Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Overall Health Benefits of Cerebrolysin
- Key Takeaways
- What is Cerebrolysin?
- How Cerebrolysin Works
- Chemical Structure of Cerebrolysin
- Research on Cerebrolysin
- Associated Side Effects and Adverse Events of Cerebrolysin
- What is Cerebrolysate?
- Is Cerebrolysin 10ml Good?
- Cerebrolysin Price
- Cortexin vs Cerebrolysin
- Cerebrolysin in Stroke and Vascular Dementia
- Cerebrolysin Buy Online
- FAQ
- Reference
Table of Contents
- Overall Health Benefits of Cerebrolysin
- Key Takeaways
- What is Cerebrolysin?
- How Cerebrolysin Works
- Chemical Structure of Cerebrolysin
- Research on Cerebrolysin
- Associated Side Effects and Adverse Events of Cerebrolysin
- What is Cerebrolysate?
- Is Cerebrolysin 10ml Good?
- Cerebrolysin Price
- Cortexin vs Cerebrolysin
- Cerebrolysin in Stroke and Vascular Dementia
- Cerebrolysin Buy Online
- FAQ
- Reference
Overall Health Benefits of Cerebrolysin
Cerebrolysin benefits include boosting cognitive function, improving mood, and enhancing the management of neurological conditions such as autism, ADHD, and cerebral palsy. It also aids in nerve repair, treats hyperthermia-induced neurotoxicity and morphine withdrawal symptoms, boosts immune function, and improves eye health.
- Boosts cognitive function [1-29]
- Improves mood [30-33]
- Improves symptoms of autism [34-36]
- Improves symptoms of attention deficit hyperactivity disorder (ADHD) [37-38]
- Improves symptoms of cerebral palsy (CP) [39-42]
- Repairs nerve damage [43-49]
- Treats hyperthermia-induced neurotoxicity [50-51]
- Treats morphine withdrawal symptoms [52-54]
- Boosts immune function [55-62]
- Improves eye health [63-64]
Key Takeaways
- Enhanced Cognitive Abilities: Cerebrolysin is known for its ability to boost cognitive function, making it a potential treatment option for enhancing memory, concentration, and overall brain performance.
- Neurological Disorder Management: It shows promise in improving symptoms associated with various neurological disorders, including autism, ADHD, and cerebral palsy, providing a multifaceted approach to neurorehabilitation.
- Neuroprotection and Repair: Cerebrolysin has properties that facilitate nerve repair and protect against further neurological damage, which is particularly beneficial in conditions like stroke or traumatic brain injuries.
- Symptom Relief in Addiction and Toxicity: The drug is effective in managing symptoms related to morphine withdrawal and counteracting neurotoxicity caused by hyperthermia, highlighting its role in complex neurochemical processes.
- Overall Health Improvement: Beyond neurological benefits, Cerebrolysin boosts immune function and improves eye health, demonstrating its versatility and broad therapeutic potential.
What is Cerebrolysin?
Cerebrolysin is a nootropic drug, which means that it has the capacity to enhance a number of cognitive functions such as memory, concentration, and thinking skills. It is used in the treatment of memory disorders, concentration disorders, and degenerative dementia, including Alzheimer’s disease. Cerebrolysin is also used in the treatment of chronic neurological disorders. The brain-boosting effects of cerebrolysin may be attributed to the neuropeptides it contains. These neuropeptides are active brain peptides (chains of amino acids) that are used by nerve cells (neurons) to enhance their communication with each other. Cerebrolysin is a natural compound derived from the brains of pigs using a safe and standardized enzymatic process. In order to achieve its therapeutic effect, cerebrolysin needs to be administered in the form of injections.
How Cerebrolysin Works
Cerebrolysin works by increasing the levels of neurotrophic factors (NFT) and brain-derived neurotrophic factors. This in turn stimulates the formation and repair of neurons (nerve cells) in the brain.
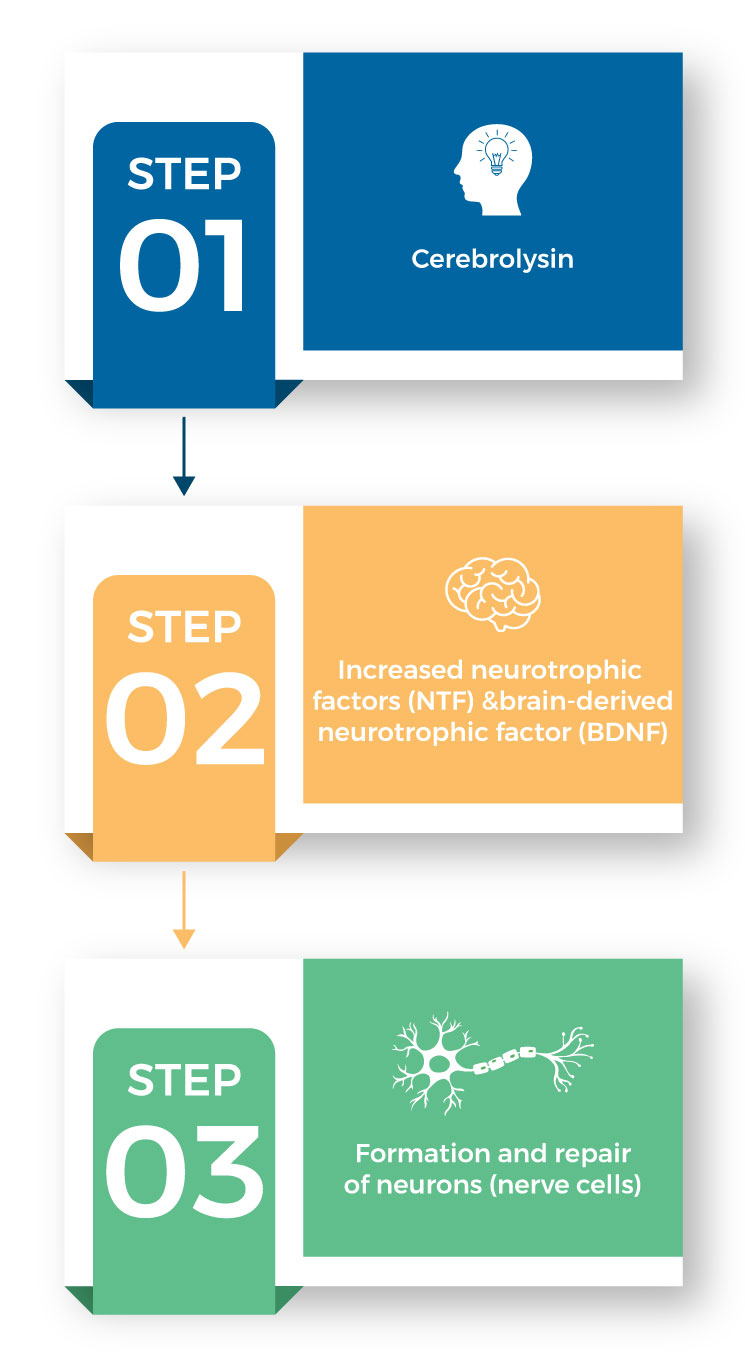
Chemical Structure of Cerebrolysin
Research on Cerebrolysin
A. Boosts Cognitive Function
There is increasing evidence that cerebrolysin may help improve cognitive function and counter the effects of certain medical conditions that lead to cognitive impairment:
- In patients with schizophrenia dominated by negative symptoms, administration of cerebrolysin in addition to antipsychotic medication appears to improve cognitive function and memory. [1]
- In healthy older adults with memory loss, the administration of a derivative of cerebrolysin, N-PEP-12, improved memory. [2]
- In healthy elderly adults, a single dose of cerebrolysin improved memory performance.[3]
- In patients with Alzheimer’s disease and dementia, high doses of cerebrolysin reduced psychological symptoms and slowed disease progression. [4]
- In animals with Alzheimer’s disease, cerebrolysin administration reduced the build-up of brain beta-amyloid plaques, which are sticky proteins known to cause the disease. [5-7]
- In patients with stroke and traumatic brain injury (TBI), cerebrolysin administration appears to improve cognitive recovery without any adverse side effects. [8-14]
- In infants with communication defects due to severe brain damage during pregnancy, cerebrolysin treatment for 3 months improved communication and social interaction.[15]
- In mouse and rat models of Parkinson’s disease, cerebrolysin promoted survival of brain cells, improved motor symptoms, and slowed disease progression. [16-18]
- The combination of cerebrolysin with recombinant tissue-Plasminogen Activator produced a more favorable response in neurological outcome measures as compared to the placebo group. [19]
- In patients with stroke who were treated with cerebrolysin (30 mL over seven days followed by 10 mL until day 30) once daily over a period of four weeks, a significant improvement in neurological and global function outcomes was observed compared to the group who received placebo treatment. [20]
- In dementia models and stroke animal models, cerebrolysin decreased the accumulation of abnormal protein structures in the brain, improved the transmission of signals by the neurons (nerve cells), restored neuron structures, induced restorative processes, decreased infarct volume (dead tissue) and formation of edema (swelling), resulting in improved cognitive and behavioral performance. [21-23]
- In patients with ischemic stroke (insufficient blood flow to the brain), the administration of cerebrolysin produced significant improvements in motor and cognitive recovery. [24-28]
- In elderly patients with vascular dementia of mild to moderate severity, cerebrolysin administration produced beneficial effects on general cognitive function as measured by mini-mental state examination (MMSE). [29]
B. Improves Mood

The brain-boosting effect of cerebrolysin also has a beneficial effect on mood, especially in depressive symptoms. Studies show that administration of cerebrolysin produces an antidepressant effect:
- In elderly patients with depression, intravenous infusions of cerebrolysin reduced symptoms of depression, anxiety, and apathy (lack of interest, enthusiasm, or concern). [30]
- In patients with Alzheimer’s disease, cerebrolysin treatment improved scores on the Cornell Depression Scale. [31]
- In patients with treatment-resistant depression, cerebrolysin therapy improved depressive symptoms without any adverse side effects. [32]
- In rats, cerebrolysin administration produced an anti-anxiety effect. [33]
C. Improves Symptoms of Autism

