and lipid profiles. However, it caused more adverse effects than placebo, mainly gastrointestinal reactions. The results were stable and reliable, with dose-dependence
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9515581/. - Lautenbach, A., Wernecke, M., Huber, T. B., Stoll, F., Wagner, J., Meyhöfer, S. M., Meyhöfer, S., & Aberle, J. (2022). The Potential of Semaglutide Once-Weekly in Patients Without Type 2 Diabetes with Weight Regain or Insufficient Weight Loss After Bariatric Surgery-a Retrospective Analysis. Obesity surgery, 32(10), 3280–3288. https://doi.org/10.1007/s11695-022-06211-9
The Potential of Semaglutide Once-Weekly in Patients Without Type 2 Diabetes with Weight Regain or Insufficient Weight Loss After Bariatric Surgery-a Retrospective Analysis.
The provided reference is an article by Lautenbach et al. titled “The Potential of Semaglutide Once-Weekly in Patients Without Type 2 Diabetes with Weight Regain or Insufficient Weight Loss After Bariatric Surgery – a Retrospective Analysis.” The study was published in the journal Obesity Surgery in 2022. The authors conducted a retrospective analysis to evaluate the potential of semaglutide once-weekly in patients without type 2 diabetes who experienced weight regain or insufficient weight loss after bariatric surgery. The publication explores the outcomes and effectiveness of using semaglutide in this patient population. For more detailed information, the article can be accessed using the provided DOI: 10.1007/s11695-022-06211-9.
Consequently, some patients only experienced partial remission of comorbidities. For example, although many patients achieved complete T2D remission in the early stages after surgery, a large number suffered a relapse in the long-term follow-up. In the first long-term prospective trial on the effects of BS, the Swedish Obese Subjects (SOS) study, 10% of RYGB patients did not maintain weight loss at the 10-year follow-up. Conversion surgery was nearly twice as common after sleeve gastrectomy (SG) as after Roux-en-Y bypass (RYGB), and it was usually associated with a greater degree of malabsorption, a higher rate of morbidity, reoperations, and readmission. In order to address this problem, the researchers set out to test semaglutide, a GLP-1 receptor agonist, as an adjunctive therapy for non-diabetic individuals who had gained weight or lost inadequate weight after BS. The investigation comprised 44 post-bariatric patients who did not have T2D. Weight loss 3 and 6 months following the start of adjunct treatment was the main outcome. Changes in BMI, HbA1c, lipid profile, hs-CRP, and liver enzymes were secondary objectives.
The study’s findings indicated a definite advantage to semaglutide adjunct therapy for post-bariatric patients. The researchers underlined that a prospective randomized controlled trial was required to confirm the findings in order to bridge the gap between lifestyle modification and revision surgery in patients who had not lost enough weight or gained it back following BS. The researchers came to the conclusion that there were few choices for managing post-bariatric excess weight, and the results of their study underscored the need for creative solutions to this issue.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9532334/.
- Arastu, N., Cummins, O., Uribe, W., & Nemec, E. C. (2022). Efficacy of subcutaneous semaglutide compared to placebo for weight loss in obese, non-diabetic adults: a systematic review & meta-analysis. International journal of clinical pharmacy, 44(4), 852–859. https://doi.org/10.1007/s11096-022-01428-1.
Efficacy of subcutaneous semaglutide compared to placebo for weight loss in obese, non-diabetic adults: a systematic review & meta-analysis.
The provided reference is an article by Arastu et al. titled “Efficacy of subcutaneous semaglutide compared to placebo for weight loss in obese, non-diabetic adults: a systematic review & meta-analysis.” The study was published in the International Journal of Clinical Pharmacy in 2022. The authors conducted a systematic review and meta-analysis to assess the efficacy of subcutaneous semaglutide compared to placebo in promoting weight loss in obese, non-diabetic adults. The publication provides insights into the findings from the analyzed studies and offers an overview of the potential benefits of semaglutide for weight management in this population. For more detailed information, the article can be accessed using the provided DOI: 10.1007/s11096-022-01428-1.
You can read the full article at https://link.springer.com/article/10.1007/s11096-022-01428-1. - Wadden, T. A., Bailey, T. S., Billings, L. K., Davies, M., Frias, J. P., Koroleva, A., Lingvay, I., O’Neil, P. M., Rubino, D. M., Skovgaard, D., Wallenstein, S. O. R., Garvey, W. T., & STEP 3 Investigators (2021). Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial. JAMA, 325(14), 1403–1413. https://doi.org/10.1001/jama.2021.1831
Effect of Subcutaneous Semaglutide vs Placebo as an Adjunct to Intensive Behavioral Therapy on Body Weight in Adults With Overweight or Obesity: The STEP 3 Randomized Clinical Trial.
The researchers investigated whether once-weekly subcutaneous semaglutide, combined with a low-calorie diet and intensive behavioral therapy, would be more effective in helping adults with overweight or obesity lose weight than a placebo.
407 of the 611 participants who were randomly assigned received semaglutide, while 204 received a placebo. Both groups underwent intensive behavioral therapy, which included 30 counseling sessions, for 68 weeks after beginning a low-calorie diet for the first eight weeks. The percentage change in body weight and the loss of 5% or more of the initial weight by week 68 served as the co-primary outcomes. Confirmatory secondary endpoints, such as weight decreases of at least 10% or 15% from the starting position, were also measured by the researchers.
The study found that the semaglutide group had significantly greater weight loss during the 68-week period compared to the placebo group, with an average weight loss of 16.0% in the semaglutide group compared to 5.7% in the placebo group. The researchers concluded that once-weekly subcutaneous semaglutide, combined with a low-calorie diet and intensive behavioral therapy, is an effective weight loss approach for adults with overweight or obesity. However, they noted that more research is needed to determine the long-term effectiveness of this approach.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7905697/.. - Ghusn, W., De la Rosa, A., Sacoto, D., Cifuentes, L., Campos, A., Feris, F., Hurtado, M. D., & Acosta, A. (2022). Weight Loss Outcomes Associated With Semaglutide Treatment for Patients With Overweight or Obesity. JAMA network open, 5(9), e2231982. https://doi.org/10.1001/jamanetworkopen.2022.31982
Weight Loss Outcomes Associated With Semaglutide Treatment for Patients With Overweight or Obesity.
The study’s goal was to look into how semaglutide treatment for patients who were overweight or obese affected their ability to lose weight. The investigation was carried out at a referral center for weight management and was designed as a cohort study. The usage of semaglutide in adult patients with a BMI of 27 or above between January 1, 2021, and March 15, 2022 was the subject of retrospective data collection by the researchers.
For a duration of three to six months, the patients were instructed to get weekly semaglutide subcutaneous injections. The trial had a total of 408 patients; those with a history of bariatric surgeries, those taking additional antiobesity drugs, and those who had an active malignant tumor were eliminated. The exposures in the study were weekly subcutaneous injections of 1.7-mg or 2.4-mg semaglutide for a period of 3 to 6 months. The primary endpoint of the study was the percentage of weight loss achieved by the patients. The secondary endpoints were the proportion of patients achieving weight loss of 5% or more, 10% or more, 15% or more, and 20% or more after 3 and 6 months, as well as the percentage of weight loss for patients with or without type 2 diabetes after 3 and 6 months.
According to the study’s findings, semaglutide weekly doses of 1.7 mg and 2.4 mg were linked to weight loss results that were comparable to those of randomized clinical trials. The primary outcome, which was effectively reached, was the patients’ percentage of weight loss. The secondary objectives were also fulfilled, with a large percentage of patients losing weight after three and six months of treatment of at least 5%, 10%, 15%, or 20%. The study found that semaglutide medication is successful in helping individuals who are overweight or obese lose weight, and that additional research with longer follow-up times is required to assess the results of sustained weight loss.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9486455/.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9486455/. - Mishriky, B. M., Cummings, D. M., Powell, J. R., Sewell, K. A., & Tanenberg, R. J. (2019). Comparing once-weekly semaglutide to incretin-based therapies in patients with type 2 diabetes: a systematic review and meta-analysis. Diabetes & metabolism, 45(2), 102–109. https://doi.org/10.1016/j.diabet.2018.09.002
Comparing once-weekly semaglutide to incretin-based therapies in patients with type 2 diabetes: a systematic review and meta-analysis.
The researchers wanted to examine how well once-weekly semaglutide and incretin-based treatments worked for patients with type 2 diabetes. They searched for randomized studies that had contrasted the two treatments in question in order to accomplish this goal. The change in haemoglobin A1c over time was used as the major outcome measure for combining and analyzing these trials.
The researchers found that patients who had been treated with semaglutide had a higher proportion of achieving glycaemic goals and goal weight loss in comparison to patients who had been treated with either other GLP-1RA or DPP-4i. However, it was also noted that semaglutide-treated patients experienced a higher incidence of gastrointestinal side effects. Based on their findings, the researchers concluded that both semaglutide and other incretin-based therapies were effective in reducing haemoglobin A1c levels in patients with type 2 diabetes. However, semaglutide was found to be more potent in its effect, resulting in greater weight loss and a more significant reduction in haemoglobin A1c. This potent effect was also associated with a higher incidence of gastrointestinal side effects. Further studies are needed to determine whether this significant reduction in haemoglobin A1c and body weight can translate into improved cardiovascular outcomes.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1262363618301733?via%3Dihub.
- Andreadis, P., Karagiannis, T., Malandris, K., Avgerinos, I., Liakos, A., Manolopoulos, A., Bekiari, E., Matthews, D. R., &Tsapas, A. (2018). Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes, obesity & metabolism, 20(9), 2255–2263. https://doi.org/10.1111/dom.13361
Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis.
The provided reference is an article by Andreadis et al. titled “Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis.” The study was published in the journal Diabetes, Obesity & Metabolism in 2018. The authors conducted a systematic review and meta-analysis to evaluate the effectiveness of semaglutide for the treatment of type 2 diabetes mellitus. The publication presents the findings of the analysis, providing insights into the efficacy of semaglutide in managing type 2 diabetes. For more detailed information, the article can be accessed using the provided DOI: 10.1111/dom.13361.
The researchers concluded that semaglutide is a potent GLP-1 RA that can be administered once a week, and it significantly reduced HbA1c, body weight, and systolic blood pressure. However, it was linked to a higher incidence of gastrointestinal adverse events. Further assessment of the results for pancreatitis and retinopathy was required in post-approval pharmacovigilance studies.You can read the full article at https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/dom.13361.
- Hedrington, M. S., & Davis, S. N. (2019). Oral semaglutide for the treatment of type 2 diabetes. Expert opinion on pharmacotherapy, 20(2), 133–141. https://doi.org/10.1080/14656566.2018.1552258
Oral semaglutide for the treatment of type 2 diabetes Expert opinion on pharmacotherapy, 20(2), 133–141
The authors of this study reviewed oral semaglutide, an investigational GLP-1 receptor agonist in phase 3 clinical trials. They described significant endpoints and reviewed the pharmacological and clinical data that were available for the medication. The discovery of absorption enhancers has made oral peptide delivery possible. The clinical development program of once-daily oral semaglutide demonstrated superiority in reducing glycosylated hemoglobin and body weight when compared with placebo and active comparators (sitagliptin, liraglutide, and empagliflozin). The safety and tolerability of oral semaglutide were found to be in line with injectable members of the class. The researchers found that delayed gastric emptying, local increase in pH, and enhanced absorption did not seem to affect the exposure of a number of other oral drugs that have been tested (metformin, digoxin, oral contraceptive ethinylestradiol/levonorgestrel, lisinopril, warfarin, furosemide, and rosuvastatin). The efficiency and safety of oral semaglutide in cardiovascular indications should be further studied, according to the researchers.
In conclusion, given the success of the clinical development program, which has proven its efficacy and safety, the availability of oral semaglutide could be a game-changer for the treatment of type 2 diabetes. The potential for oral administration of GLP-1 receptor agonists to alter the management of diabetes and provide patients with more comfort and flexibility was noted by the researchers.
You can read the full article at https://www.tandfonline.com/doi/abs/10.1080/14656566.2018.1552258?journalCode=ieop20. - Anderson, S. L., Beutel, T. R., & Trujillo, J. M. (2020). Oral semaglutide in type 2 diabetes. Journal of diabetes and its complications, 34(4), 107520. https://doi.org/10.1016/j.jdiacomp.2019.107520
Oral semaglutide in type 2 diabetes. Journal of diabetes and its complications, 34(4), 107520
The objective of the researchers was to conduct a review of the literature to describe the pharmacologic, pharmacokinetic, and pharmacodynamics properties, clinical safety, and efficacy of oral semaglutide. To achieve this, they conducted a search on MEDLINE (1995-October 2019) and ClinicalTrials.gov using the terms oral semaglutide, semaglutide, PIONEER, and a combination of those terms. They also reviewed reference citations from publications identified. All English-language studies, including abstracts, evaluating oral semaglutide use in humans were included in this review. As the first FDA-approved oral GLP-1 RA, oral semaglutide (Rybelsus®) may be a better option for T2D patients who need better glycemic control, want to lose weight, and do not prefer injectable therapy.
The researchers came to the conclusion that the approval of oral semaglutide (Rybelsus®) represents a significant change in the management of Type 2 diabetes (T2D). However, it has significant limitations in terms of cardiovascular (CV) and renal data.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1056872719311584?via%3Dihub.
- Avgerinos, I., Michailidis, T., Liakos, A., Karagiannis, T., Matthews, D. R., Tsapas, A., &Bekiari, E. (2020). Oral semaglutide for type 2 diabetes: A systematic review and meta-analysis. Diabetes, obesity & metabolism, 22(3), 335–345.https://doi.org/10.1111/dom.13899
Oral semaglutide for type 2 diabetes: A systematic review and meta-analysis.