Autism, or autism spectrum disorder (ASD), refers to a wide range of medical conditions that affect social interaction, behavior, speech, and nonverbal communication. Since cerebrolysin has the capacity to enhance a number of cognitive functions, this nootropic drug has also been studied for its beneficial effect on autism:
- In patients with childhood autism and Asperger’s syndrome (one of the autism spectrum disorders), cerebrolysin therapy improved various areas of cognitive function (expressive and receptive speech, fine motoring, and playing). [34]
- In children with autism, cerebrolysin therapy improved symptoms and scores on the Childhood Autism Rating Scale (CARS). [35]
- In a rat model of autism, cerebrolysin therapy improved behavior and brain cell communication. [36]
D. Improves Symptoms of Attention Deficit Hyperactivity Disorder (ADHD)

ADHD is a mental disorder characterized by hyperactivity, impulsivity, and short attention span. This mental disorder affects children and teens and can transition into adulthood. Studies show that administration of cerebrolysin may help reduce symptoms of ADHD:
- In children with ADHD, cerebrolysin administration at a dose of 1 ml per 10 kg of weight intramuscularly for 1 month improved the core symptoms of ADHD. [37]
- In patients with ADHD of unknown cause, cerebrolysin treatment reduced impulsivity and hyperactivity. [38]
E. Improves Symptoms of Cerebral Palsy (CP)

- In patients with CP, intramuscular administration of cerebrolysin as a single daily dose of 0.1 cc/kg for 10 days and then continued weekly for 4 months appears to improve gross motor function. [39]
- In patients with infantile cerebral paralysis, the administration of cerebrolysin improved symptoms without any adverse side effects. [40]
- In pediatric patients living with CP, the combination of standard rehabilitation therapy with cerebrolysin improved gross motor skills. [41]
- In children with traumatic brain injury and cerebral palsy, cerebrolysin therapy induced the repair of nerve cells of the brain. [42]
F. Repairs Nerve Damage

With nerve damage, there can be a broad range of symptoms depending on the location and types of nerves affected. In addition, chronic nerve damage may impair the sensation or function of the affected body part. Interestingly, numerous studies support the therapeutic benefits of cerebrolysin in different medical conditions associated with nerve damage:
- In patients with diabetic neuropathy (nerve damage caused by diabetes), daily cerebrolysin infusion over a period of 10 days alleviated pain and tingling sensations. [43]
- In a mouse model of diabetic neuropathy, the administration of cerebrolysin improved dysfunction of the sciatic nerve (the largest single nerve located on each side of the lower spine going all the way down to the foot). [44]
- In patients with different nerve injuries, cerebrolysin treatment was associated with rapid neurological recovery after various peripheral nerve lesions than conventional therapies. [45]
- In aged rats, cerebrolysin treatment ameliorated performance deficits and nerve damage. [46]
- In mice, low-dose cerebrolysin administration promoted regeneration of injured spinal motor neurons. [47]
- In animals with nerve injury related to microsurgical suturing, cerebrolysin administration promoted regeneration of the injured peripheral nerve. [48]
- In patients with various forms of nerve injury, cerebrolysin was more associated with rapid recovery of neurological functions than other therapies such as steroids and supportive therapies such as vitamins and antioxidants. [49]
G. Treats Hyperthermia-Induced Neurotoxicity
Adverse environmental circumstances such as heat stress related to hot climates can lead to disturbed mental function. This condition is known as hyperthermia-induced neurotoxicity. Researchers suggest that one of the suitable therapeutic strategies to treat heat-induced mental anomalies related to this condition is cerebrolysin administration. Studies show that cerebrolysin exerts its therapeutic effect through the following important mechanisms:
- In rats exposed to whole body hyperthermia (increased temperature), cerebrolysin administration induced a marked reduction in brain toxicity, thus preventing mental dysfunction related to heat stress. [50]
- In rats suffering from heat stroke, cerebrolysin exerted superior neuroprotective effects as compared to other neuroprotective agents. [51]
H. Treats Morphine Withdrawal Symptoms

Prolonged use of morphine changes the way nerve receptors in the brain work. As a result, sudden withdrawal from this drug can lead to debilitating symptoms such as sleep problems, restlessness, anxiety, digestive problems, high blood pressure, rapid heartbeat, and vision problems. Studies suggest that cerebrolysin can be considered a therapeutic option for morphine withdrawal symptoms:
- A study reported that morphine withdrawal can activate heat shock protein which triggers unpleasant symptoms, suggesting that cerebrolysin can produce beneficial effects through its ability to suppress the activation of these proteins. [52]
- Rat studies showed that cerebrolysin counteracted the activation of heat shock protein, thus alleviating the negative effects of morphine withdrawal. [53-54]
I. Boosts Immune Function
There is mounting evidence that cerebrolysin may help heighten the immune response and prevent a wide array of diseases. Studies show that cerebrolysin positively affects the production of different cells of the immune system:
- In mice, cerebrolysin treatment increased the production of Thy-1 cells. [55]
- In patients with mental developmental delay, cerebrolysin therapy at a dose of 0.1 mg/1kg of body mass for 42 days improved immune status. [56]
- In patients with minimal cerebral dysfunction, intrasmuscular cerebrolysin administration at a dose of 1 ml per 10 kg of body weight for 1 month increased the blood levels of CD19(+) and CD16(+) cells with a simultaneous normalization of blood IgG, IgA, and natural killer cell levels. [57]
- A laboratory study showed that cerebrolysin exerts its immune-boosting properties by increasing the production of T- and B-lymphocytes, and promoting the survival of immunocompetent cells. [58]
- Studies found that cerebrolysin can help decrease the levels of free radicals, which are unstable molecules that damage cells and impair the function of immune system cells. [59-62]
J. Improves Eye Health
IMG
Cerebrolysin has the capacity to stimulate the regeneration of various nerves and cells in the body. Studies show that this regenerative ability may help maintain visual health:
- In a rat model of optic nerve crush, the injection of cerebrolysin promoted the survival of retinal cells. [63]
- In a patient with vision loss in both eyes, intense photophobia (light sensitivity), and eye pain, cerebrolysin administration appears to reduce eye pressure and improve visual acuity. [64]
Associated Side Effects and Adverse Events of Cerebrolysin
Cerebrolysin side effects are very uncommon, but adverse events have been reported. There is also a potential for serious adverse events with Cerebrolysin use, including an increase in non-fatal serious adverse events. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on cerebrolysin. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of cerebrolysin. Despite this, it was listed as a side effect associated with cerebrolysin even though these associated side effects are very uncommon.
Side effects associated with cerebrolysin may include the following:
- Agitation
- Changes in blood pressure
- Confusion
- Constipation
- Diarrhea
- Fatigue
- Feeling hot
- Nausea
- Seizure
- Vertigo
- Vomiting
What is Cerebrolysate?
Cerebrolysate, commonly known as Cerebrolysin, is a peptide-based, neurotrophic treatment derived from pig brain proteins. It contains a balanced composition of free amino acids and biologically active neuropeptides. The formulation is specifically designed to mimic the natural neurotrophic factors in the human brain that regulate the survival, development, and function of neurons. Cerebrolysin is used extensively in neurology and psychiatry to treat a variety of brain health issues and neurodegenerative disorders, including traumatic brain injury. Reviews in the database of systematic reviews have assessed its efficacy and safety for these applications, often finding little to no difference in outcomes compared to other treatments. Despite its use, some studies have reported little to no difference in clinical improvement between patients treated with Cerebrolysin and those receiving standard care. Continued research and systematic reviews are necessary to further evaluate its potential benefits and to understand why some findings indicate little to no difference in therapeutic efficacy.
The therapeutic action of Cerebrolysate centers on its neuroprotective and neurorestorative effects. It works by enhancing brain metabolism and plasticity, which leads to improved brain function and reduced symptoms in various neurological conditions, such as stroke, traumatic brain injury, Alzheimer’s disease, and other forms of dementia. Research and clinical applications suggest that Cerebrolysate helps improve symptoms in these disorders, offering hope for symptom management and quality of life improvement in patients with conditions like autism, ADHD, and cerebral palsy, as documented in the database of systematic reviews. The inclusion criteria for these studies ensure that only relevant and high-quality research is considered, providing robust evidence for Cerebrolysate’s efficacy. Inclusion criteria also play a crucial role in determining the applicability of findings to different patient populations. Additionally, the inclusion criteria help standardize the assessment of outcomes, facilitating a clearer understanding of the therapeutic benefits of Cerebrolysate across various neurological conditions.
Administration of Cerebrolysate is typically done through injections or infusions, considering it is a peptide that may be broken down if taken orally. The treatment regimen varies depending on the patient’s condition and the severity of symptoms, usually involving a series of doses over a period of time. Effective in managing symptoms of traumatic brain injury, Cerebrolysate’s wide usage and potential benefits are under continuous research to better understand its full scope and mechanism of action within neurotherapeutic applications, particularly for traumatic brain injury. The ability of Cerebrolysate to counteract pathologies associated with traumatic brain injury is a key area of investigation, with numerous entries in the database of systematic reviews supporting its clinical use. Further studies aim to elucidate how Cerebrolysate can counteract pathologies in other neurological conditions. Continued research is crucial to determine how effectively Cerebrolysate can counteract pathologies across various neurotherapeutic applications.
Is Cerebrolysin 10ml Good?
Cerebrolysin, available in 10ml vials among other dosages, is regarded positively in many clinical settings for its potential neuroprotective and neurotrophic effects. It is often administered to improve cognitive function, treat neurodegenerative disorders, and aid recovery in patients suffering from stroke or traumatic brain injuries. Moderate certainty evidence supports its use in these contexts, highlighting its benefits in enhancing cognitive recovery and overall neurological health. The 10ml dosage allows for controlled administration of the treatment, tailored to meet the specific needs and medical conditions of patients, ensuring both efficacy and safety, particularly in those with chronic neurological disorders. Additionally, moderate certainty evidence suggests that Cerebrolysin can be effective in managing symptoms of chronic neurological conditions, contributing to improved patient outcomes. This moderate certainty evidence underscores the importance of dosage control and personalized treatment plans in achieving optimal therapeutic effects with Cerebrolysin.
The effectiveness of the 10ml dosage of Cerebrolysin is supported by various studies, which suggest improvements in symptoms of Alzheimer’s disease, dementia, and other cognitive impairments when used as part of a comprehensive treatment plan for chronic neurological disorders. However, some studies indicate an unclear risk in terms of the consistency of these improvements. Patients often report improvements in memory, focus, and overall cognitive ability, as well as enhanced mood and functionality. Despite these positive reports, there remains an unclear risk regarding the long-term efficacy and safety of Cerebrolysin. Moreover, Cerebrolysin’s ability to promote neuronal growth and repair is a critical aspect of its therapeutic value, potentially slowing the progression of chronic neurological disorders and neurodegenerative diseases, though some findings suggest an unclear risk of bias in the reported outcomes.
However, the use of Cerebrolysin, including the 10ml dosage, is not without concerns, especially in the treatment of chronic neurological disorders. It requires careful handling and administration, typically via injection or infusion, which can be a limitation for some patients. Side effects, although generally mild, can include fatigue, headache, and dizziness. As with any medical treatment, the decision to use Cerebrolysin should be made in consultation with a healthcare provider, taking into account the individual’s medical history and specific health needs related to chronic neurological disorders. Overall, while Cerebrolysin 10ml has demonstrated beneficial effects for many, its suitability and effectiveness can vary depending on the patient and the condition being treated.
Cerebrolysin Price
The price of Cerebrolysin can vary widely depending on several factors, including the country of purchase, the purchasing source (hospital, pharmacy, or online distributor), and the dosage form. Generally, Cerebrolysin is considered a premium medication, primarily due to its complex manufacturing process and the biological origin of its ingredients. It is typically more expensive in regions where it is not widely distributed or where medical treatments tend to have higher overall costs, particularly when used for conditions like acute ischaemic stroke. However, the efficacy of Cerebrolysin in treating acute ischaemic stroke is based on low certainty evidence, which impacts the overall perceived value of the treatment. The need for acute ischaemic stroke treatments such as Cerebrolysin emphasizes its value despite higher costs, even though the supporting data is often of low certainty evidence. Further research and systematic reviews are necessary to strengthen the evidence base, as current findings are often characterized by low certainty evidence, influencing both clinical decisions and healthcare costs.
In terms of specific costs, the price of a 10ml vial of Cerebrolysin can range significantly. For example, in the United States and Western Europe, prices are typically higher due to regulatory practices and market conditions. In contrast, in Eastern European countries, where Cerebrolysin is more commonly used and locally produced, especially for treating acute ischaemic stroke, the prices can be somewhat lower. Online pharmacies might offer competitive pricing, but buyers should be cautious of the source to ensure they are obtaining a legitimate and safe product for acute ischaemic stroke treatment. The potential benefit for acute ischaemic stroke recovery can justify the effort and expense of sourcing Cerebrolysin.
The price of Cerebrolysin can vary widely depending on several factors, including the country of purchase, the purchasing source (hospital, pharmacy, or online distributor), and the dosage form. Generally, Cerebrolysin is considered a premium medication, primarily due to its complex manufacturing process and the biological origin of its ingredients. It is typically more expensive in regions where it is not widely distributed or where medical treatments tend to have higher overall costs, particularly when used for conditions like acute ischaemic stroke. However, the efficacy of Cerebrolysin in treating acute ischaemic stroke is based on low certainty evidence, which impacts the overall perceived value of the treatment. The need for acute ischaemic stroke treatments such as Cerebrolysin emphasizes its value despite higher costs, even though the supporting data is often of low certainty evidence. Further research and systematic reviews are necessary to strengthen the evidence base, as current findings are often characterized by low certainty evidence, influencing both clinical decisions and healthcare costs.
Cortexin vs Cerebrolysin
Cortexin and Cerebrolysin are both peptide-based drugs used primarily in neurology for their neuroprotective and neurotrophic effects, but they have distinct compositions and slightly different applications. Cortexin is derived from the cerebral cortex of pigs and contains a complex of polypeptide fractions along with amino acids that influence the central nervous system. It is administered to improve brain function, protect against damage, and enhance recovery from various neurological conditions. Unlike Cerebrolysin, which is derived from pig brain proteins and contains a mixture of low-molecular-weight peptides and free amino acids, Cortexin’s active components are smaller and potentially more focused in their action.