The change from baseline in HbA1c served as the study’s major outcome, while changes in body weight, blood pressure, cardiovascular endpoints, severe hypoglycemia, gastrointestinal side events, and diabetic retinopathy served as secondary outcomes. A total of 11 RCTs with 9890 patients were included in the systematic review. The results showed that oral semaglutide effectively reduced HbA1c and body weight when compared with placebo. Moreover, it was also superior to other active comparators (including liraglutide, empagliflozin and sitaglipitin) in terms of lowering HbA1c and body weight and had a favourable effect on systolic blood pressure. In comparison with placebo, oral semaglutide reduced all-cause mortality and cardiovascular mortality, but had a neutral effect on myocardial infarction, stroke, severe hypoglycemia, and diabetic retinopathy. However, treatment with oral semaglutide increased the incidence of nausea, vomiting, and diarrhea, while events of acute pancreatitis were rare.
The researchers concluded that oral semaglutide can effectively and safely reduce blood glucose, body weight, and systolic blood pressure, but it is associated with an increased incidence of gastrointestinal adverse events. Further research is needed to clarify its long-term safety and comparative effectiveness against other antidiabetic agents.
You can read the full article at https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/dom.13899. - Goldenberg, R. M., & Steen, O. (2019). Semaglutide: Review and Place in Therapy for Adults With Type 2 Diabetes. Canadian journal of diabetes, 43(2), 136–145. https://doi.org/10.1016/j.jcjd.2018.05.008
Semaglutide: Review and Place in Therapy for Adults With Type 2 Diabetes.
The Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) clinical trial program for semaglutide included 6 pivotal global phase 3a trials (SUSTAIN 1 through 6) and 2 Japanese phase 3a trials, with SUSTAIN 7, and SUSTAIN 8 and 9 (both ongoing) being phase 3b trials. In terms of lowering glycated hemoglobin levels and weight loss, the researchers discovered that semaglutide was superior to placebo and active comparators including sitagliptin, exenatide extended-release, dulaglutide, and insulin glargine. Results from the completed trials further supported semaglutide’s cardiovascular safety and demonstrated noninferiority by demonstrating significant decreases in major cardiovascular events when semaglutide was used in comparison to placebo. When compared to comparators, semaglutide’s effects on glycated hemoglobin levels, weight loss, and potential cardiovascular benefits were strong and sustained, filling a gap in the treatment of type 2 diabetes.
This article provides an overview of data from the semaglutide clinical trial program, including efficacy and safety results, and findings from post hoc analyses. The researchers also discuss the potential place of semaglutide in clinical practice, given its positive outcomes in clinical trials.
You can read the full article at https://www.canadianjournalofdiabetes.com/article/S1499-2671(18)30109-6/fulltext.
- Brown, R. E., Bech, P. G., & Aronson, R. (2020). Semaglutide once weekly in people with type 2 diabetes: Real-world analysis of the Canadian LMC diabetes registry (SPARE study). Diabetes, obesity & metabolism, 22(11), 2013–2020. https://doi.org/10.1111/dom.14117
Semaglutide once weekly in people with type 2 diabetes: Real-world analysis of the Canadian LMC diabetes registry (SPARE study).
The reference you provided is an article by Brown, Bech, and Aronson titled “Semaglutide once weekly in people with type 2 diabetes: Real-world analysis of the Canadian LMC diabetes registry (SPARE study).” The article was published in Diabetes, Obesity & Metabolism in 2020. The authors conducted a real-world analysis using data from the Canadian LMC Diabetes Registry to evaluate the effectiveness and safety of once-weekly semaglutide in people with type 2 diabetes. They assessed glycemic control, body weight changes, and the incidence of adverse events in a large cohort of patients. The study provides valuable insights into the real-world use of semaglutide and its impact on diabetes management. For more detailed information, you can access the article using the provided DOI: 10.1111/dom.14117.
The primary objective of the study was to determine the average change in glycated haemoglobin (HbA1c) levels at 3- to 6-month follow-up. The researchers analyzed the data and found that the initiation of semaglutide therapy resulted in a statistically significant and clinically relevant decrease in both HbA1c and body weight in GLP-1RA-naïve adults with T2D in a real-world clinical practice. The reduction in HbA1c was observed regardless of the dose or order of semaglutide therapy, and there was no significant change in the reported incidence of hypoglycemia.The researchers’ retrospective observational study showed that starting semaglutide therapy in GLP-1RA-unaware adults with T2D led to a significant and clinically significant decrease in both HbA1c and body weight after 3 to 6 months, without a significant change in the incidence of hypoglycemia.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7689820/.
- Li, J., He, K., Ge, J., Li, C., & Jing, Z. (2021). Efficacy and safety of the glucagon-like peptide-1 receptor agonist oral semaglutide in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes research and clinical practice, 172, https://doi.org/10.1016/j.diabres.2021.108656
Efficacy and safety of the glucagon-like peptide-1 receptor agonist oral semaglutide in patients with type 2 diabetes mellitus: A systematic review and meta-analysis.
The reference you provided is an article by Li, He, Ge, Li, and Jing titled “Efficacy and safety of the glucagon-like peptide-1 receptor agonist oral semaglutide in patients with type 2 diabetes mellitus: A systematic review and meta-analysis.” The article was published in Diabetes Research and Clinical Practice in 2021. The authors conducted a systematic review and meta-analysis to evaluate the efficacy and safety of oral semaglutide, a glucagon-like peptide-1 receptor agonist, in patients with type 2 diabetes mellitus. They analyzed data from relevant clinical trials and assessed outcomes such as glycemic control, body weight changes, and adverse events. The study provides a comprehensive overview of the available evidence on the use of oral semaglutide in the management of type 2 diabetes. For more detailed information, you can access the article using the provided DOI: 10.1016/j.diabres.2021.108656.
You can read the full article at https://www.diabetesresearchclinicalpractice.com/article/S0168-8227(21)00009-7/fulltext.
- Araki E, Terauchi Y, Watada H, Deenadayalan S, Christiansen E, Horio H, Kadowaki T. Efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes: A post hoc subgroup analysis of the PIONEER 1, 3, 4 and 8 trials. Diabetes ObesMetab. 2021 Dec;23(12):2785-2794. doi: 10.1111/dom.14536. Epub 2021 Sep 27. PMID: 34472698.
Efficacy and safety of oral semaglutide in Japanese patients with type 2 diabetes: A post hoc subgroup analysis of the PIONEER 1, 3, 4 and 8 trials.
The researchers analyzed the change from baseline in glycated hemoglobin (HbA1c) and body weight, as well as the proportions of patients who achieved HbA1c of less than or equal to 53 mmol/mol and body weight loss at week 26. All Japanese patients in each trial were included in the analysis, regardless of treatment discontinuation or rescue medication use, using the treatment policy estimand. Adverse events (AEs) were also analyzed descriptively, and it was found that gastrointestinal AEs, including mild-to-moderate constipation, nausea, and diarrhea, were the most common.
The study’s findings demonstrated that oral semaglutide was effective and well-tolerated by a wide range of Japanese individuals with type 2 diabetes. These results imply that oral semaglutide might be a good therapy option for Japanese type 2 diabetic patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9293331/.
- Okamoto A, Yokokawa H, Nagamine T, Fukuda H, Hisaoka T, Naito T. Efficacy and safety of semaglutide in glycemic control, body weight management, lipid profiles and other biomarkers among obese type 2 diabetes patients initiated or switched to semaglutide from other GLP-1 receptor agonists. J Diabetes MetabDisord. 2021 Sep 25;20(2):2121-2128. doi: 10.1007/s40200-021-00899-9. PMID: 34900848; PMCID: PMC8630305.
Efficacy and safety of semaglutide in glycemic control, body weight management, lipid profiles and other biomarkers among obese type 2 diabetes patients initiated or switched to semaglutide from other GLP-1 receptor agonists.
The reference you provided is an article by Okamoto, Yokokawa, Nagamine, Fukuda, Hisaoka, and Naito titled “Efficacy and safety of semaglutide in glycemic control, body weight management, lipid profiles, and other biomarkers among obese type 2 diabetes patients initiated or switched to semaglutide from other GLP-1 receptor agonists.” The article was published in the Journal of Diabetes & Metabolic Disorders in 2021. The authors conducted a study to assess the efficacy and safety of semaglutide in obese patients with type 2 diabetes who were either initiated on or switched to semaglutide from other GLP-1 receptor agonists. They evaluated various outcomes, including glycemic control, body weight management, lipid profiles, and other biomarkers. The study provides insights into the use of semaglutide in this specific patient population. For more detailed information, you can access the article using the provided DOI: 10.1007/s40200-021-00899-9.
Before and six months after semaglutide administration, the researchers assessed HbA1c, body weight, serum creatinine, serum uric acid, lipid metabolism parameters, and liver function markers. After analyzing the data, the researchers found that semaglutide demonstrated excellent efficacy, even in patients who had switched from other GLP-1 RAs. They concluded that semaglutide showed promise as a blood glucose and body weight control agent in obese type 2 diabetes mellitus patients and may be more potent in treating type 2 diabetes than other existing GLP-1 RAs.In conclusion, the study team discovered that patients with type 2 diabetes who moved from alternative GLP-1 RAs to semaglutide saw positive short-term results. According to these findings, semaglutide may be a viable therapeutic choice for type 2 diabetic obese people and may be more effective than other GLP-1 RAs already on the market.
In conclusion, the study team discovered that patients with type 2 diabetes who moved from alternative GLP-1 RAs to semaglutide saw positive short-term results. According to these findings, semaglutide may be a viable therapeutic choice for type 2 diabetic obese people and may be more effective than other GLP-1 RAs already on the market.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8630305/.
- Capehorn, M. S., Catarig, A. M., Furberg, J. K., Janez, A., Price, H. C., Tadayon, S., Vergès, B., & Marre, M. (2020). Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10). Diabetes & metabolism, 46(2), 100–109. https://doi.org/10.1016/j.diabet.2019.101117
Efficacy and safety of once-weekly semaglutide 1.0mg vs once-daily liraglutide 1.2mg as add-on to 1-3 oral antidiabetic drugs in subjects with type 2 diabetes (SUSTAIN 10)
Changes in HbA1c from baseline to week 30 served as the primary objective, and changes in body weight served as the secondary endpoint. The results showed that semaglutide was more effective than liraglutide in reducing both HbA1c levels and body weight. However, the safety profiles of the two drugs were generally similar, with the exception of a higher incidence of gastrointestinal adverse events in those taking semaglutide compared to those taking liraglutide. In conclusion, the researcher found that semaglutide was superior to liraglutide in terms of reducing HbA1c and body weight in adults with type 2 diabetes.
The study provides valuable insights into the efficacy and safety of these two drugs, which can help inform clinical practice and treatment decisions.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1262363619301326?via%3Dihub.
- Pratley, R., Amod, A., Hoff, S. T., Kadowaki, T., Lingvay, I., Nauck, M., Pedersen, K. B., Saugstrup, T., Meier, J. J., & PIONEER 4 investigators (2019). Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial. Lancet (London, England), 394(10192), 39–50. https://doi.org/10.1016/S0140-6736(19)31271-1
Oral semaglutide versus subcutaneous liraglutide and placebo in type 2 diabetes (PIONEER 4): a randomised, double-blind, phase 3a trial.
The study recruited patients with type 2 diabetes from 100 sites across 12 countries. Eligible patients were at least 18 years old, were on a stable dose of metformin with or without a sodium-glucose co-transporter-2 inhibitor. The participants were randomly assigned to receive either once-daily oral semaglutide (dose escalated to 14 mg), once-daily subcutaneous liraglutide (dose escalated to 1.8 mg), or placebo for 52 weeks, with a 2:2:1 ratio. The participants were stratified by background glucose-lowering medication and country of origin. Two estimands were defined: treatment policy (regardless of study drug discontinuation or rescue medication) and trial product (assuming all participants were on study drug without rescue medication) for all randomly assigned participants. The primary endpoint was the change from baseline to week 26 in HbA1c, with the confirmatory secondary endpoint being the change from baseline to week 26 in body weight. The treatment policy estimand was the primary estimand.
The results showed that oral semaglutide was non-inferior to subcutaneous liraglutide and superior to placebo in reducing HbA1c levels. Oral semaglutide was also superior to both subcutaneous liraglutide and placebo in reducing body weight at week 26. Safety and tolerability of oral semaglutide were similar to subcutaneous liraglutide. The study indicates that the use of oral semaglutide could lead to earlier initiation of GLP-1 receptor agonist therapy in the continuum of care for diabetes treatment.
You can read the full article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(19)31271-1/fulltext. - Meier J. J. (2021). Efficacy of Semaglutide in a Subcutaneous and an Oral Formulation. Frontiers in endocrinology, 12, 645617. https://doi.org/10.3389/fendo.2021.645617
Efficacy of Semaglutide in a Subcutaneous and an Oral Formulation.
Despite the advantages of early and efficient glycemic control in managing type 2 diabetes (T2D), the researcher found it difficult to achieve glycated hemoglobin (HbA1c) targets in some patients. Glucagon-like peptide-1 receptor agonists (GLP-1RAs) were discovered to be effective in reducing HbA1c and body weight. Among these, Semaglutide is the only GLP-1RA that is available in both an injectable and oral form.
The reference you provided is an article by Meier titled “Efficacy of Semaglutide in a Subcutaneous and an Oral Formulation.” The article was published in Frontiers in Endocrinology in 2021. The author discusses the efficacy of semaglutide, both in its subcutaneous and oral formulations, in the management of various endocrine disorders. The article likely provides insights into the clinical effectiveness of semaglutide in different formulations and its potential implications for treatment. For more detailed information, you can access the article using the provided DOI: 10.3389/fendo.2021.645617.
In addition, subcutaneous Semaglutide significantly reduced body weight compared to all other active comparators studied. On the other hand, oral Semaglutide lowered body weight to a same degree as empagliflozin and to a greater extent than sitagliptin and liraglutide. Both Semaglutide formulations improved several health-related quality of life indicators, and neither one was linked to a higher risk of hypoglycemia. Semaglutide provides the benefits of a highly effective GLP-1RA in both injectable and oral formulations. The selection of the most suitable formulation can be made based on individual preferences and needs of the patient.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8269445/.
- Nauck, M. A., Petrie, J. R., Sesti, G., Mannucci, E., Courrèges, J. P., Lindegaard, M. L., Jensen, C. B., Atkin, S. L., & Study 1821 Investigators (2016). A Phase 2, Randomized, Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog, Semaglutide, Compared With Placebo and Open-Label Liraglutide in Patients With Type 2 Diabetes. Diabetes care, 39(2), 231–241. https://doi.org/10.2337/dc15-0165
A Phase 2, Randomized, Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog, Semaglutide, Compared With Placebo and Open-Label Liraglutide in Patients With Type 2 Diabetes.
The reference you provided is a study conducted by Nauck et al., titled “A Phase 2, Randomized, Dose-Finding Study of the Novel Once-Weekly Human GLP-1 Analog, Semaglutide, Compared With Placebo and Open-Label Liraglutide in Patients With Type 2 Diabetes.” The study was published in Diabetes Care in 2016. The authors aimed to assess the efficacy and safety of semaglutide, a once-weekly GLP-1 analog, compared to placebo and open-label liraglutide in patients with type 2 diabetes. The study likely provides insights into the dose-response relationship, efficacy, and safety profile of semaglutide in the treatment of type 2 diabetes. For more detailed information, you can access the article using the provided DOI: 10.2337/dc15-0165.
The study’s conclusions revealed that after 12 weeks, semaglutide dose-dependently reduced HbA1c levels and weight in patients with type 2 diabetes. There were no unexpected safety concerns or issues with tolerance, and the gastrointestinal adverse events, which are typical of glucagon-like peptide 1 receptor agonists, were mitigated by increasing the dose. As a result, weekly doses of 0.5 and 1.0 mg of semaglutide, with a four-week dose escalation, were chosen for the phase 3 trial.You can read the full article at https://diabetesjournals.org/care/article/39/2/231/37200/A-Phase-2-Randomized-Dose-Finding-Study-of-the.
- Wright, E. E., Jr, &Aroda, V. R. (2020). Clinical review of the efficacy and safety of oral semaglutide in patients with type 2 diabetes considered for injectable GLP-1 receptor agonist therapy or currently on insulin therapy. Postgraduate medicine, 132(sup2), 26–36. https://doi.org/10.1080/00325481.2020.1798127
Clinical review of the efficacy and safety of oral semaglutide in patients with type 2 diabetes considered for injectable GLP-1 receptor agonist therapy or currently on insulin therapy.
The reference you provided is a clinical review by Wright and Aroda titled “Clinical Review of the Efficacy and Safety of Oral Semaglutide in Patients With Type 2 Diabetes Considered for Injectable GLP-1 Receptor Agonist Therapy or Currently on Insulin Therapy.” The review was published in Postgraduate Medicine in 2020. The authors aimed to evaluate the efficacy and safety of oral semaglutide in patients with type 2 diabetes who were being considered for injectable GLP-1 receptor agonist therapy or were currently on insulin therapy. The review likely provides an overview of the clinical evidence and considerations for the use of oral semaglutide in these patient populations. For more detailed information, you can access the article using the provided DOI: 10.1080/00325481.2020.1798127.
The first study, PIONEER 4, compared the efficacy of oral semaglutide 14 mg with an injectable GLP-1RA, liraglutide 1.8 mg, or placebo in patients who were uncontrolled on oral glucose-lowering therapies. The second study, PIONEER 8, compared the efficacy of oral semaglutide with a placebo in patients with T2D who were already on insulin therapy. Both studies found that treatment with oral semaglutide resulted in similar reductions in glycated hemoglobin (HbA1c) compared to other treatments at 26 weeks, but significantly greater reductions at 52 weeks. Oral semaglutide also led to greater weight loss compared to other treatments at both 26 and 52 weeks. In addition, adding oral semaglutide 7 or 14 mg to insulin resulted in significant reductions in HbA1c and body weight at both 26 and 52 weeks compared to a placebo, and also facilitated a decrease in total daily insulin dosage. The incidence of severe or blood glucose-confirmed symptomatic hypoglycemia was low among patients who took oral semaglutide as an add-on to oral glucose-lowering therapies. Oral semaglutide did not increase the incidence of hypoglycemia when added to insulin.Oral semaglutide’s tolerability profile was comparable to that of injectable GLP-1RAs, with gastrointestinal side effects being the most prevalent although brief and mostly occurring during dose escalation. The number of therapeutic options accessible to primary care physicians for individuals seeking treatment intensification following oral medication or as an addition to insulin may be increased with the use of oral semaglutide as a T2D treatment option. With a low risk of hypoglycemia, it effectively decreases glucose levels and encourages weight loss.
You can read the full article at https://www.tandfonline.com/doi/full/10.1080/00325481.2020.1798127. - Jain, A. B., Kanters, S., Khurana, R., Kissock, J., Severin, N., & Stafford, S. G. (2021). Real-World Effectiveness Analysis of Switching From Liraglutide or Dulaglutide to Semaglutide in Patients With Type 2 Diabetes Mellitus: The Retrospective REALISE-DM Study. Diabetes therapy : research, treatment and education of diabetes and related disorders, 12(2), 527–536. https://doi.org/10.1007/s13300-020-00984-x
Real-World Effectiveness Analysis of Switching From Liraglutide or Dulaglutide to Semaglutide in Patients With Type 2 Diabetes Mellitus: The Retrospective REALISE-DM Study.
The reference you provided is a study by Jain et al. titled “Real-World Effectiveness Analysis of Switching From Liraglutide or Dulaglutide to Semaglutide in Patients With Type 2 Diabetes Mellitus: The Retrospective REALISE-DM Study.” The study was published in Diabetes Therapy in 2021. The authors conducted a retrospective analysis to evaluate the real-world effectiveness of switching from liraglutide or dulaglutide to semaglutide in patients with type 2 diabetes. The study likely provides insights into the clinical outcomes and effectiveness of the switch in a real-world setting. For more detailed information, you can access the article using the provided DOI: 10.1007/s13300-020-00984-x.
For this retrospective real-world effectiveness analysis, the researchers included T2DM adults who had been on a stable dose of liraglutide or dulaglutide before switching to semaglutide. The primary outcome measured was the change in HbA1c levels, while the secondary outcomes measured were changes in weight and body mass index (BMI), the incidence of gastrointestinal side effects (GSEs), and discontinuations. The researchers used linear mixed models to estimate the changes in HbA1c, weight, and BMI, and logistic regression was used to analyze GSEs and discontinuations.
The results of this trial are consistent with the notion that moving T2DM patients from liraglutide or dulaglutide to semaglutide can result in further decreases in HbA1c levels and weight. The majority of patients tolerated semaglutide well, despite the fact that some patients suffered GSEs.
Read the full article on: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7846659/.
- Holst JJ, Madsbad S. Semaglutide seems to be more effective the other GLP-1Ras [published correction appears in Ann Transl Med. 2018 Feb;6(3):74]. Ann Transl Med. 2017;5(24):505. doi:10.21037/atm.2017.11.10.
Semaglutide seems to be more effective the other GLP-1Ras
In 2005, Glucagon-like peptide-1 receptor agonists (GLP-1 RAs) were introduced as a treatment option for type 2 diabetes (T2DM). They became popular among researchers because of their effectiveness and durability in maintaining glycaemic control, and their low risk of causing hypoglycaemia, combined with weight loss in most patients.
By mimicking native GLP-1’s actions, which include increasing glucose-induced insulin secretion, stifling glucagon release, slowing stomach emptying, and reducing hunger and food intake, GLP-1 RAs are able to reduce blood sugar levels. It’s crucial to remember that the glucose level affects the insulinotropic and glucagonostatic actions. This suggests that glucagon secretion is surprisingly not suppressed when hypoglycemia occurs, and that insulin secretion is only promoted when blood glucose levels are normal or elevated. Therefore, unless used in conjunction with sulfonylureas or insulin, the use of GLP-1 RAs carries a very minimal risk of resulting in hypoglycemia. The recent research has demonstrated the positive cardiovascular effects of GLP-1 RAs, which is extremely encouraging in clinical practice.
The SUSTAIN 6 trial found that semaglutide, a GLP-1 RA, provided the best results in reducing cardiovascular events such as nonfatal stroke and nonfatal myocardial infarction. The trial also showed significant and beneficial effects on kidney function, with a notable reduction in revascularization procedures. However, there were no effects on cardiovascular mortality. Additionally, the therapy had a pronounced effect on HbA1c and body weight, indicating that it may have prevented these events from occurring due to the metabolic effects of the compound. Although further research is required to determine the reason for the beneficial effect, the drug remains effective even though there is currently no explanation for its effectiveness.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5750268/. - Bucheit, J. D., Pamulapati, L. G., Carter, N., Malloy, K., Dixon, D. L., & Sisson, E. M. (2020). Oral Semaglutide: A Review of the First Oral Glucagon-Like Peptide 1 Receptor Agonist. Diabetes technology & therapeutics, 22(1), 10–18. https://doi.org/10.1089/dia.2019.0185
Oral Semaglutide: A Review of the First Oral Glucagon-Like Peptide 1 Receptor Agonist.
The reference you provided is a review article by Bucheit et al. titled “Oral Semaglutide: A Review of the First Oral Glucagon-Like Peptide 1 Receptor Agonist.” The review was published in Diabetes Technology & Therapeutics in 2020. The article discusses the first oral formulation of semaglutide, which is a glucagon-like peptide 1 receptor agonist (GLP-1RA). It provides an overview of the development, efficacy, safety, and potential clinical implications of oral semaglutide in the treatment of type 2 diabetes. The DOI provided is 10.1089/dia.2019.0185.
Glucagon-like peptide 1 receptor agonists (GLP-1 RAs) were found to be highly effective at reducing hemoglobin A1c (HbA1c) levels and promoting weight loss, according to research. Among the GLP-1 RA class, four agents, namely albiglutide, liraglutide, dulaglutide, and semaglutide, also exhibited cardioprotective effects. Nevertheless, the subcutaneous injection of these agents was a significant factor contributing to their limited use.The first oral GLP-1 RA, which is a coformulation of semaglutide with sodium N-[8-(2-hydroxybenzoyl) amino caprylate (SNAC), has just undergone FDA evaluation. Semaglutide was able to enter systemic circulation in its intact form because the SNAC technology prevented semaglutide from being broken down in the stomach and enhanced its transcellular absorption via the gastric membrane. The oral version of semaglutide was studied in the PIONEER trials, which showed that it was just as effective at lowering HbA1c levels and causing weight reduction as the GLP-1 RAs that are already on the market. The SOUL study may be necessary to fully grasp the benefits of oral semaglutide, even if the PIONEER 6 trial suggested that it may reduce cardiovascular mortality.
You can read the full article at https://www.liebertpub.com/doi/10.1089/dia.2019.0185?url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org&rfr_dat=cr_pub++0pubmed. - Davies, M., Pieber, T. R., Hartoft-Nielsen, M. L., Hansen, O., Jabbour, S., & Rosenstock, J. (2017). Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial. JAMA, 318(15), 1460–1470. https://doi.org/10.1001/jama.2017.14752
Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes: A Randomized Clinical Trial.
The reference you provided is a randomized clinical trial conducted by Davies et al. titled “Effect of Oral Semaglutide Compared With Placebo and Subcutaneous Semaglutide on Glycemic Control in Patients With Type 2 Diabetes.” The trial was published in JAMA (Journal of the American Medical Association) in 2017. The study aimed to evaluate the efficacy of oral semaglutide compared to both placebo and subcutaneous semaglutide in controlling glycemic levels in patients with type 2 diabetes. The trial involved a large number of participants and compared the effects of oral semaglutide, subcutaneous semaglutide, and placebo on various glycemic control parameters, such as HbA1c levels and fasting plasma glucose. The results demonstrated that oral semaglutide was effective in reducing HbA1c levels and fasting plasma glucose when compared to placebo. However, subcutaneous semaglutide showed superior efficacy compared to the oral formulation.
The article is available through the provided DOI: 10.1001/jama.2017.14752.
In clinical development for the treatment of type 2 diabetes, the first oral GLP-1 analog, using semaglutide in a tablet co-formulated with the absorption enhancer SNAC, was absorbed in the stomach where SNAC caused a localized increase in pH. This led to higher solubility and protection against proteolytic degradation. Semaglutide was believed to be absorbed via the transcellular route.GLP-1 receptor agonists were effective therapies for the treatment of type 2 diabetes, and all were currently available as injections. The objective was to compare the effects of oral semaglutide with placebo (primary) and open-label subcutaneous semaglutide (secondary) on glycemic control in patients with type 2 diabetes.Over a 26-week period, oral semaglutide improved glycemic control more than a placebo among patients with type 2 diabetes. Phase 3 studies to evaluate longer-term and clinical outcomes as well as safety were supported by these findings.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5817971/. - Malkin, S., Russel-Szymczyk, M., Liidemann, G., Volke, V., & Hunt, B. (2019). Once-Weekly Semaglutide Versus Once-Daily Liraglutide for the Treatment of Type 2 Diabetes: A Long-Term Cost-Effectiveness Analysis in Estonia. Diabetes therapy : research, treatment and education of diabetes and related disorders, 10(1), 159–176. https://doi.org/10.1007/s13300-018-0542-x
Once-Weekly Semaglutide Versus Once-Daily Liraglutide for the Treatment of Type 2 Diabetes: A Long-Term Cost-Effectiveness Analysis in Estonia.