In terms of clinical use, both Cortexin and Cerebrolysin are utilized to treat similar conditions, including traumatic brain injuries, stroke recovery, and cognitive disorders such as Alzheimer’s disease. However, the specific indications and the perceived efficacy can vary. Cortexin is often noted for its neuroprotective properties and its ability to stabilize cell membranes and reduce oxidative stress. Cerebrolysin, on the other hand, is more explicitly recognized for its role in enhancing cognitive functions and supporting neuronal growth and repair, making it particularly useful in the treatment of dementia and similar degenerative conditions.
The choice between Cortexin and Cerebrolysin often comes down to clinical objectives, patient response to treatment, and the preferences of the healthcare provider. Both medications are administered via injection, requiring similar protocols for use. Side effects for both drugs are generally mild but can include discomfort at the injection site, dizziness, and headaches. While both treatments are supported by a substantial amount of research, the body of evidence is more robust for Cerebrolysin, particularly in its effects on a broad range of neurodegenerative and cognitive disorders, as documented in the database of systematic reviews. Ultimately, the decision to use Cortexin or Cerebrolysin should be tailored to the individual patient’s condition and needs, often guided by the experience and observation of their healthcare team. Consulting the database of systematic reviews can provide valuable insights into the comparative efficacy and safety of these treatments. Additionally, continuous reference to the database of systematic reviews helps ensure that treatment decisions are based on the most comprehensive and up-to-date evidence available.
Cerebrolysin in Stroke and Vascular Dementia
Cerebrolysin has garnered attention in the medical community for its potential therapeutic effects in treating stroke and vascular dementia. This peptide-based treatment is believed to confer neuroprotective and neurotrophic benefits, making it a viable option for promoting neural repair and functional recovery post-stroke. The neurotrophic factors present in Cerebrolysin are thought to enhance neurogenesis, reduce inflammation, and improve synaptic connectivity, which can be critical in the acute phase following a stroke. Clinical trials and studies have shown that when administered soon after a stroke, Cerebrolysin can help improve neurological function and reduce the extent of brain damage, with a low incidence of serious adverse events.
In the context of vascular dementia, Cerebrolysin’s ability to improve cognitive functions and protect neural structures offers a promising approach to managing this condition. Vascular dementia, which results from impaired blood flow to the brain leading to cognitive decline, can benefit from Cerebrolysin’s mechanisms that enhance cerebral blood flow and neuronal resilience. Patients treated with Cerebrolysin have reported improvements in memory, attention, and executive function. Continuous research and clinical trials suggest that Cerebrolysin not only helps in stabilizing the symptoms of vascular dementia but may also slow its progression, offering a better quality of life for patients, with minimal serious adverse events.
Despite the promising outcomes, the use of Cerebrolysin in stroke recovery and vascular dementia must be carefully considered by healthcare professionals. The treatment involves a series of injections or infusions, which require monitoring and management by medical personnel. Side effects, though generally mild, can include headache, nausea, and dizziness. The risk of serious adverse events, while low, requires careful monitoring. Reviews in the Cochrane Database of Systematic Reviews highlight the importance of such vigilance in clinical practice. The cost of treatment and the variability in patient responses also pose challenges. Therefore, while Cerebrolysin offers a potentially effective treatment modality for stroke and vascular dementia, it should be part of a comprehensive therapeutic plan that includes other medical interventions and lifestyle adjustments tailored to individual patient needs. Ongoing vigilance for any serious adverse events is critical, as emphasized by findings from the Cochrane Database of Systematic Reviews. Additionally, systematic reviews and meta-analyses, such as those found in the Cochrane Database of Systematic Reviews, provide valuable insights into the efficacy and safety of Cerebrolysin, guiding healthcare professionals in making informed decisions about its use in diverse patient populations.
Cerebrolysin Buy Online
Purchasing Cerebrolysin online is a convenient option for many, offering the ability to source this treatment from various global suppliers. However, potential buyers should proceed with caution. Cerebrolysin requires precise storage conditions to maintain its efficacy and safety, such as specific temperature ranges which might not be guaranteed during international shipping. Additionally, the authenticity of the product is crucial as counterfeit medications can pose significant health risks. Buyers should seek out reputable pharmacies or distributors that provide clear product sourcing information and have good reviews from other users, potentially verified through resources like the Cochrane Database of Systematic Reviews. It’s important to reference the Cochrane Database of Systematic Reviews for verified information on product efficacy and safety, as this resource is known for its rigorous assessment of clinical trials and treatments. Finally, consulting the Cochrane Database of Systematic Reviews can help ensure that the purchased Cerebrolysin meets high standards and has been evaluated in reliable studies.
Before buying Cerebrolysin online, it’s important to verify the legal implications of importing prescription drugs into your country. Regulations can vary widely; some countries strictly prohibit importing drugs without a local prescription, while others may allow it under certain conditions. Consulting with a healthcare professional is also recommended to ensure that Cerebrolysin is an appropriate treatment option for your condition. They can provide guidance on the correct dosage and administration, and monitor treatment progress and safety, with standards often reflected in the Cochrane database of systematic reviews.
Additionally, price comparisons can reveal significant differences in cost, which can be influenced by factors like origin of manufacture, shipping fees, and import taxes. Websites offering extremely low prices should be approached with suspicion as they may be selling counterfeit or expired products. Secure payment options and a clear return policy are also important to consider when purchasing medications online, with the Cochrane database of systematic reviews often providing data on efficacy and safety that can help guide purchasing decisions. Ultimately, while the convenience of buying Cerebrolysin online is appealing, ensuring the safety and legality of the purchase should be the top priority, supported by evidence from the Cochrane database of systematic reviews.
FAQ
What is Cerebrolysin used for?
Cerebrolysin is used for treating various neurological conditions such as Alzheimer’s disease, stroke, traumatic brain injuries, and certain forms of dementia. Clinical studies have shown that moderate certainty evidence suggests it effectively improves outcomes in these serious conditions by promoting neurogenesis and enhancing synaptic connectivity. It is also explored for improving symptoms in developmental disorders like cerebral palsy, ADHD, and autism. Clinical studies indicate that here too, moderate certainty evidence suggests that cerebrolysin like agents started can significantly alleviate symptoms and enhance quality of life for patients dealing with these challenges. Additionally, cerebrolysin may have potential benefits in reducing the risk of pulmonary embolism in certain patient populations, although further research is needed to confirm its efficacy in this regard.
Does Cerebrolysin repair the brain?
Cerebrolysin is believed to aid in brain repair by promoting neurogenesis, synaptic connectivity, and neuronal survival, which can help in the recovery of brain functions after injuries or in degenerative conditions, particularly in dementia patients. Randomised controlled trials have supported these effects, demonstrating unclear risk Cerebrolysin’s potential to enhance neurological recovery in dementia patients. Further evidence from additional randomised controlled trials continues to underscore the therapeutic benefits of this treatment in various neurologic contexts, including its use among dementia patients. Securing research grants to conduct larger-scale studies can facilitate the exploration of Cerebrolysin’s efficacy and safety profile in diverse patient populations and clinical settings.
Does Cerebrolysin require a prescription?
Yes, Cerebrolysin requires a prescription as it is administered through injections and intended for use under medical supervision, particularly in a clinical setting focused on brain protection. Information from the Cochrane Stroke Trials Register supports the importance of such supervision to monitor the effects and manage any potential complications related to brain protection. Evidence from the Cochrane Stroke Trials Register also plays a crucial role in guiding the use of Cerebrolysin in clinical settings, ensuring that it is administered safely and effectively for optimal brain protection.
How do you administer Cerebrolysin?
Cerebrolysin, an intravenous or intramuscularly administered neurotrophic treatment, contains amino acids derived from brain proteins. The administration method may depend on the patient’s condition and treatment protocol. As a neurotrophic treatment, cerebrolysin like peptide mixtures is designed to support neural growth and cognitive enhancement, requiring careful administration to ensure optimal therapeutic effects.
What is the mechanism of action of Cerebrolysin in stroke patients?
In stroke patients, Cerebrolysin works by enhancing neuroplasticity, promoting neuronal repair, and improving synaptic connectivity. It also helps to reduce inflammation and protect neurons from further damage, aiding in the recovery of neurological functions. In evaluating the effectiveness of Cerebrolysin in acute ischaemic stroke, review authors independently applied standardized criteria to assess its impact on neuroplasticity, neuronal repair, and synaptic connectivity. Such rigorous bias assessment analysis ensures a comprehensive understanding of its therapeutic mechanisms and potential benefits in acute ischaemic stroke management.
How safe is Cerebrolysin?
Cerebrolysin is generally considered safe when used under medical supervision, but like any medication, it can have side effects, including ischaemic stroke. Common side effects include headache, dizziness, and flu-like symptoms. It should be used cautiously in patients with ischaemic stroke, kidney conditions, or those who are prone to seizures. When assessing its usage, applied grade criteria should be considered to evaluate its appropriateness for individual patients.
What is the role of Cerebrolysin in stroke patients?
In stroke patients, Cerebrolysin plays a role in enhancing brain repair and recovery. It supports neuroprotection, neurogenesis, and functional recovery, helping patients regain cognitive and motor functions more effectively, as demonstrated in randomized controlled trials of assessed trial quality. Further investigation into its benefits, with an emphasis on assessed trial quality, is necessary to fully understand its potential in stroke rehabilitation. Incomplete outcome data, particularly in long-term studies, may provide valuable insights into the sustained effects of Cerebrolysin treatment, potentially reducing the risk of early death.
Is Cerebrolysin a multi target drug for recovery after stroke?
Yes, Cerebrolysin is considered a multi-target drug for recovery after stroke due to its complex mechanism of action that targets various aspects of neuronal repair, protection, and regeneration. Inclusion criteria for studies evaluating Cerebrolysin’s efficacy in stroke recovery often focus on specific patient populations to ensure homogeneity in the research cohorts. Information about ongoing trials registers can provide valuable insights into the current research landscape and potential future directions in investigating Cerebrolysin’s role in stroke rehabilitation.
What does Cerebrolysin do to the brain?
Cerebrolysin stimulates neuronal growth and connectivity, enhances cognitive function, and helps in the repair and protection of brain cells. It works by mimicking the action of natural growth factors in the brain, which are crucial for neuronal health. However, the available data supporting these claims may have very low certainty evidence, emphasizing the need for further research to better understand the effectiveness of Cerebrolysin. Studies investigating the safety and efficacy of Cerebrolysin in pregnant patients are particularly scarce, highlighting the importance of dedicated research in this population. Conducting a meta analysis could provide a comprehensive overview of existing studies on Cerebrolysin’s effects, shedding light on its potential benefits and limitations. Furthermore, meta analysis studies can help identify trends and patterns across various research findings, offering valuable insights into the overall efficacy and safety profile of Cerebrolysin. Given the complexity of neurological disorders and the diverse patient populations they affect, conducting multiple meta analysis studies focusing on different aspects of Cerebrolysin’s effects could provide a more nuanced understanding of its therapeutic potential. By synthesizing data from multiple studies, meta analysis can enhance the reliability and robustness of findings, ultimately guiding clinical decision-making and informing future research directions. Therefore, investing in well-designed meta analysis studies is essential for advancing our understanding of Cerebrolysin and optimizing its use in clinical practice.
What are the contraindications for Cerebrolysin?
Cerebrolysin should not be used in patients who have a known allergy to the drug or any of its components. It is also contraindicated in individuals with severe renal impairment due to the risk of fluid overload and electrolyte imbalance. Caution is advised in patients with epileptic disorders due to potential excitatory effects on the nervous system, impacting neural transmission. However, in cases where Cerebrolysin is deemed appropriate, it may be administered to counteract pathologies associated with neurological disorders, under close medical supervision, targeting neural transmission for therapeutic benefit.
What are the contraindications for Cerebrolysin?
Cerebrolysin should not be used in patients with severe renal failure, allergy to any of its components, or a history of seizure disorders, particularly in cases of stroke onset. It is crucial to evaluate these contraindications before administering the drug, especially in conditions like traumatic brain injury, where stroke onset may exacerbate existing neurological complications. Furthermore, consulting reputable scientific databases like the Science Citation Index can provide valuable insights into the latest research and evidence regarding placebo group cumulative dose Cerebrolysin’s efficacy and safety profile.
Can Cerebrolysin be used for spinal cord injury?
Yes, Cerebrolysin has been studied for its effects on spinal cord injury. Research indicates that it may aid in cognitive recovery, poor functional outcome, and neuroprotection in patients with spinal cord injury, although it may also result in poor functional outcome in some cases. Exploring scholarly sources such as the Science Core Collection can offer comprehensive insights into the latest studies and findings regarding add on therapy placebo group Cerebrolysin’s efficacy in spinal cord injury treatment. However, the available data supporting these claims may have moderate certainty evidence, highlighting the need for further research to strengthen the evidence base.
Why is selective outcome reporting judgement important in Cerebrolysin studies?
Selective outcome reporting judgment is essential in assessing the risk of bias in studies related to Cerebrolysin-supported ischemic stroke. Evaluating the public availability of study protocols and reported outcomes helps ensure the reliability of the findings, especially in the context of neuropsychiatric disease. Factors such as blinding, allocation sequence, incomplete outcome data, and other potential biases must also be considered. Ensuring transparency and rigor in research methodologies is crucial for advancing our understanding of Cerebrolysin’s efficacy in managing neuropsychiatric disease. Additionally, careful consideration of secondary outcome measures is important for comprehensively evaluating the impact of Cerebrolysin-supported ischemic stroke in neuropsychiatric disease management. Comparisons with different neuroprotective drugs can further elucidate the relative efficacy and safety of Cerebrolysin-supported ischemic stroke in managing neuropsychiatric disease.
How many days should you take Cerebrolysin?
Yes, Cerebrolysin has been studied for its effects on spinal cord injury. Research indicates that it may aid in cognitive recovery, poor functional outcome, and neuroprotection in patients with spinal cord injury, although it may also result in poor functional outcome in some cases. The risk ratio of these outcomes is a key consideration in evaluating its effectiveness. As one of the neuroprotective drugs, Cerebrolysin is part of ongoing research into neuroprotective drugs aimed at improving outcomes for patients with spinal cord injuries, with comparing Cerebrolysin to different neuroprotective drugs being a crucial factor in these systematic reviews studies. Additionally, research into its effects on ischemic stroke is crucial, as ischemic stroke represents a significant portion of stroke cases, and the neuroprotective potential of Cerebrolysin could be highly beneficial. Evaluating its efficacy in ischemic stroke through systematic reviews will help determine its relative benefits and guide clinical practices.
Does Cerebrolysin improve memory?
Yes, Cerebrolysin has been shown to improve memory, as indicated by clinical data. It is used to treat cognitive impairments in conditions like Alzheimer’s disease, stroke, and traumatic brain injuries. It enhances memory by promoting synaptic connectivity and neuronal growth, which are critical for memory formation and retrieval. However, in some studies, the risk of bias regarding the reported memory improvement may be unclear, emphasizing the need for further investigation and analysis to validate these findings through systematic reviews robust clinical data. Utilizing cattle brain as a model for studying memory mechanisms may offer insights into the specific neurobiological processes underlying Cerebrolysin’s effects on memory enhancement. Additionally, comparing the effects of Cerebrolysin with other neuroprotective agents using cattle brain models can provide valuable information regarding its relative efficacy and safety profile in improving memory function.
What are the side effects of Cerebrolysin peptide?
The side effects of Cerebrolysin peptide are generally mild but can include headache, dizziness, agitation, and flu-like symptoms such as fever and fatigue, especially in patients with acute stroke. Less commonly, it may cause gastrointestinal disturbances like nausea or diarrhea in those recovering from acute stroke. In systematic reviews, it’s important to monitor for allergic reactions, particularly in acute stroke patients, and administer under medical supervision to manage any adverse effects effectively. In cases of acute stroke, careful monitoring is crucial to ensure safety and efficacy. Overall, while Cerebrolysin is used in various conditions, its administration in acute stroke requires special attention to potential side effects, which can be assessed further in a randomized trial.
Reference
Xiao S, Xue H, Li G. Therapeutic effects of cerebrolysin added to risperidone in patients with schizophrenia dominated by negative symptoms. The Australian and New Zealand journal of psychiatry. 2012; 46(2):153-60. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22311531
Therapeutic effects of cerebrolysin added to risperidone in patients with schizophrenia dominated by negative symptoms
The study by Xiao, Xue, and Li, published in the Australian and New Zealand Journal of Psychiatry in 2012, explored the effects of adding cerebrolysin to risperidone for treating schizophrenia patients with predominant negative symptoms. The research was a double-blind, placebo-controlled trial involving 109 patients. Although both the cerebrolysin and placebo groups showed improvements in psychiatric symptoms, cognitive, and memory performance, cerebrolysin did not significantly enhance the effectiveness of risperidone in treating psychotic symptoms. However, cerebrolysin was found to be safe and potentially beneficial in improving cognitive and memory functions in these patients.