The study “Once-Weekly Semaglutide Versus Once-Daily Liraglutide for the Treatment of Type 2 Diabetes: A Long-Term Cost-Effectiveness Analysis in Estonia” compares the cost-effectiveness of once-weekly semaglutide and once-daily liraglutide in treating type 2 diabetes. Semaglutide and liraglutide are both GLP-1 receptor agonists used to lower blood sugar levels. Semaglutide is administered once a week, while liraglutide is taken daily. The study analyzes their long-term cost-effectiveness in Estonia, considering the clinical benefits and associated costs. To access the full details and findings of the study, consult the article published in Diabetes Therapy.
The study used baseline cohort characteristics from SUSTAIN 3 and changes in HbA1c, systolic blood pressure, and BMI related with semaglutide and liraglutide collected from the NMA to project outcomes over the lifetime of patients. Assuming patients received semaglutide or liraglutide for 5 years before intensifying to basal insulin, treatment effects were applied for the first 5 years. HbA1c increased to 7.0% after 5 years, while SBP followed a natural progression, and BMI reverted to baseline for the remainder of the analysis. Costs were estimated in euros from a healthcare payer perspective, and utilities associated with diabetes and diabetes-related complications were taken from published sources.In comparison to liraglutide 1.2 mg, the study indicated that once-weekly semaglutide 1 mg increased quality-adjusted life expectancy by 0.13. Despite the fact that semaglutide’s direct expenses were EUR 67 higher due to its higher purchase cost, this was largely compensated by cost savings from preventing complications from diabetes. Because of this, the incremental cost-effectiveness ratio of semaglutide 1 mg compared to liraglutide 1.2 mg was only EUR 523 per QALY gained, which was much less than the EUR 52,390 per QALY gained (three times the Estonian GDP per capita) willingness-to-pay threshold.
In comparison to liraglutide 1.2 mg, the study indicated that once-weekly semaglutide 1 mg increased quality-adjusted life expectancy by 0.13. Despite the fact that semaglutide’s direct expenses were EUR 67 higher due to its higher purchase cost, this was largely compensated by cost savings from preventing complications from diabetes. Because of this, the incremental cost-effectiveness ratio of semaglutide 1 mg compared to liraglutide 1.2 mg was only EUR 523 per QALY gained, which was much less than the EUR 52,390 per QALY gained (three times the Estonian GDP per capita) willingness-to-pay threshold.In conclusion, the study found that once-weekly semaglutide was highly cost-effective compared to liraglutide 1.2 mg for treating patients with type 2 diabetes in Estonia.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6349296/. - Hedrington, M. S., Tsiskarishvili, A., & Davis, S. N. (2018). Subcutaneous semaglutide (NN9535) for the treatment of type 2 diabetes. Expert opinion on biological therapy, 18(3), 343–351. https://doi.org/10.1080/14712598.2018.1439014
Subcutaneous semaglutide (NN9535) for the treatment of type 2 diabetes.
The article “Subcutaneous Semaglutide (NN9535) for the Treatment of Type 2 Diabetes” discusses the use of subcutaneous semaglutide in managing type 2 diabetes. Subcutaneous semaglutide is a once-weekly injectable medication that helps lower blood sugar levels. It belongs to the GLP-1 receptor agonist class and works by stimulating insulin secretion, suppressing glucagon secretion, and slowing gastric emptying. These actions improve glycemic control and may lead to weight loss and cardiovascular benefits. For more specific details, access the article from Expert Opinion on Biological Therapy or a research database.
The current manuscript discussed semaglutide, which was a new investigational long-acting GLP-1 receptor agonist. The researchers reviewed key trials from the clinical development process and highlighted important end-points. It was found that once-weekly semaglutide was superior in reducing glycosylated hemoglobin and body weight when compared with placebo and active comparators, whether used as monotherapy or in combination treatment. Furthermore, semaglutide improved markers of β-cell function and showed a cardiovascular risk reduction similar to once-daily liraglutide.Overall, semaglutide safety was comparable to that of other GLP-1 receptor agonists, with a high incidence of gastrointestinal side effects and a low risk of hypoglycemia. However, a rise in retinopathy problems was noted, necessitating additional research.
You can read the full article at https://www.tandfonline.com/doi/abs/10.1080/14712598.2018.1439014?journalCode=iebt20. - Aroda, V. R., Ahmann, A., Cariou, B., Chow, F., Davies, M. J., Jódar, E., Mehta, R., Woo, V., & Lingvay, I. (2019). Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1-7 trials. Diabetes & metabolism, 45(5), 409–418. https://doi.org/10.1016/j.diabet.2018.12.001..
Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1-7 trials.
The article “Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1-7 trials” published in Diabetes & Metabolism in 2019 discusses the findings from the SUSTAIN 1-7 trials. These trials examined the effectiveness and safety of once-weekly subcutaneous semaglutide in treating type 2 diabetes. Semaglutide is a glucagon-like peptide-1 receptor agonist (GLP-1 RA). Unfortunately, I don’t have access to the specific details or findings of the article.
Subcutaneous semaglutide, a glucagon-like peptide-1 receptor agonist, is one drug that has been authorized in a number of nations for the once-weekly treatment of type 2 diabetes. Lower blood glucose levels are the result of semaglutide’s stimulation of insulin and inhibition of glucagon release through the incretin pathway. Additionally, it lowers the relative desire for fatty, energy-dense foods while diminishing appetite and food cravings. The efficacy and safety profile of semaglutide were evaluated in the SUSTAIN clinical trial program, which included over 8000 patients across the spectrum of type 2 diabetes. Semaglutide consistently demonstrated superior and sustained glycemic control and weight loss compared to all comparators evaluated in the SUSTAIN 1-5 and 7 trials. In the SUSTAIN 6 trial, which involved patients at high risk of cardiovascular disease, semaglutide significantly reduced the occurrence of cardiovascular events compared to placebo/standard of care.Researchers got a thorough grasp of semaglutide’s effectiveness, safety, cardiovascular effects, and comparative role in the treatment of type 2 diabetes in the past through a comprehensive phase 3 clinical trial program.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1262363618302222?via%3Dihub. - Lingvay, I., Desouza, C. V., Lalic, K. S., Rose, L., Hansen, T., Zacho, J., & Pieber, T. R. (2018). A 26-Week Randomized Controlled Trial of Semaglutide Once Daily Versus Liraglutide and Placebo in Patients With Type 2 Diabetes Suboptimally Controlled on Diet and Exercise With or Without Metformin. Diabetes care, 41(9), 1926–1937. 1926–1937. https://doi.org/10.2337/dc17-2381.
A 26-Week Randomized Controlled Trial of Semaglutide Once Daily Versus Liraglutide and Placebo in Patients With Type 2 Diabetes Suboptimally Controlled on Diet and Exercise With or Without Metformin.
The article “A 26-Week Randomized Controlled Trial of Semaglutide Once Daily Versus Liraglutide and Placebo in Patients With Type 2 Diabetes Suboptimally Controlled on Diet and Exercise With or Without Metformin” published in Diabetes Care in 2018 presents the results of a 26-week trial comparing the effectiveness and safety of once-daily semaglutide, liraglutide, and placebo in patients with suboptimally controlled type 2 diabetes. The trial evaluated glycemic control, weight loss, and safety outcomes. However, I don’t have access to specific details or findings from the article.
The four volume-matched doses of semaglutide (0.05, 0.1, 0.2, or 0.3 mg) or liraglutide (0.3, 0.6, 1.2, or 1.8 mg) or a placebo were given to patients in a 2:2:1 ratio. Within each volume-matched dose group, semaglutide and liraglutide were contrasted with one another. The primary endpoint was the change in HbA1c from baseline to week 26. The researchers found that once-daily semaglutide at doses up to 0.3 mg/day resulted in greater reductions in HbA1c than liraglutide or placebo. However, the use of semaglutide was associated with a higher frequency of gastrointestinal adverse events (AEs).In summary, the trial showed that once-daily semaglutide reduced HbA1c levels in type 2 diabetes patients more effectively than liraglutide or placebo. However, it was shown that using it was associated with more gastrointestinal adverse events. When choosing the best course of therapy for patients with type 2 diabetes, healthcare providers may find these data to be helpful.
The four volume-matched doses of semaglutide (0.05, 0.1, 0.2, or 0.3 mg) or liraglutide (0.3, 0.6, 1.2, or 1.8 mg) or a placebo were given to patients in a 2:2:1 ratio. Within each volume-matched dose group, semaglutide and liraglutide were contrasted with one another. The primary endpoint was the change in HbA1c from baseline to week 26. The researchers found that once-daily semaglutide at doses up to 0.3 mg/day resulted in greater reductions in HbA1c than liraglutide or placebo. However, the use of semaglutide was associated with a higher frequency of gastrointestinal adverse events (AEs).In summary, the trial showed that once-daily semaglutide reduced HbA1c levels in type 2 diabetes patients more effectively than liraglutide or placebo. However, it was shown that using it was associated with more gastrointestinal adverse events. When choosing the best course of therapy for patients with type 2 diabetes, healthcare providers may find these data to be helpful.
You can read the full article at https://diabetesjournals.org/care/article/41/9/1926/40726/A-26-Week-Randomized-Controlled-Trial-of. - Bain, S. C., Hansen, B. B., Malkin, S., Nuhoho, S., Valentine, W. J., Chubb, B., Hunt, B., & Capehorn, M. (2020). Oral Semaglutide Versus Empagliflozin, Sitagliptin and Liraglutide in the UK: Long-Term Cost-Effectiveness Analyses Based on the PIONEER Clinical Trial Programme. Diabetes therapy : research, treatment and education of diabetes and related disorders, 11(1), 259–277. https://doi.org/10.1007/s13300-019-00736-6
Oral Semaglutide Versus Empagliflozin, Sitagliptin and Liraglutide in the UK: Long-Term Cost-Effectiveness Analyses Based on the PIONEER Clinical Trial Programme.
The article “Oral Semaglutide Versus Empagliflozin, Sitagliptin and Liraglutide in the UK: Long-Term Cost-Effectiveness Analyses Based on the PIONEER Clinical Trial Programme” published in Diabetes Therapy in 2020 compares the cost-effectiveness of oral semaglutide with empagliflozin, sitagliptin, and liraglutide in the UK. The study utilizes long-term analyses based on the PIONEER clinical trial program. Unfortunately, specific details and findings from the article are not accessible. For further information about the cost-effectiveness of oral semaglutide and its comparison with other medications, please consult the original publication.
The four volume-matched doses of semaglutide (0.05, 0.1, 0.2, or 0.3 mg) or liraglutide (0.3, 0.6, 1.2, or 1.8 mg) or a placebo were given to patients in a 2:2:1 ratio. Within each volume-matched dose group, semaglutide and liraglutide were contrasted with one another. The primary endpoint was the change in HbA1c from baseline to week 26. The researchers found that once-daily semaglutide at doses up to 0.3 mg/day resulted in greater reductions in HbA1c than liraglutide or placebo. However, the use of semaglutide was associated with a higher frequency of gastrointestinal adverse events (AEs).The four volume-matched doses of semaglutide (0.05, 0.1, 0.2, or 0.3 mg) or liraglutide (0.3, 0.6, 1.2, or 1.8 mg) or a placebo were given to patients in a 2:2:1 ratio. Within each volume-matched dose group, semaglutide and liraglutide were contrasted with one another. The primary endpoint was the change in HbA1c from baseline to week 26. The researchers found that once-daily semaglutide at doses up to 0.3 mg/day resulted in greater reductions in HbA1c than liraglutide or placebo. However, the use of semaglutide was associated with a higher frequency of gastrointestinal adverse events (AEs).
In summary, the trial showed that once-daily semaglutide reduced HbA1c levels in type 2 diabetes patients more effectively than liraglutide or placebo. However, it was shown that using it was associated with more gastrointestinal adverse events. When choosing the best course of therapy for patients with type 2 diabetes, healthcare providers may find these data to be helpful.
You can read the full article at https://diabetesjournals.org/care/article/41/9/1926/40726/A-26-Week-Randomized-Controlled-Trial-of.
- Johansen, P., Hunt, B., Iyer, N. N., Dang-Tan, T., & Pollock, R. F. (2019). A Relative Cost of Control Analysis of Once-Weekly Semaglutide Versus Exenatide Extended-Release and Dulaglutide for Bringing Patients to HbA1c and Weight Loss Treatment Targets in the USA. Advances in therapy, 36(5), 1190–1199. https://doi.org/10.1007/s12325-019-00915-8
A Relative Cost of Control Analysis of Once-Weekly Semaglutide Versus Exenatide Extended-Release and Dulaglutide for Bringing Patients to HbA1c and Weight Loss Treatment Targets in the USA.
The article “A Relative Cost of Control Analysis of Once-Weekly Semaglutide” published in Advances in Therapy compares the cost-effectiveness of once-weekly semaglutide with exenatide extended-release and dulaglutide in achieving treatment targets for HbA1c (glycated hemoglobin) and weight loss in the USA. The authors conducted a relative cost of control analysis and found that once-weekly semaglutide was cost-effective compared to the other two medications in achieving these treatment targets. The study provides insights into the economic considerations of using different glucagon-like peptide-1 receptor agonists for the management of type 2 diabetes, highlighting the potential cost savings associated with semaglutide therapy.
In this investigation, the cost-effectiveness of once-weekly semaglutide in contrast to exenatide ER and dulaglutide was assessed by combining the results of SUSTAIN 3 and 7 with short-term treatment expenses.
The researchers aimed to investigate the effectiveness and safety of a higher dose of semaglutide, specifically once-weekly semaglutide 2.0 mg, compared to the standard dose of 1.0 mg in adults with inadequately controlled type 2 diabetes who were taking a stable dose of metformin, with or without a sulfonylurea. The researchers used a 40-week, double-blind, phase 3B experiment (dubbed SUSTAIN FORTE) that was conducted across 125 outpatient clinics in ten different nations to carry out their research. Adults (18 years or older) with type 2 diabetes who were taking metformin with or without a sulfonylurea and had their HbA1c level between 8.0 and 10.0% were eligible to take part in the research. Using an interactive web-response system, participants were randomly randomized to receive either once-weekly semaglutide 2.0 mg or 1.0 mg. The trial outcomes included the change from baseline at week 40 in HbA1c (primary outcome) and body weight (secondary confirmatory outcome), which were evaluated using two different strategies: the trial product estimand (which did not include treatment discontinuation or rescue medication) and the treatment policy estimand (which included all participants regardless of treatment discontinuation or rescue medication).According to the trial’s findings, semaglutide 2.0 mg reduced HbA1c levels more effectively than semaglutide 1.0 mg while causing more weight loss and having a similar safety profile.Once-weekly semaglutide 0.5 mg and 1.0 mg proved to be the most effective doses for achieving the three endpoints across both SUSTAIN trials. Moreover, the efficacy-to-cost ratios of both doses of once-weekly semaglutide surpassed all comparators when analyzing the single endpoint of HbA1c < 7.0% and two composite endpoints, including weight loss and hypoglycemia. In terms of accomplishing single and composite goals, the study indicated that once-weekly semaglutide 0.5 mg and 1.0 mg is more cost-effective than exenatide ER and dulaglutide, as shown by the analysis of retrieved dropout data. As a result, once-weekly semaglutide doses offer fair value in the USA, especially when it comes to achieving the varied T2D treatment goals. You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6824375/.
- Frías, J. P., Auerbach, P., Bajaj, H. S., Fukushima, Y., Lingvay, I., Macura, S., Søndergaard, A. L., Tankova, T. I., Tentolouris, N., & Buse, J. B. (2021). Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial. The lancet. Diabetes & endocrinology, 9(9), 563–574. https://doi.org/10.1016/S2213-8587(21)00174-1
Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE): a double-blind, randomised, phase 3B trial.
The article titled “Efficacy and safety of once-weekly semaglutide 2·0 mg versus 1·0 mg in patients with type 2 diabetes (SUSTAIN FORTE)” compared the effectiveness and safety of two doses of once-weekly semaglutide in patients with type 2 diabetes. The study found that both doses (2.0 mg and 1.0 mg) led to significant reductions in HbA1c levels and weight loss. The higher dose (2.0 mg) showed greater efficacy in lowering HbA1c. Adverse events were primarily gastrointestinal and similar between the two dose groups.
The researchers aimed to investigate the effectiveness and safety of a higher dose of semaglutide, specifically once-weekly semaglutide 2.0 mg, compared to the standard dose of 1.0 mg in adults with inadequately controlled type 2 diabetes who were taking a stable dose of metformin, with or without a sulfonylurea. The researchers used a 40-week, double-blind, phase 3B experiment (dubbed SUSTAIN FORTE) that was conducted across 125 outpatient clinics in ten different nations to carry out their research. Adults (18 years or older) with type 2 diabetes who were taking metformin with or without a sulfonylurea and had their HbA1c level between 8.0 and 10.0% were eligible to take part in the research. Using an interactive web-response system, participants were randomly randomized to receive either once-weekly semaglutide 2.0 mg or 1.0 mg. The trial outcomes included the change from baseline at week 40 in HbA1c (primary outcome) and body weight (secondary confirmatory outcome), which were evaluated using two different strategies: the trial product estimand (which did not include treatment discontinuation or rescue medication) and the treatment policy estimand (which included all participants regardless of treatment discontinuation or rescue medication).According to the trial’s findings, semaglutide 2.0 mg reduced HbA1c levels more effectively than semaglutide 1.0 mg while causing more weight loss and having a similar safety profile.As a result, patients with type 2 diabetes who need more glycemic control while using semaglutide have a choice with the larger dose of the drug. The study’s findings support the use of semaglutide 2.0 mg as a therapy intensification option for type 2 diabetes patients who are already on semaglutide, the researchers write in their conclusion.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/34293304/. - Hall, S., Isaacs, D., & Clements, J. N. (2018). Pharmacokinetics and Clinical Implications of Semaglutide: A New Glucagon-Like Peptide (GLP)-1 Receptor Agonist. Clinical pharmacokinetics, 57(12), 1529–1538. https://doi.org/10.1007/s40262-018-0668-z
Pharmacokinetics and Clinical Implications of Semaglutide: A New Glucagon-Like Peptide (GLP)-1 Receptor Agonist.
The article titled “Pharmacokinetics and Clinical Implications of Semaglutide: A New GLP-1 Receptor Agonist” explores the pharmacokinetics of semaglutide, a medication used for treating type 2 diabetes. It discusses semaglutide’s absorption, distribution, metabolism, and elimination, emphasizing its long half-life and renal elimination. The study underscores the importance of appropriate dosing and administration instructions for optimizing therapeutic effects and considers potential drug interactions.
Semaglutide is administered as a subcutaneous injection once a week, and a dose of 0.5 or 1 mg has a half-life of 7 days, thereby reaching steady state in 4-5 weeks. The medication has few drug interactions, and no dose adjustments are necessary. However, it may delay gastric emptying and impact the absorption of oral medications, like other GLP-1 RAs.On the basis of clinical trials, the researcher contrasted semaglutide with placebo, sitagliptin, exenatide extended release, and insulin glargine as monotherapy or add-on therapy. After 30-56 weeks, the medicine has been shown to reduce glycosylated hemoglobin A1c by 1.5-1.9% and cause a weight loss of 5–10% compared to baseline. Semaglutide has a minimal risk of hypoglycemia and may be an effective treatment for T2DM patients, particularly those who need to lose weight.Based on efficacy and safety data, semaglutide’s pharmacokinetics have been evaluated, and its application to clinical practice has been summarized. The researcher concludes that semaglutide can be another acceptable option for patients with T2DM, given its potential role in weight loss management and the low risk of hypoglycemia.
You can read the full article at https://link.springer.com/article/10.1007/s40262-018-0668-z. - Gibbons, C., Blundell, J., Tetens Hoff, S., Dahl, K., Bauer, R., & Baekdal, T. (2021). Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes. Diabetes, obesity & metabolism, 23(2), 581–588. https://doi.org/10.1111/dom.14255
Effects of oral semaglutide on energy intake, food preference, appetite, control of eating and body weight in subjects with type 2 diabetes.
The article titled “Effects of oral semaglutide on energy intake, food preference, appetite, control of eating, and body weight in subjects with type 2 diabetes” investigates the impact of oral semaglutide on eating behavior and weight management in individuals with type 2 diabetes. The study found that oral semaglutide led to reduced energy intake, decreased appetite, improved control of eating, and significant weight loss. These findings suggest that oral semaglutide holds promise as a therapeutic option for weight management in individuals with type 2 diabetes.
The researchers measured energy intake during an ad libitum lunch, evening meal, and snack box after a standard breakfast. They also measured appetite ratings using a visual analogue scale after standard and fat-rich breakfasts. Other assessments included eating and craving control (using the Control of Eating Questionnaire), and changes in body weight and composition.The findings showed that 13 out of 15 subjects had a significantly lower total daily ad libitum calorie consumption with oral semaglutide compared to placebo following a typical meal. There were significant differences between oral semaglutide and placebo for measures of satiety, hunger, and overall appetite score after a breakfast high in fat, but not after a typical breakfast. In addition, oral semaglutide showed superior eating control than placebo in terms of reduced food cravings. The loss of body fat mass was the main cause of the mean body weight dropping by 2.7 kg with oral semaglutide and 0.1 kg with placebo.
In conclusion, after 12 weeks of treatment, ad libitum energy intake was lower with oral semaglutide compared to placebo. This reduction in energy intake resulted in reduced body fat mass and was associated with increased satiety and fullness after a fat-rich breakfast, and improved eating control. The study suggests that oral semaglutide could be an effective treatment for individuals with T2D who struggle with managing their appetite and energy intake.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7839771/. - Xourgia E, Papazafiropoulou A, Melidonis A. Antidiabetic treatment on memory and spatial learning: From the pancreas to the neuron. World J Diabetes. 2019;10(3):169-180. doi:10.4239/wjd.v10.i3.169.
Antidiabetic treatment on memory and spatial learning: From the pancreas to the neuron.
The article titled “Antidiabetic treatment on memory and spatial learning: From the pancreas to the neuron” published in the World Journal of Diabetes explores the effects of antidiabetic treatments on memory and spatial learning. The authors discuss the potential impact of various antidiabetic medications on cognitive function, focusing on the connection between pancreatic function and neuronal health. The article highlights the importance of considering the cognitive effects of antidiabetic medications in addition to their glycemic control properties.
The adverse impacts of persistent hyperglycemia on neural function have been rigorously assessed in the context of diabetes mellitus, both quantitatively and qualitatively. Among the prominent characteristics of diabetic encephalopathy (DE) are compromised synaptic adaptation and reduced spatial learning capability. The continuous and gradual decline in cognitive function, fueled by various reinforcing mechanisms in individuals with diabetes, contributes to the emergence of early-onset dementia and Alzheimer’s disease.
Despite the comprehensive description of the various clinical manifestations of DE and the clarification of its underlying pathophysiology in both type 1 and type 2 diabetes mellitus, an effective therapeutic strategy remains elusive. Hence, this review aims to succinctly summarize the accumulating body of evidence regarding the impact of existing antidiabetic treatment options on individuals with and without DE.
You can read the full article at Antidiabetic treatment on memory and spatial learning: From the pancreas to the neuron – PMC (nih.gov).
- Grieco M, Giorgi A, Gentile MC, et al. Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases. Front Neurosci. 2019;13:1112. Published 2019 Oct 18. doi:10.3389/fnins.2019.01112.
Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases.
The article titled “Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases” explores the potential of glucagon-like peptide-1 (GLP-1) in treating neurodegenerative diseases. GLP-1 has shown neuroprotective effects and may benefit conditions like Alzheimer’s, Parkinson’s, and Huntington’s diseases. The study discusses the mechanisms through which GLP-1 exerts its effects and reviews the use of GLP-1 agonists in clinical and preclinical settings. It concludes that GLP-1 and its agonists hold promise for neurodegenerative disease treatment, but more research is needed.
Drawing attention as a potential bridge between metabolic disruptions and cognitive deficits, the gut-released hormone glucagon-like peptide-1 (GLP-1) emerges. Research has illuminated GLP-1’s impact on diverse neuronal functions including thermogenesis, blood pressure regulation, neurogenesis, neurodegeneration, retinal restoration, and energy equilibrium. Furthermore, GLP-1’s modulation holds potential to influence amyloid β peptide aggregation in Alzheimer’s disease (AD) and dopamine (DA) levels in Parkinson’s disease (PD).Amid these observations, GLP-1 receptor agonists (GLP-1RAs) showcase commendable impacts on cerebral ischemia in animal models. They manifest through reduction in cerebral infarct area and amelioration of neurological deficits, primarily by curbing oxidative stress, inflammation, and apoptosis. Moreover, these agents exhibit potential in mitigating cognitive decline triggered by diabetes or obesity, promoting learning and memory enhancement through synaptic plasticity modulation. Hippocampal neurodegeneration is also alleviated by GLP-1RAs.
Significantly, mounting evidence underscores the neuroprotective potential of these agonists beyond diabetes. In animal models of neurodegenerative diseases, GLP-1RAs demonstrate the ability to safeguard motor function and dopaminergic neurons in PD models, while in AD models, they display promise in ameliorating various neuropathological aspects and enhancing cognitive functions.
Though further human clinical investigations are essential, GLP-1RAs exhibit promise as a potential therapy for diabetes-associated cognitive decline.
You can read the full article at Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases – PMC (nih.gov).
- Available athttps://www.frontiersin.org/articles/10.3389/fnins.2019.01112/full..
Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases
The article “Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases” explores the potential of glucagon-like peptide-1 (GLP-1) in treating neurodegenerative diseases. It highlights GLP-1’s neuroprotective effects and its relevance in conditions like Alzheimer’s, Parkinson’s, and Huntington’s diseases. The study reviews the use of GLP-1 agonists and their potential benefits in improving cognitive function and reducing disease progression. Although promising, further research is needed to understand the underlying mechanisms and optimize treatment strategies. Overall, GLP-1 and its agonists show promise as therapeutic targets for neurodegenerative diseases.
Capturing attention as a potential nexus between metabolic disturbances and cerebral decline, glucagon-like peptide-1 (GLP-1) emerges from the gut as a hormone of interest. Diverse investigations unveil GLP-1’s far-reaching influence on neuronal functions encompassing thermogenesis, blood pressure regulation, neurogenesis, neurodegeneration, retinal restoration, and maintenance of energy equilibrium. Further, GLP-1 modulation bears the capacity to impact amyloid β peptide aggregation in Alzheimer’s disease (AD) and dopamine (DA) levels in Parkinson’s disease (PD).Expanding its influence, GLP-1 receptor agonists (GLP-1RAs) surface as agents of promise in the realm of cerebral ischemia, as demonstrated in animal models. Their actions encompass reducing the area of cerebral infarction and enhancing neurological deficits through robust inhibition of oxidative stress, inflammation, and apoptosis. Beyond this, GLP-1RAs potentially yield benefits in countering cognitive impairment stemming from diabetes or obesity. This is achieved through the modulation of synaptic plasticity, bolstering learning, memory, and cognitive function. Furthermore, they contribute to the attenuation of hippocampal neurodegeneration.
Intriguingly, emerging evidence highlights the neuroprotective potential of these agonists beyond the bounds of diabetes. In models of neurodegenerative disorders, GLP-1RAs manifest their prowess by safeguarding motor activity and dopaminergic neurons in PD models, while concurrently exhibiting potential in enhancing various neuropathological attributes and cognitive functions in AD models.
As we tread forward, the necessity for additional clinical exploration of GLP-1RAs in human subjects remains paramount. Despite this, the trajectory suggests that these agents hold substantial promise as a therapeutic avenue in addressing the cognitive decline entwined with diabetes.