For more details https://www.ncbi.nlm.nih.gov/pubmed/22311531
Crook TH, Ferris SH, Alvarez XA, Laredo M, Moessler H. Effects of N-PEP-12 on memory among older adults. International clinical psychopharmacology. 2005; 20(2):97-100. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15729085
Effects of N-PEP-12 on memory among older adults
The study by Crook, Ferris, Alvarez, Laredo, and Moessler, published in International Clinical Psychopharmacology in 2005, examined the effects of N-PEP-12, a neuropeptide supplement, on memory among older adults. The double-blind, placebo-controlled trial involved 54 participants aged 50 or older who reported memory problems. The results suggested that N-PEP-12 may have a beneficial effect on memory performance in older adults with subjective memory complaints.
For more details https://www.ncbi.nlm.nih.gov/pubmed/15729085
Alvarez XA, Lombardi VR, Corzo L. Oral Cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects. Journal of neural transmission. Supplementum. 2000; 59:315-28. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10961443
Oral Cerebrolysin enhances brain alpha activity and improves cognitive performance in elderly control subjects
The study by Alvarez, Lombardi, and Corzo, published in the Journal of Neural Transmission Supplementum in 2000, investigated the effects of oral Cerebrolysin on brain alpha activity and cognitive performance in elderly subjects. This research observed an enhancement in brain alpha activity and an improvement in cognitive performance among elderly control subjects who were administered oral Cerebrolysin. The study contributes to understanding the potential cognitive benefits of Cerebrolysin in the elderly population.
For more details https://www.ncbi.nlm.nih.gov/pubmed/10961443
Allegri RF, Guekht A. Cerebrolysin improves symptoms and delays progression in patients with Alzheimer’s disease and vascular dementia. Drugs of today (Barcelona, Spain: 1998). 2012; 48 Suppl A:25-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22514793
Cerebrolysin improves symptoms and delays progression in patients with Alzheimer’s disease and vascular dementia.
The study by Allegri and Guekht, published in 2012 in “Drugs of Today”, examined the effects of Cerebrolysin on patients with Alzheimer’s disease and vascular dementia. The research suggested that Cerebrolysin not only improves symptoms but also delays the progression of these conditions. This study highlights the potential therapeutic benefits of Cerebrolysin in treating neurodegenerative diseases.
For more details https://www.ncbi.nlm.nih.gov/pubmed/22514793
Rockenstein E, Torrance M, Mante M. Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease. Journal of neuroscience research. 2006; 83(7):1252-61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16511867
Cerebrolysin decreases amyloid-beta production by regulating amyloid protein precursor maturation in a transgenic model of Alzheimer’s disease
The study by Rockenstein, Torrance, and Mante, published in the Journal of Neuroscience Research in 2006, investigated the impact of Cerebrolysin on amyloid-beta production in a transgenic model of Alzheimer’s disease. The research focused on the regulation of amyloid protein precursor maturation, finding that Cerebrolysin can reduce amyloid-beta production. This study contributes to the understanding of potential treatments for Alzheimer’s disease by targeting amyloid-beta pathways.
For more details https://www.ncbi.nlm.nih.gov/pubmed/16511867
Rockenstein E, Mallory M, Mante M. Effects of Cerebrolysin on amyloid-beta deposition in a transgenic model of Alzheimer’s disease. Journal of neural transmission. Supplementum. 2002. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12456076.
Effects of Cerebrolysin on amyloid-beta deposition in a transgenic model of Alzheimer’s disease
The study by Rockenstein, Mallory, and Mante, published in 2002 in the Journal of Neural Transmission Supplementum, examined the effects of Cerebrolysin on amyloid-beta deposition in a transgenic model of Alzheimer’s disease. This research contributes to the understanding of how Cerebrolysin might influence the pathogenesis of Alzheimer’s disease, particularly in relation to amyloid-beta accumulation.
For more details https://www.ncbi.nlm.nih.gov/pubmed/12456076
Rockenstein E, Adame A, Mante M, Moessler H, Windisch M, Masliah E. The neuroprotective effects of Cerebrolysin in a transgenic model of Alzheimer’s disease are associated with improved behavioral performance. Journal of neural transmission (Vienna, Austria : 1996). 2003; 110(11):1313-27. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14628195
The neuroprotective effects of Cerebrolysin in a transgenic model of Alzheimer’s disease are associated with improved behavioral performance.
The study by Rockenstein, Adame, Mante, Moessler, Windisch, and Masliah, published in the Journal of Neural Transmission in 2003, focused on the neuroprotective effects of Cerebrolysin in a transgenic model of Alzheimer’s disease. The research demonstrated that Cerebrolysin is associated with improved behavioral performance, highlighting its potential as a therapeutic agent in Alzheimer’s disease management.
For more details https://www.ncbi.nlm.nih.gov/pubmed/14628195
Ladurner G, Kalvach P, Moessler H, .Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial. Journal of neural transmission (Vienna, Austria : 1996). 2005; 112(3):415-28. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15583955
Neuroprotective treatment with cerebrolysin in patients with acute stroke: a randomised controlled trial
The study by Ladurner, Kalvach, and Moessler, published in the Journal of Neural Transmission in 2005, investigated the neuroprotective effects of Cerebrolysin in patients with acute stroke in a randomised controlled trial. The research aimed to evaluate the potential benefits of Cerebrolysin in acute stroke management.
For more details https://www.ncbi.nlm.nih.gov/pubmed/15583955
Muresanu DF, Heiss W-D, Hoemberg V, et al. Cerebrolysin and Recovery After Stroke (CARS): A Randomized, Placebo-Controlled, Double-Blind, Multicenter Trial. Stroke; a Journal of Cerebral Circulation. 2016;47(1):151-159. doi:10.1161/STROKEAHA.115.009416. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4689177/.
Cerebrolysin and Recovery After Stroke (CARS): A Randomized, Placebo-Controlled, Double-Blind, Multicenter Trial
The study by Muresanu, Heiss, Hoemberg, and colleagues, published in 2016 in the journal “Stroke,” was a randomized, placebo-controlled, double-blind, multicenter trial titled “Cerebrolysin and Recovery After Stroke (CARS).” It focused on evaluating the efficacy of Cerebrolysin in stroke recovery. The trial aimed to determine how Cerebrolysin affects the recovery process after a stroke.
For more details https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4689177/
Guekht A, Vester J, Heiss WD. Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta-analysis of the CARS trials. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2017; 38(10):1761-1769. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28707130.
Safety and efficacy of Cerebrolysin in motor function recovery after stroke: a meta-analysis of the CARS trials
The 2017 study by Guekht, Vester, and Heiss, published in Neurological Sciences, is a meta-analysis of the CARS trials focusing on the safety and efficacy of Cerebrolysin in motor function recovery after stroke. This comprehensive analysis consolidates findings from various studies to evaluate the overall impact of Cerebrolysin on stroke recovery, particularly in terms of motor function.
For more details https://www.ncbi.nlm.nih.gov/pubmed/28707130
Chen CC, Wei ST, Tsaia SC, Chen XX, Cho DY. Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study. British journal of neurosurgery. 2013; 27(6):803-7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23656173.
Cerebrolysin enhances cognitive recovery of mild traumatic brain injury patients: double-blind, placebo-controlled, randomized study
The study by Chen, Wei, Tsaia, Chen, and Cho, published in the British Journal of Neurosurgery in 2013, was a double-blind, placebo-controlled, randomized study that investigated the effects of Cerebrolysin on cognitive recovery in patients with mild traumatic brain injury. The study demonstrated that Cerebrolysin enhances cognitive recovery in these patients.