You can read the full article at Frontiers | Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases (frontiersin.org).
- Zhang, L., Zhang, L., Li, L., &Hölscher, C. (2019). Semaglutide is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson’s Disease. Journal of Parkinson’s disease, 9(1), 157–171.https://doi.org/10.3233/JPD-181503
Semaglutide is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson’s Disease.
The article titled “Semaglutide is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson’s Disease” investigates the potential neuroprotective effects of semaglutide in a mouse model of Parkinson’s disease. The study shows that semaglutide protects against neurodegeneration, reduces the loss of dopaminergic neurons, and lowers α-synuclein levels. These findings suggest that semaglutide may have therapeutic potential for Parkinson’s disease. Further research is needed to fully understand the underlying mechanisms and to determine the clinical implications of these results.
In a notable stride, a phase II clinical trial featuring the GLP-1 receptor agonist exendin-4 exhibited promising protective effects among PD patients. Building on these insights, the present study delves into investigating the neuroprotective capacities of GLP-1 analogues: semaglutide (administered at 25 nmol/kg intraperitoneally once every two days for 30 days) and liraglutide (administered at 25 nmol/kg intraperitoneally once daily for 30 days). Both of these compounds are currently approved for Type II diabetes treatment.Findings from our investigation underscore that both semaglutide and liraglutide yielded improvements in motor impairments stemming from the chronic MPTP mouse model of PD. Furthermore, both compounds exhibited the ability to counteract the decrease in tyrosine hydroxylase (TH) levels, mitigate α-syn accumulation, alleviate chronic brain inflammation, reduce lipid peroxidation, and suppress the mitochondrial mitophagy signaling pathway. Additionally, they heightened the expression of the pivotal growth factor GDNF, known to safeguard dopaminergic neurons within the substantia nigra (SN) and striatum.
Remarkably, the extended-action GLP-1 analogue, semaglutide, showcased heightened efficacy compared to once-daily liraglutide across numerous parameters measured in this study. The outcomes of our research spotlight semaglutide as a potential beacon of hope in PD treatment. With an impending clinical trial focused on testing semaglutide’s impact on PD patients, promising avenues for the therapeutic landscape seem to be on the horizon.
You can read the abstract of this article at Semaglutide is Neuroprotective and Reduces α-Synuclein Levels in the Chronic MPTP Mouse Model of Parkinson’s Disease – PubMed (nih.gov).
- McClean PL, Parthsarathy V, Faivre E, et al. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J Neurosci 2011;31:6587-94. 10.1523/JNEUROSCI.0529-11.2011.
The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease.
Type 2 diabetes increases the risk of Alzheimer’s disease. This might be because the brain’s insulin signaling is not working well. There’s a hormone called GLP-1 that helps with insulin signaling, and there are new versions of it, like liraglutide, used to treat diabetes. GLP-1 seems to protect the brain’s cells in studies. They tested liraglutide on mice with Alzheimer’s-like problems. The drug went into their brains and was given for 8 weeks. It helped the mice remember things and improved their brain connections. It also reduced harmful brain plaques and inflammation, which are common in Alzheimer’s. The drug didn’t affect normal mice much, but it made their brain connections better. This suggests that GLP-1 drugs could be a new way to treat Alzheimer’s disease.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6622662/. - Harkavyi A, Abuirmeileh A, Lever R, et al. Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease. J Neuroinflammation 2008;5:19. 10.1186/1742-2094-5-19.
Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease.
The study titled “Glucagon-like peptide 1 receptor stimulation reverses key deficits in distinct rodent models of Parkinson’s disease” investigates the effects of GLP-1 receptor stimulation in rodent models of Parkinson’s disease. The study demonstrates that GLP-1 receptor stimulation can reverse motor impairment, neuroinflammation, and oxidative stress associated with the disease. These findings suggest the therapeutic potential of GLP-1 receptor stimulation for Parkinson’s disease. Further research is needed to understand the underlying mechanisms and validate these results in humans.
In Parkinson’s disease (PD), inflammation in the brain can be a problem. A hormone called GLP-1 might help protect the brain and promote the growth of new brain cells. Scientists tested a drug called exendin-4 (EX-4), which activates the GLP-1 receptor, in animal models of PD.They used two methods to make rats have PD-like symptoms: injecting a toxin called 6-hydroxydopamine (6-OHDA) or a substance called lipopolysaccharide (LPS). Then, they gave the rats EX-4 a week later and observed their behavior, brain chemicals, and brain tissues.
The rats given EX-4 showed less circling behavior when given a challenge. Their brain chemical dopamine was better preserved in the striatum, which is a part of the brain affected in PD. The staining of a protein called tyrosine hydroxylase (TH) was better in the brain’s substantia nigra, another important area. EX-4 also helped maintain dopamine levels in the striatum of freely moving rats.
These findings suggest that EX-4 could slow down or even reverse brain damage in PD. Unlike other experimental drugs, EX-4 is already used for diabetes and can reach the brain. This means it might be a potential treatment for PD, and testing its effects on real patients with PD could be done soon.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2426681/. - Teramoto S, Miyamoto N, Yatomi K, et al. Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia. J Cereb Blood Flow Metab 2011;31:1696-705. 10.1038/jcbfm.2011.51.
Exendin-4, a glucagon-like peptide-1 receptor agonist, provides neuroprotection in mice transient focal cerebral ischemia.
Glucagon-like peptide-1 (GLP-1) is a hormone that helps release insulin in response to glucose. A similar hormone, exendin-4, is used to treat type 2 diabetes. This study looked at whether exendin-4 could protect the brain from damage caused by reduced blood flow (ischemia) and then restored blood flow (reperfusion).
The study titled “Exendin-4 provides neuroprotection in mice with transient focal cerebral ischemia” investigates the effects of Exendin-4, a glucagon-like peptide-1 receptor agonist, in a mouse model of cerebral ischemia. Exendin-4 treatment demonstrates neuroprotective effects by reducing infarct size, preserving brain tissue integrity, and improving neurological outcomes. These findings suggest the potential of Exendin-4 as a therapeutic agent for cerebral ischemia. Further research is needed to understand the underlying mechanisms and to validate these findings in clinical settings.
Mice were given exendin-4 after a 60-minute period of reduced blood flow to the brain. The effects were measured at different times after the event. The mice treated with exendin-4 had smaller brain damage, better brain function, and less oxidative stress and inflammation after the blood flow was restored. The levels of a molecule called cyclic AMP (cAMP), which is involved in cell signaling, were slightly higher in the exendin-4 group. Insulin and glucose levels were not significantly affected.In summary, exendin-4 seems to protect the brain from damage caused by reduced blood flow and reperfusion. This could be due to increased cAMP levels. The study suggests that exendin-4 could be useful in treating acute ischemic stroke.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3170947/. - Basalay, M. V., Davidson, S. M., &Yellon, D. M. (2019). Neuroprotection in Rats Following Ischaemia-Reperfusion Injury by GLP-1 Analogues-Liraglutide and Semaglutide. Cardiovascular drugs and therapy, 33(6), 661–667. https://doi.org/10.1007/s10557-019-06915-8.
Neuroprotection in Rats Following Ischaemia-Reperfusion Injury by GLP-1 Analogues-Liraglutide and Semaglutide.
The study titled “Neuroprotection in rats following ischemia-reperfusion injury by GLP-1 analogues – liraglutide and semaglutide” investigates the neuroprotective effects of liraglutide and semaglutide in a rat model of ischemia-reperfusion injury. Both GLP-1 analogues demonstrate neuroprotective effects by reducing infarct size and preserving brain tissue integrity. These findings suggest the potential of liraglutide and semaglutide as therapeutic options for neuroprotection. Further research is needed to understand the underlying mechanisms and validate these findings in clinical settings.
They used rats without diabetes and induced stroke for different lengths of time. They gave the rats either liraglutide or semaglutide through injections at the start of reperfusion or just before it. They measured the size of brain damage (infarct size) and how well the rats functioned after 24 hours or 72 hours.Liraglutide, given as a single injection at the start of reperfusion, reduced brain damage by up to 90% and improved brain function after 24 hours for strokes lasting 90 minutes. Semaglutide and liraglutide, given as a subcutaneous injection, reduced brain damage by 63% and 48%, respectively, and improved brain function after 72 hours for strokes lasting 90 minutes. Blocking GLP-1 receptors with a substance called exendin(9-39) eliminated the neuroprotective effects of semaglutide.
In conclusion, liraglutide’s protective effects vary with the dose and time of administration. Semaglutide is also protective and works through GLP-1 receptors. This research suggests that these GLP-1 analogues could be potential treatments to limit brain damage caused by ischemic stroke.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6994526/. - Zhang, L., Zhang, L., Li, L., &Hölscher, C. (2018). Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model. Neuropeptides, 71, 70–80. https://doi.org/10.1016/j.npep.2018.07.003
Neuroprotective effects of the novel GLP-1 long acting analogue semaglutide in the MPTP Parkinson’s disease mouse model.
The article titled “Neuroprotective effects of the novel GLP-1 long-acting analogue semaglutide in the MPTP Parkinson’s disease mouse model” investigates the neuroprotective effects of semaglutide in a mouse model of Parkinson’s disease induced by MPTP. The study shows that semaglutide has neuroprotective properties by reducing dopaminergic neuron loss, inflammation, and oxidative stress in the brain. These findings suggest that semaglutide may have therapeutic potential for Parkinson’s disease. Further research is needed to understand the underlying mechanisms and to explore the clinical implications of semaglutide in the treatment of Parkinson’s disease.
Both semaglutide and liraglutide were found to improve motor problems caused by PD in the mouse model. They also helped maintain the levels of a key enzyme, tyrosine hydroxylase (TH), which is linked to dopamine production. Both compounds reduced inflammation, decreased damage from oxidative stress, prevented cell death, and boosted cellular cleaning processes called autophagy in the brain areas affected by PD.Notably, semaglutide, which is a longer-acting GLP-1 analogue, showed better results compared to liraglutide in most measures. This suggests that semaglutide holds promise as a potential treatment for Parkinson’s disease.
In summary, this study suggests that the new GLP-1 analogue, semaglutide, could be a hopeful treatment option for Parkinson’s disease, with its potentially superior benefits compared to liraglutide.
You can read the abstract of abstract at https://www.sciencedirect.com/science/article/abs/pii/S0143417918300684?via%3Dihub.
- Chang, Y. F., Zhang, D., Hu, W. M., Liu, D. X., & Li, L. (2020). Semaglutide-mediated protection against Aβ correlated with enhancement of autophagy and inhibition of apotosis. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia, 81, 234–239. https://doi.org/10.1016/j.jocn.2020.09.054
Semaglutide-mediated protection against Aβ correlated with enhancement of autophagy and inhibition of apotosis.
The study titled “Semaglutide-mediated protection against Aβ correlated with enhancement of autophagy and inhibition of apoptosis” explores the protective effects of semaglutide against Aβ (amyloid-beta) in cellular models. The study demonstrates that semaglutide enhances autophagy, a cellular process that clears toxic proteins, and inhibits apoptosis, a form of programmed cell death. These effects correlate with protection against Aβ, suggesting that semaglutide may have potential therapeutic benefits in conditions related to Aβ accumulation, such as Alzheimer’s disease. Further research is needed to validate these findings and understand the underlying mechanisms in clinical settings.
The study used a cell line called SH-SY5Y and damaged it using a substance associated with Alzheimer’s (Aβ25-35). Then, they treated the damaged cells with semaglutide. They looked at certain proteins related to autophagy (cellular cleaning) and apoptosis (cell death) to understand how semaglutide might work against Aβ25-35.The results showed that semaglutide increased autophagy by boosting the levels of specific proteins (LC3II, Atg7, Beclin-1, and P62) that were suppressed by Aβ25-35. Additionally, semaglutide reduced apoptosis by decreasing the expression of a protein called Bax, which was increased by Aβ25-35, and increasing the expression of another protein called Bcl2, which was reduced by Aβ25-35.
In conclusion, this study suggests that semaglutide might protect against the effects of Aβ25-35, which is associated with Alzheimer’s disease, by enhancing autophagy (cellular cleaning) and inhibiting apoptosis (cell death). This could potentially provide insights into how semaglutide might have a positive impact on Alzheimer’s disease.
You can read the full article at https://www.jocn-journal.com/article/S0967-5868(20)31533-2/fulltext. - Maskery, M. P., Holscher, C., Jones, S. P., Price, C. I., Strain, W. D., Watkins, C. L., Werring, D. J., &Emsley, H. C. (2021). Glucagon-like peptide-1 receptor agonists as neuroprotective agents for ischemic stroke: a systematic scoping review. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 41(1), 14–30. https://doi.org/10.1177/0271678X20952011
Glucagon-like peptide-1 receptor agonists as neuroprotective agents for ischemic stroke: a systematic scoping review.
The article titled “Glucagon-like peptide-1 receptor agonists as neuroprotective agents for ischemic stroke: a systematic scoping review” provides an overview of the potential neuroprotective effects of glucagon-like peptide-1 receptor agonists (GLP-1RAs) in the context of ischemic stroke. The scoping review examines the existing literature and identifies evidence supporting the neuroprotective properties of GLP-1RAs in experimental models of stroke. While the review highlights promising findings regarding the neuroprotective effects of GLP-1RAs, further research is needed to determine their clinical efficacy and safety in human stroke patients. The article serves as a foundation for future investigations into the therapeutic potential of GLP-1RAs in ischemic stroke.
The review looked at 35 pre-clinical studies, 11 studies based on retrospective data, 7 trials focused on cardiovascular outcomes, and 4 prospective clinical studies. In laboratory studies, GLP-1RAs demonstrated neuroprotection even when given up to 24 hours after inducing stroke in models with normal blood sugar levels. Benefits included reducing brain damage, cell death, oxidative stress, and inflammation, while promoting the growth of new brain cells, blood vessels, and blood flow. Improved brain function and a potential increase in survival were also observed. Cardiovascular trials found that semaglutide and dulaglutide significantly reduced the incidence of stroke. Retrospective studies hinted at potential neuroprotection. Ongoing clinical trials are testing these drugs for effectiveness and safety, with early indications of positive tolerability.The review concludes that repurposing GLP-1RAs for stroke treatment is promising, but well-designed trials are necessary to determine their actual clinical benefits and cost-effectiveness.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7747170/. - Femminella, G. D., Frangou, E., Love, S. B., Busza, G., Holmes, C., Ritchie, C., Lawrence, R., McFarlane, B., Tadros, G., Ridha, B. H., Bannister, C., Walker, Z., Archer, H., Coulthard, E., Underwood, B. R., Prasanna, A., Koranteng, P., Karim, S., Junaid, K., McGuinness, B., … Edison, P. (2019). Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study). Trials, 20(1), 191. https://doi.org/10.1186/s13063-019-3259-x
Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomised controlled trial (ELAD study).
The article titled “Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: study protocol for a randomized controlled trial (ELAD study)” outlines the study protocol for a clinical trial investigating the effects of liraglutide, a GLP-1 analogue, in individuals with Alzheimer’s disease. The study aims to evaluate the potential therapeutic benefits of liraglutide on cognitive function, biomarkers, and disease progression in Alzheimer’s patients. The article provides a detailed description of the study design, methodology, and outcome measures. The trial results will contribute to our understanding of the potential role of liraglutide in the treatment of Alzheimer’s disease and may have implications for future therapeutic approaches.
The study involves 206 participants with mild Alzheimer’s who will be randomly assigned to either receive liraglutide or a placebo through daily injections for a year. The primary focus is on changes in brain glucose metabolism in specific brain regions. Secondary outcomes include changes in clinical and cognitive measures, safety assessments, brain imaging parameters, and levels of substances related to Alzheimer’s.This research is important because current treatments for Alzheimer’s only address symptoms, and finding treatments that can actually modify the disease’s progression is crucial. If successful, liraglutide and similar drugs could become an important option for future Alzheimer’s treatments.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6448216/
- Yamamoto, H., Lee, C. E., Marcus, J. N., Williams, T. D., Overton, J. M., Lopez, M. E., Hollenberg, A. N., Baggio, L., Saper, C. B., Drucker, D. J., & Elmquist, J. K. (2002). Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons. The Journal of clinical investigation, 110(1), 43–52. https://doi.org/10.1172/JCI15595
Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons.
The study titled “Glucagon-like peptide-1 receptor stimulation increases blood pressure and heart rate and activates autonomic regulatory neurons” investigates the effects of glucagon-like peptide-1 receptor (GLP-1R) stimulation on cardiovascular parameters and autonomic regulation. The study demonstrates that GLP-1R stimulation leads to an increase in blood pressure and heart rate, and activates autonomic regulatory neurons. These findings highlight the cardiovascular effects of GLP-1R activation and its potential implications for cardiovascular regulation. Further research is needed to better understand the underlying mechanisms and the clinical relevance of these findings.
This study found that when GLP-1 receptor agonists were given either centrally (in the brain) or peripherally (outside the brain), they increased blood pressure and heart rate in a dose-dependent manner. Activation of GLP-1 receptors led to the activation of specific brain regions controlling autonomic functions like heart rate and blood pressure. It also triggered changes in gene expression related to the production of important neurotransmitters in the brainstem.In summary, GLP-1 doesn’t just influence insulin production; it also plays a role in regulating heart rate, blood pressure, and other cardiovascular responses through its effects on the brain’s autonomic control centers.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC151031/.
- Andreadis, P., Karagiannis, T., Malandris, K., Avgerinos, I., Liakos, A., Manolopoulos, A., Bekiari, E., Matthews, D. R., &Tsapas, A. (2018). Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetes, obesity & metabolism, 20(9), 2255–2263. https://doi.org/10.1111/dom.13361
A systematic review and meta-analysis. Diabetes, obesity & metabolism, 20(9), 2255–2263.
The article titled “Semaglutide for type 2 diabetes mellitus: A systematic review and meta-analysis” presents a comprehensive analysis of the efficacy and safety of semaglutide in the treatment of type 2 diabetes mellitus. The systematic review and meta-analysis examine multiple clinical studies to evaluate the effects of semaglutide on various outcomes related to glycemic control, body weight, and adverse events. The findings suggest that semaglutide is effective in reducing HbA1c levels and body weight, with a favorable safety profile. The study provides valuable insights into the use of semaglutide as a treatment option for type 2 diabetes mellitus and contributes to the existing evidence base.
Researchers conducted a comprehensive search of various sources for randomized controlled trials comparing semaglutide with either placebo or other diabetes medications. They looked at outcomes such as changes in HbA1c (a measure of blood sugar control), body weight, blood pressure, heart rate, hypoglycemia (low blood sugar), gastrointestinal side effects, pancreatitis, and diabetic retinopathy.The study included data from multiple trials involving subcutaneous (under the skin) administration of semaglutide, with both 0.5 mg and 1 mg doses. The results showed that semaglutide significantly lowered HbA1c levels, reduced body weight, and had a positive effect on systolic blood pressure. While there was no major increase in hypoglycemia, there was a higher incidence of gastrointestinal side effects like nausea, vomiting, and diarrhea. Occurrences of pancreatitis were rare, and the impact of semaglutide on diabetic retinopathy needed further investigation through post-approval studies.
In conclusion, semaglutide is an effective weekly GLP-1 RA that helps control blood sugar, body weight, and blood pressure. However, it may lead to more gastrointestinal side effects. The safety aspects of pancreatitis and retinopathy require further evaluation through ongoing monitoring.
You can read the abstract of this article at https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/dom.13361.
- Inzucchi S. E., Bergenstal R. M., Buse J. B., Diamant M., Ferrannini E., Nauck M., et al. . (2012). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 35, 1364–1379. 10.2337/dc12-0413.
Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD).
The article titled “Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD)” provides a comprehensive guideline for the management of hyperglycemia in type 2 diabetes. The position statement emphasizes a patient-centered approach, taking into account individualized treatment goals, preferences, and needs. It covers various aspects of diabetes management, including lifestyle modifications, oral medications, injectable therapies, and insulin therapy. The article serves as a valuable resource for healthcare professionals involved in the care of individuals with type 2 diabetes, providing evidence-based recommendations for optimal management strategies.
The recommendations emphasize individualized treatment based on each patient’s needs, preferences, and tolerances. They acknowledge the increased cardiovascular risks in type 2 diabetes and stress the importance of managing other risk factors like blood pressure, lipid levels, and smoking. The guidelines aim to provide a flexible approach, as there is limited comparative research in this area. The document encourages healthcare providers to consider the progressive nature of type 2 diabetes, the role of each drug, patient factors, and the influence of age and other health conditions. The implementation of these guidelines requires clinicians to integrate current evidence with patient-specific factors and circumstances.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3357214/. - Dugan J. A. (2017). Standards of care and treatment in diabetes. Phys. Assist. Clin. 2, 13–23. 10.1016/j.cpha.2016.08.004.
Standards of care and treatment in diabetes.
The article titled “Standards of care and treatment in diabetes” provides an overview of the current standards of care for diabetes management. It discusses key aspects of diabetes treatment, including lifestyle modifications, pharmacotherapy, and monitoring. The article aims to guide healthcare professionals, specifically physician assistants, in providing optimal care to individuals with diabetes. By highlighting evidence-based guidelines and best practices, the article helps ensure that patients receive appropriate and comprehensive care.
You can read the full article at Standards of Care in Diabetes—2023 Abridged for Primary Care Providers | Clinical Diabetes | American Diabetes Association (diabetesjournals.org). - Shi FH, Li H, Cui M, Zhang ZL, Gu ZC, Liu XY. Efficacy and Safety of Once-Weekly Semaglutide for the Treatment of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Pharmacol. 2018;9:576. Published 2018 Jun 4. doi:10.3389/fphar.2018.00576.
Efficacy and Safety of Once-Weekly Semaglutide for the Treatment of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front Pharmacol.
The article titled “Efficacy and Safety of Once-Weekly Semaglutide for the Treatment of Type 2 Diabetes: A Systematic Review and Meta-Analysis of Randomized Controlled Trials” presents a systematic review and meta-analysis that evaluates the effectiveness and safety of once-weekly semaglutide in the treatment of type 2 diabetes. The study analyzes data from multiple randomized controlled trials to assess the impact of semaglutide on glycemic control, body weight, and adverse events. The findings indicate that once-weekly semaglutide is effective in improving glycemic control and reducing body weight, with a generally favorable safety profile. The article provides valuable insights into the use of once-weekly semaglutide as a treatment option for type 2 diabetes, based on a comprehensive analysis of available clinical evidence.
The findings indicated that semaglutide led to significant improvements in various measures of glycemic control and weight reduction compared to other therapies. These improvements included reductions in glycosylated hemoglobin, fasting plasma glucose, self-monitoring of plasma glucose, body weight, body mass index, and systolic blood pressure. However, there was a slight increase in pulse rate and no significant effect on diastolic blood pressure. Safety-wise, semaglutide did not raise the risk of adverse events, hypoglycemia, or pancreatitis. However, it did increase the risk of gastrointestinal disorders compared to other therapies.In conclusion, semaglutide showed effectiveness and acceptability in treating T2DM, with notable improvements in glycemic control and weight reduction. Its impact on these aspects appeared more effective than other GLP-1 receptor agonists like exenatide and dulaglutide. The study emphasized that while semaglutide demonstrated positive outcomes, there was a higher risk of gastrointestinal disorders associated with its use.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5994433/. - Røder ME. Clinical potential of treatment with semaglutide in type 2 diabetes patients. Drugs Context. 2019;8:212585. Published 2019 Dec 2. doi:10.7573/dic.212585.
Clinical potential of treatment with semaglutide in type 2 diabetes patients.
The article titled “Clinical potential of treatment with semaglutide in type 2 diabetes patients” explores the therapeutic potential of semaglutide in the management of type 2 diabetes. It discusses the pharmacological properties of semaglutide and its mechanism of action as a glucagon-like peptide-1 receptor agonist. The article also reviews clinical studies that have evaluated the efficacy and safety of semaglutide in improving glycemic control and reducing cardiovascular risk factors in patients with type 2 diabetes. The findings suggest that semaglutide has promising clinical potential as a treatment option for type 2 diabetes. The article provides insights into the current understanding of semaglutide and its role in the management of this chronic condition.
Studies show that semaglutide significantly reduces HbA1c, fasting plasma glucose, body weight, and blood pressure. It has been compared favorably to other GLP-1RAs and insulin, with minimal hypoglycemia risk. A cardiovascular outcome trial demonstrated semaglutide’s superiority in reducing cardiovascular events. Gastrointestinal side effects are common, but they decrease over time.A potential concern arose regarding increased retinopathy risk, observed in one study (SUSTAIN 6), possibly due to rapid glycemic improvement. Additional research is needed to better understand this effect. Overall, semaglutide appears to be a promising and effective treatment option for type 2 diabetes, but ongoing investigations will help address remaining questions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6905643/. - Marso, S. P., Bain, S. C., Consoli, A., Eliaschewitz, F. G., Jódar, E., Leiter, L. A., Lingvay, I., Rosenstock, J., Seufert, J., Warren, M. L., Woo, V., Hansen, O., Holst, A. G., Pettersson, J., Vilsbøll, T., & SUSTAIN-6 Investigators (2016). Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine, 375(19), 1834–1844.https://doi.org/10.1056/NEJMoa1607141
Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes.
The article titled “Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes” reports the results of the SUSTAIN-6 clinical trial, which investigated the cardiovascular outcomes of semaglutide in patients with type 2 diabetes. The study compared semaglutide to placebo and evaluated its effect on major adverse cardiovascular events (MACE) such as cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. The findings showed that treatment with semaglutide led to a significant reduction in the risk of MACE compared to placebo. The article provides important evidence supporting the cardiovascular safety profile of semaglutide in patients with type 2 diabetes.
You can read the full article at https://www.nejm.org/doi/10.1056/NEJMoa1607141?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov. - Husain M, Bain SC, Holst AG, Mark T, Rasmussen S, Lingvay I. Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: combined post hoc analysis of the SUSTAIN and PIONEER trials. CardiovascDiabetol. 2020;19(1):156. Published 2020 Sep 30. doi:10.1186/s12933-020-01106-4.
Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: combined post hoc analysis of the SUSTAIN and PIONEER trials.
The article titled “Effects of semaglutide on risk of cardiovascular events across a continuum of cardiovascular risk: combined post hoc analysis of the SUSTAIN and PIONEER trials” presents a combined analysis of the SUSTAIN and PIONEER clinical trials to evaluate the effects of semaglutide on cardiovascular risk in patients with type 2 diabetes. The study analyzed data across a spectrum of cardiovascular risk levels and assessed the incidence of major adverse cardiovascular events (MACE) such as cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke. The findings demonstrated that treatment with semaglutide resulted in a reduced risk of MACE across different cardiovascular risk groups. This analysis provides valuable insights into the cardiovascular benefits of semaglutide in patients with type 2 diabetes across various risk categories.
Read the full article on:https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7526237/ - Thethi, T. K., Pratley, R., & Meier, J. J. (2020). Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: The PIONEER programme. Diabetes, obesity & metabolism, 22(8), 1263–1277. https://doi.org/10.1111/dom.14054
Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: The PIONEER programme.
The article “Efficacy, safety and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes: The PIONEER programme” discusses the results of the PIONEER clinical program, which evaluated the efficacy, safety, and cardiovascular outcomes of once-daily oral semaglutide in patients with type 2 diabetes. The program included a series of randomized controlled trials that assessed the effects of oral semaglutide on various endpoints such as glycemic control, body weight, cardiovascular events, and safety parameters. The findings indicated that oral semaglutide demonstrated significant improvements in glycemic control, body weight reduction, and a favorable cardiovascular safety profile. This study highlights the potential of oral semaglutide as an effective treatment option for patients with type 2 diabetes.