For more details https://www.ncbi.nlm.nih.gov/pubmed/23656173
Bornstein N, Poon WS. Accelerated recovery from acute brain injuries: clinical efficacy of neurotrophic treatment in stroke and traumatic brain injuries. Drugs of today (Barcelona, Spain : 1998). 2012; 48 Suppl A:43-61. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22514794
Accelerated recovery from acute brain injuries: clinical efficacy of neurotrophic treatment in stroke and traumatic brain injuries
The study by Bornstein and Poon, published in 2012 in “Drugs of Today,” discusses the clinical efficacy of neurotrophic treatment in accelerating recovery from acute brain injuries, particularly focusing on stroke and traumatic brain injuries. The research provides insights into the potential benefits of such treatments in enhancing recovery processes.
For more details https://www.ncbi.nlm.nih.gov/pubmed/22514794
Wong GK, Zhu XL, Poon WS. Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study. Actaneurochirurgica. Supplement. 2005; 95:59-60. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16463821.
Beneficial effect of cerebrolysin on moderate and severe head injury patients: result of a cohort study
The study by Wong, Zhu, and Poon, published in “Acta Neurochirurgica Supplement” in 2005, explored the beneficial effects of cerebrolysin on patients with moderate and severe head injuries. This cohort study contributes to the understanding of cerebrolysin’s role in treating head injury cases.
For more details https://www.ncbi.nlm.nih.gov/pubmed/16463821
Zhang D, Dong Y, Li Y, Chen J, Wang J, Hou L. Efficacy and Safety of Cerebrolysin for Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials. BioMed Research International. 2017;2017:4191670. doi:10.1155/2017/4191670. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5474547/.
Efficacy and Safety of Cerebrolysin for Acute Ischemic Stroke: A Meta-Analysis of Randomized Controlled Trials
The 2017 study by Zhang, Dong, Li, Chen, Wang, and Hou, published in BioMed Research International, is a meta-analysis of randomized controlled trials assessing the efficacy and safety of Cerebrolysin for treating acute ischemic stroke. This comprehensive analysis aims to provide insights into the therapeutic benefits and safety profile of Cerebrolysin in this context.
For more details https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5474547/
Hassanein SM, Deifalla SM, El-Houssinie M, Mokbel SA. Safety and Efficacy of Cerebrolysin in Infants with Communication Defects due to Severe Perinatal Brain Insult: A Randomized Controlled Clinical Trial. Journal of clinical neurology (Seoul, Korea). 2016; 12(1):79-84. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26365023.Ozkizilcik A, Sharma A, Muresanu DF. Timed Release of Cerebrolysin Using Drug-Loaded TitanateNanospheres Reduces Brain Pathology and Improves Behavioral Functions in Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28875428.
Safety and Efficacy of Cerebrolysin in Infants with Communication Defects due to Severe Perinatal Brain Insult: A Randomized Controlled Clinical Trial
The study by Hassanein, Deifalla, El-Houssinie, and Mokbel, published in the Journal of Clinical Neurology in 2016, was a randomized controlled clinical trial investigating the safety and efficacy of Cerebrolysin in infants with communication defects due to severe perinatal brain insult. The study contributes to the understanding of potential treatments for infants affected by these conditions.
For more details https://www.ncbi.nlm.nih.gov/pubmed/26365023
Ozkizilcik A, Sharma A, Muresanu DF. Timed Release of Cerebrolysin Using Drug-Loaded TitanateNanospheres Reduces Brain Pathology and Improves Behavioral Functions in Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28875428.
Timed Release of Cerebrolysin Using Drug-Loaded TitanateNanospheres Reduces Brain Pathology and Improves Behavioral Functions in Parkinson’s Disease
The 2017 study by Ozkizilcik, Sharma, and Muresanu, published in Molecular Neurobiology, focused on the use of drug-loaded titanate nanospheres for the timed release of Cerebrolysin in Parkinson’s disease. This innovative approach aimed to reduce brain pathology and improve behavioral functions in Parkinson’s disease, marking a significant advancement in the treatment strategies for this condition.
For more details https://www.ncbi.nlm.nih.gov/pubmed/28875428
Requejo C, Ruiz-Ortega JA, Cepeda H. Nanodelivery of Cerebrolysin and Rearing in Enriched Environment Induce Neuroprotective Effects in a Preclinical Rat Model of Parkinson’s Disease. Molecular neurobiology. 2017. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28840482.
Nanodelivery of Cerebrolysin and Rearing in Enriched Environment Induce Neuroprotective Effects in a Preclinical Rat Model of Parkinson’s Disease
The 2017 study by Requejo, Ruiz-Ortega, and Cepeda, published in Molecular Neurobiology, explores the neuroprotective effects of nanodelivery of Cerebrolysin combined with rearing in an enriched environment in a preclinical rat model of Parkinson’s disease. This innovative approach aimed to study the combined effects of advanced drug delivery and environmental factors on Parkinson’s disease pathology.
For more details https://www.ncbi.nlm.nih.gov/pubmed/28840482
Noor NA, Mohammed HS, Mourad IM, Khadrawy YA, AboulEzz HS. A promising therapeutic potential of cerebrolysin in 6-OHDA rat model of Parkinson’s disease. Life sciences. 2016; 155:174-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27210889
A promising therapeutic potential of cerebrolysin in 6-OHDA rat model of Parkinson’s disease.
The 2016 study by Noor, Mohammed, Mourad, Khadrawy, and AboulEzz, published in Life Sciences, investigated the therapeutic potential of cerebrolysin in a 6-OHDA rat model of Parkinson’s disease. The study focused on assessing the effectiveness of cerebrolysin in treating Parkinson’s disease symptoms and its potential as a therapeutic option.
For more details https://www.ncbi.nlm.nih.gov/pubmed/27210889
KalynIaB, Safarova TP, Sheshenin VC, Gavrilova SI. [Comparative efficacy and safety of antidepressant mono- and multimodal therapy in elderly patients with depression (a clinical experience in a psychogeriatric hospital)]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2014; 114(6 Pt 2):20-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25042499.
Comparative efficacy and safety of antidepressant mono- and multimodal therapy in elderly patients with depression (a clinical experience in a psychogeriatric hospital)
The 2014 study by Kalyn, Safarova, Sheshenin, and Gavrilova, published in the Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova, focused on comparing the efficacy and safety of antidepressant mono- and multimodal therapy in elderly patients with depression, based on clinical experiences in a psychogeriatric hospital. This research provides valuable insights into the treatment strategies for depression in the elderly.
For more details https://www.ncbi.nlm.nih.gov/pubmed/25042499
Gharagozli, K., Harandi, A. A., Houshmand, S., Akbari, N., Muresanu, D. F., Vester, J., Winter, S., & Moessler, H. (2017). Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo-controlled, double-blinded, multicenter clinical trial. Journal of medicine and life, 10(3), 153–160.
Efficacy and safety of Cerebrolysin treatment in early recovery after acute ischemic stroke: a randomized, placebo-controlled, double-blinded, multicenter clinical trial
The study by Gharagozli, Harandi, Houshmand, Akbari, Muresanu, Vester, Winter, and Moessler, published in the Journal of Medicine and Life in 2017, is a randomized, placebo-controlled, double-blinded, multicenter clinical trial that examined the efficacy and safety of Cerebrolysin treatment in the early recovery phase after acute ischemic stroke. This research contributes to the understanding of Cerebrolysin’s role in stroke recovery.
For more details https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5652261/
Masliah, E., & Díez-Tejedor, E. (2012). The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders. Drugs of today (Barcelona, Spain : 1998), 48 Suppl A, 3–24. https://doi.org/10.1358/dot.2012.48(Suppl.A).1739716
The pharmacology of neurotrophic treatment with Cerebrolysin: brain protection and repair to counteract pathologies of acute and chronic neurological disorders
The 2012 study by Masliah and Díez-Tejedor, published in “Drugs of Today,” explores the pharmacology of neurotrophic treatment with Cerebrolysin, emphasizing its potential in providing brain protection and aiding in repair processes. This study addresses the effectiveness of Cerebrolysin in combating both acute and chronic neurological disorders, offering insights into its therapeutic role and mechanism of action.
For more details https://doi.org/10.1358/dot.2012.48(Suppl.A).1739716
Fiani, B., Covarrubias, C., Wong, A., Doan, T., Reardon, T., Nikolaidis, D., & Sarno, E. (2021). Cerebrolysin for stroke, neurodegeneration, and traumatic brain injury: review of the literature and outcomes. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 42(4), 1345–1353. https://doi.org/10.1007/s10072-021-05089-2
Cerebrolysin for stroke, neurodegeneration, and traumatic brain injury: review of the literature and outcomes.