Oral semaglutide, taken once daily at 7mg and 14mg doses, consistently reduced HbA1c, body weight, and improved fasting plasma glucose in T2D patients over up to 78 weeks of treatment. It demonstrated greater effectiveness compared to placebos and commonly used T2D drugs like sitagliptin, empagliflozin, liraglutide, and dulaglutide. Side effects were generally mild and related to the gastrointestinal system.The trials also confirmed the cardiovascular safety of oral semaglutide in patients at high cardiovascular risk. Overall, the PIONEER program indicated that oral semaglutide is effective and well-tolerated for managing blood sugar in T2D. Its availability could expand treatment options and encourage the use of GLP-1RAs earlier in T2D management.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7384149/ - Husain, M., Bain, S. C., Jeppesen, O. K., Lingvay, I., Sørrig, R., Treppendahl, M. B., &Vilsbøll, T. (2020). Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk. Diabetes, obesity & metabolism, 22(3), 442–451.https://doi.org/10.1111/dom.13955
Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk.
The article “Semaglutide (SUSTAIN and PIONEER) reduces cardiovascular events in type 2 diabetes across varying cardiovascular risk” investigates the impact of semaglutide on cardiovascular events in patients with type 2 diabetes. The study combines data from the SUSTAIN and PIONEER trials, examining the effects of semaglutide on cardiovascular outcomes in individuals with different levels of cardiovascular risk. The findings demonstrate that semaglutide treatment reduces the occurrence of cardiovascular events, including cardiovascular death, non-fatal myocardial infarction, and non-fatal stroke, across varying levels of cardiovascular risk. This research emphasizes the potential of semaglutide as an effective intervention to improve cardiovascular outcomes in individuals with type 2 diabetes.
When combining the results of SUSTAIN 6 and PIONEER 6, semaglutide showed a significant reduction in overall MACE risk (with a hazard ratio of 0.76) compared to placebo, driven primarily by a reduction in nonfatal stroke risk. The risk of hospitalization for heart failure, however, did not show a significant difference between semaglutide and placebo (hazard ratio of 1.03). Subgroup analyses indicated that semaglutide’s effect on MACE was consistent across different cardiovascular risk levels, except for individuals with prior heart failure.In the broader glycaemic efficacy trials, the effect of semaglutide on MACE was not statistically significant.
In conclusion, the combined analysis of SUSTAIN and PIONEER trials demonstrated that semaglutide, a glucagon-like peptide-1 analogue, consistently reduced the risk of major adverse cardiovascular events compared to comparators across varying levels of cardiovascular risk. However, semaglutide did not have a significant impact on MACE outcomes in individuals with prior heart failure.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7064975/. - Leiter, L. A., Bain, S. C., Hramiak, I., Jódar, E., Madsbad, S., Gondolf, T., Hansen, T., Holst, I., &Lingvay, I. (2019). Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial. Cardiovascular diabetology, 18(1), 73. https://doi.org/10.1186/s12933-019-0871-8.
Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial.
The study focused on analyzing the effects of once-weekly semaglutide (0.5 and 1.0 mg) versus placebo on major adverse cardiovascular events (MACE) in subjects with type 2 diabetes (T2D) and high cardiovascular risk, taking into account gender, age, and baseline cardiovascular risk factors.
Subjects were grouped based on gender, age (50-65 years and >65 years), and baseline cardiovascular risk profile (prior myocardial infarction or stroke vs no prior MI or stroke, and established cardiovascular disease vs CV risk factors alone, including chronic kidney disease). The study examined time to MACE and its components, hospitalizations for angina or heart failure, and revascularization (coronary and peripheral). Additional analyses looked at changes in HbA1c and body weight, as well as tolerability, based on gender and age.
The study included 3297 subjects, with a majority being male (60.7%), and significant proportions aged >65 years (43%) and having a history of MI or stroke (41.5%). Most had established cardiovascular disease (76.8%). Semaglutide consistently reduced the risk of MACE and its components in all subgroups (gender, age, and baseline cardiovascular risk). Revascularizations, HbA1c, and body weight were also consistently reduced across subgroups. Gastrointestinal adverse events were more common among women, but treatment discontinuation rates were similar for both genders.
The post hoc analysis of the SUSTAIN 6 trial revealed that once-weekly semaglutide reduced the risk of major adverse cardiovascular events in all subjects, regardless of gender, age, or baseline cardiovascular risk. This study suggests that semaglutide is effective across various patient profiles in reducing cardiovascular risk.
You can read the full article at Cardiovascular risk reduction with once-weekly semaglutide in subjects with type 2 diabetes: a post hoc analysis of gender, age, and baseline CV risk profile in the SUSTAIN 6 trial – PMC (nih.gov).
- Jódar, E., Michelsen, M., Polonsky, W., Réa, R., Sandberg, A., Vilsbøll, T., Warren, M., Harring, S., Ziegler, U., & Bain, S. (2020). Semaglutide improves health-related quality of life versus placebo when added to standard of care in patients with type 2 diabetes at high cardiovascular risk (SUSTAIN 6). Diabetes, obesity & metabolism, 22(8), 1339–1347. https://doi.org/10.1111/dom.14039
Semaglutide improves health-related quality of life versus placebo when added to standard of care in patients with type 2 diabetes at high cardiovascular risk (SUSTAIN 6).
The study aimed to understand the factors influencing changes in health-related quality of life (HRQoL) among individuals with type 2 diabetes in the SUSTAIN 6 trial and to identify potential mediators of the treatment effect of semaglutide on HRQoL scores.
The Short Form (SF)-36v2® questionnaire, which includes physical component summary (PCS) and mental component summary (MCS) scores, was used to assess HRQoL changes from baseline to week 104 in a predetermined analysis. This post-hoc analysis evaluated changes in PCS and MCS scores by considering various factors as parameters or covariates. These factors included major adverse cardiac events, hypoglycemia, gastrointestinal adverse events, episodes of nausea, vomiting, or diarrhea, and changes in glycated hemoglobin (HbA1c), body weight, blood pressure, heart rate, and estimated glomerular filtration rate.
Semaglutide showed an improvement in overall HRQoL compared to placebo, with a greater increase in PCS (+1.0 vs. +0.4) and MCS (+0.5 vs. -0.2) scores. The treatment effect of semaglutide on PCS was reduced when adjusted for changes in HbA1c and body weight. Similarly, the treatment effect on MCS was reduced when adjusted for HbA1c change. Adjusting for other parameters separately did not significantly alter the estimated effects of semaglutide on PCS and MCS.
Semaglutide led to improved health-related quality of life compared to placebo. The observed improvements with semaglutide were possibly influenced, at least in part, by changes in HbA1c and body weight. The study highlights the potential mediating role of these factors in the treatment effect of semaglutide on HRQoL.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7383680/ - Hamal, S., Cherukuri, L., Shaikh, K., Kinninger, A., Doshi, J., Birudaraju, D., &Budoff, M. J. (2020). Effect of semaglutide on coronary atherosclerosis progression in patients with type II diabetes: rationale and design of the semaglutide treatment on coronary progression trial. Coronary artery disease, 31(3), 306–314.https://doi.org/10.1097/MCA.0000000000000830
Effect of semaglutide on coronary atherosclerosis progression in patients with type II diabetes: rationale and design of the semaglutide treatment on coronary progression trial.
Patients with type 2 diabetes mellitus face significant cardiovascular risks. Semaglutide, an injectable glucagon-like peptide-1 receptor agonist, has shown promise in reducing cardiovascular events, but its exact mechanism of benefit remains unknown. This study aims to investigate whether semaglutide can reduce the progression of noncalcified coronary atherosclerotic plaque volume, as measured by serial coronary computed tomography angiography (CTA), over a one-year period compared to a placebo, in individuals with diabetes.
The study plans to enroll 140 patients who will undergo baseline and 12-month coronary artery calcium scoring and coronary CTA. Participants will be randomly assigned to receive either semaglutide or a placebo as an add-on to their standard care. Follow-up will occur over 12 months, with an additional phone call 30 days after treatment discontinuation.
By July 2019, the study had enrolled approximately 30% of its intended participants, with an estimated completion of enrollment by the first quarter of 2020 and the study concluding by the first quarter of 2021. As of July 23, 2019, 30 patients had been enrolled, and preliminary data regarding demographics and clinical characteristics were summarized.
This ongoing study aims to provide valuable imaging-based insights into the effects of semaglutide on coronary atherosclerotic plaque progression in individuals with type 2 diabetes. The study’s results will complement existing clinical outcomes data from related trials and contribute to a better understanding of semaglutide’s potential cardiovascular benefits.
- Doggrell S. A. (2020). Will oral semaglutide be used to reduce cardiovascular risk in subjects with type 2 diabetes instead of subcutaneous semaglutide?. Expert opinion on biological therapy, 20(5), 489–492. https://doi.org/10.1080/14712598.2020.1724952
Will oral semaglutide be used to reduce cardiovascular risk in subjects with type 2 diabetes instead of subcutaneous semaglutide?
The introduction discusses how “glutides” stimulate the glucagon-like peptide 1 (GLP-1) receptor to enhance insulin secretion, and these compounds are used to treat type 2 diabetes. The oral form of semaglutide is the first of its kind among these drugs. The PIONEER series of clinical trials, called Peptide Innovation for Early Diabetes Treatment, aimed to establish the clinical role of oral semaglutide.
The article focuses on PIONEER 6, a phase 3a non-inferiority trial. The primary goal of PIONEER 6 was to compare the time to the first major adverse cardiovascular event between oral semaglutide and placebo. The trial demonstrated that oral semaglutide was non-inferior to placebo in this aspect.
In the expert opinion section, the author suggests that once-daily oral semaglutide might not replace the once-weekly subcutaneous semaglutide in treating type 2 diabetes. This perspective is based on a few reasons. Firstly, subcutaneous semaglutide has already been proven to be superior to placebo in reducing cardiovascular risk, while the data on oral semaglutide’s cardiovascular benefits won’t be available until 2024. Secondly, there’s a debate about whether patients would find the daily regimen of taking oral semaglutide more convenient compared to the weekly subcutaneous option.
You can read the abstract at https://www.tandfonline.com/doi/abs/10.1080/14712598.2020.1724952?journalCode=iebt20.
- Marso, S. P., Holst, A. G., &Vilsbøll, T. (2017). Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. The New England journal of medicine, 376(9), 891–892. https://doi.org/10.1056/NEJMc1615712
Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes.
This study aimed to assess the cardiovascular safety of semaglutide, a glucagon-like peptide 1 analogue, in patients with type 2 diabetes. The trial included 3297 patients receiving either once-weekly semaglutide or placebo over 104 weeks. The primary outcome was the occurrence of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke.
Among patients with established cardiovascular disease or chronic kidney disease at baseline, semaglutide was found to be noninferior to placebo. The primary outcome occurred in 6.6% of the semaglutide group and 8.9% of the placebo group. Nonfatal myocardial infarction and stroke rates were similar between the groups, with a potential trend towards reduction in semaglutide group. Rates of death from cardiovascular causes were comparable. However, semaglutide was associated with lower rates of new or worsening nephropathy but higher rates of retinopathy complications. Serious adverse events were fewer in the semaglutide group, though more patients discontinued treatment due to gastrointestinal adverse events.
In patients with type 2 diabetes at high cardiovascular risk, semaglutide demonstrated significantly lower rates of cardiovascular death, nonfatal myocardial infarction, or nonfatal stroke compared to placebo, establishing the noninferiority of semaglutide. The study suggests potential cardiovascular benefits of semaglutide in this patient population.
You can read the full article at https://www.nejm.org/doi/10.1056/NEJMoa1607141?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200www.ncbi.nlm.nih.gov.
- Verma, S., Bain, S. C., Monk Fries, T., Mazer, C. D., Nauck, M. A., Pratley, R. E., Rasmussen, S., Saevereid, H. A., Zinman, B., &Buse, J. B. (2019). Duration of diabetes and cardiorenal efficacy of liraglutide and semaglutide: A post hoc analysis of the LEADER and SUSTAIN 6 clinical trials. Diabetes, obesity & metabolism, 21(7), 1745–1751. https://doi.org/10.1111/dom.13698
Duration of diabetes and cardiorenal efficacy of liraglutide and semaglutide: A post hoc analysis of the LEADER and SUSTAIN 6 clinical trials.
This post hoc analysis examined the impact of diabetes duration on the cardiovascular and kidney-related effectiveness of liraglutide and semaglutide, two human glucagon-like peptide-1 analogues used to reduce cardiovascular risk in patients with type 2 diabetes. The analysis utilized data from the LEADER and SUSTAIN 6 cardiovascular outcome trials.
The analysis grouped patients based on diabetes duration at baseline: <5 years, 5 to <15 years, 15 to <25 years, and ≥25 years. The proportion of patients in each duration category varied in both trials. Longer diabetes duration correlated with older age, a higher proportion of females, a history of stroke, peripheral arterial disease, insulin use, and poorer kidney function. Longer duration was also linked to increased occurrences of major adverse cardiovascular events (MACE), expanded MACE, and nephropathy events. Interestingly, despite differences in diabetes duration and baseline characteristics, both liraglutide and semaglutide consistently reduced the risk of cardiorenal outcomes across all diabetes duration categories. The interaction between treatment effect and diabetes duration was not significant for any of the analyzed endpoints. In summary, this analysis indicated that the cardiorenal benefits of liraglutide and semaglutide were consistent across varying diabetes durations, demonstrating their efficacy in reducing cardiovascular and kidney-related risks in patients with type 2 diabetes. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6619033/.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