The 2021 study by Fiani, Covarrubias, Wong, Doan, Reardon, Nikolaidis, and Sarno, published in Neurological Sciences, provides a comprehensive review of Cerebrolysin’s use in treating stroke, neurodegeneration, and traumatic brain injury. This literature review and analysis of outcomes contribute to the understanding of Cerebrolysin’s therapeutic potential in these neurological conditions.
For more details https://doi.org/10.1007/s10072-021-05089-2
Zhang, C., Chopp, M., Cui, Y., Wang, L., Zhang, R., Zhang, L., Lu, M., Szalad, A., Doppler, E., Hitzl, M., & Zhang, Z. G. (2010). Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke. Journal of neuroscience research, 88(15), 3275–3281. https://doi.org/10.1002/jnr.22495.
Cerebrolysin enhances neurogenesis in the ischemic brain and improves functional outcome after stroke
The 2010 study by Zhang, Chopp, Cui, Wang, Zhang, Lu, Szalad, Doppler, Hitzl, and Zhang, published in the Journal of Neuroscience Research, examines the role of Cerebrolysin in enhancing neurogenesis in the ischemic brain and its impact on functional outcomes after stroke. The study contributes to understanding the therapeutic potential of Cerebrolysin in stroke recovery and rehabilitation.
For more details https://doi.org/10.1002/jnr.22495
Bornstein, N. M., Guekht, A., Vester, J., Heiss, W. D., Gusev, E., Hömberg, V., Rahlfs, V. W., Bajenaru, O., Popescu, B. O., & Muresanu, D. (2018). Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology, 39(4), 629–640. https://doi.org/10.1007/s10072-017-3214-0.
Safety and efficacy of Cerebrolysin in early post-stroke recovery: a meta-analysis of nine randomized clinical trials.
The 2018 study by Bornstein, Guekht, Vester, Heiss, Gusev, Hömberg, Rahlfs, Bajenaru, Popescu, and Muresanu, published in Neurological Sciences, is a meta-analysis of nine randomized clinical trials assessing the safety and efficacy of Cerebrolysin in early post-stroke recovery. This comprehensive analysis synthesizes data from various studies to evaluate the overall impact of Cerebrolysin on stroke recovery.
For more details https://doi.org/10.1007/s10072-017-3214-0
Heiss, W. D., Brainin, M., Bornstein, N. M., Tuomilehto, J., Hong, Z., & Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators (2012). Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke, 43(3), 630–636. https://doi.org/10.1161/STROKEAHA.111.628537.
Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial.
The 2012 study by Heiss, Brainin, Bornstein, Tuomilehto, Hong, and the Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators, published in “Stroke,” was a double-blind, placebo-controlled randomized trial. It focused on the effects of Cerebrolysin in patients with acute ischemic stroke in Asia. The study contributes to the understanding of Cerebrolysin’s role in stroke treatment in this specific demographic.
For more details https://doi.org/10.1161/STROKEAHA.111.628537
Chang, W. H., Lee, J., Shin, Y. I., Ko, M. H., Kim, D. Y., Sohn, M. K., Kim, J., & Kim, Y. H. (2021). Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits. Journal of personalized medicine, 11(6), 545. https://doi.org/10.3390/jpm11060545
Cerebrolysin Combined with Rehabilitation Enhances Motor Recovery and Prevents Neural Network Degeneration in Ischemic Stroke Patients with Severe Motor Deficits.
The 2021 study by Chang, Lee, Shin, Ko, Kim, Sohn, Kim, and Kim, published in the Journal of Personalized Medicine, investigated the impact of combining Cerebrolysin with rehabilitation on motor recovery and prevention of neural network degeneration in ischemic stroke patients with severe motor deficits. This study provides insights into the potential benefits of integrating pharmacological and rehabilitation therapies for stroke recovery.
For more details https://doi.org/10.3390/jpm11060545
Tran, L., Alvarez, X. A., Le, H. A., Nguyen, D. A., Le, T., Nguyen, N., Nguyen, T., Nguyen, T., Vo, T., Tran, T., Duong, C., Nguyen, H., Nguyen, S., Nguyen, H., Le, T., Nguyen, M., & Nguyen, T. (2022). Clinical Efficacy of Cerebrolysin and Cerebrolysin plus Nootropics in the Treatment of Patients with Acute Ischemic Stroke in Vietnam. CNS & neurological disorders drug targets, 21(7), 621–630. https://doi.org/10.2174/1871527320666210820091655
Clinical Efficacy of Cerebrolysin and Cerebrolysin plus Nootropics in the Treatment of Patients with Acute Ischemic Stroke in Vietnam
The 2022 study by Tran, Alvarez, Le, and colleagues, published in CNS & Neurological Disorders Drug Targets, evaluated the clinical efficacy of Cerebrolysin alone and in combination with nootropics for treating patients with acute ischemic stroke in Vietnam. The study provides important insights into treatment strategies for ischemic stroke, particularly in the context of these specific medical interventions.
For more details https://doi.org/10.2174/1871527320666210820091655
Stan, A., Birle, C., Blesneag, A., & Iancu, M. (2017). Cerebrolysin and early neurorehabilitation in patients with acute ischemic stroke: a prospective, randomized, placebo-controlled clinical study. Journal of medicine and life, 10(4), 216–222.
Cerebrolysin and early neurorehabilitation in patients with acute ischemic stroke: a prospective, randomized, placebo-controlled clinical study.
The 2017 study by Stan, Birle, Blesneag, and Iancu, published in the Journal of Medicine and Life, was a prospective, randomized, placebo-controlled clinical study assessing the effects of Cerebrolysin combined with early neurorehabilitation in patients with acute ischemic stroke. This research contributes to the understanding of the potential benefits of integrating pharmacological treatments with rehabilitation strategies in the early stages of stroke recovery.
For more details https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5771251/
Chen, N., Yang, M., Guo, J., Zhou, M., Zhu, C., & He, L. (2013). Cerebrolysin for vascular dementia. The Cochrane database of systematic reviews, (1), CD008900. https://doi.org/10.1002/14651858.CD008900.pub2.
Cerebrolysin for vascular dementia
The 2013 study by Chen, Yang, Guo, Zhou, Zhu, and He, published in The Cochrane Database of Systematic Reviews, conducted a review on the use of Cerebrolysin for treating vascular dementia. This comprehensive analysis aimed to evaluate the effectiveness and safety of Cerebrolysin in this context, contributing to the broader understanding of treatment options for vascular dementia.
For more details https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD008900.pub2/abstract
Lang, W., Stadler, C. H., Poljakovic, Z., Fleet, D., & Lyse Study Group (2013). A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy of combined treatment with alteplase (rt-PA) and Cerebrolysin in acute ischaemic hemispheric stroke. International journal of stroke : official journal of the International Stroke Society, 8(2), 95–104. https://doi.org/10.1111/j.1747-4949.2012.00901.x.
A prospective, randomized, placebo-controlled, double-blind trial about safety and efficacy of combined treatment with alteplase (rt-PA) and Cerebrolysin in acute ischaemic hemispheric stroke.
The 2013 study by Lang, Stadler, Poljakovic, Fleet, and the Lyse Study Group, published in the International Journal of Stroke, was a prospective, randomized, placebo-controlled, double-blind trial. It evaluated the safety and efficacy of a combined treatment with alteplase (rt-PA) and Cerebrolysin in acute ischemic hemispheric stroke. This research provides insights into the potential benefits of this combination therapy in stroke treatment.
For more details https://doi.org/10.1111/j.1747-4949.2012.00901.x
Panisset M, Gauthier S, Moessler H, Windisch M, .Cerebrolysin in Alzheimer’s disease: a randomized, double-blind, placebo-controlled trial with a neurotrophic agent. Journal of neural transmission (Vienna, Austria : 1996). 2002; 109(7-8):1089-104. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12111446.
Cerebrolysin in Alzheimer’s disease: a randomized, double-blind, placebo-controlled trial with a neurotrophic agent
The 2002 study by Panisset, Gauthier, Moessler, and Windisch, published in the Journal of Neural Transmission, was a randomized, double-blind, placebo-controlled trial investigating the efficacy of Cerebrolysin in Alzheimer’s disease. This trial aimed to assess the potential benefits of Cerebrolysin, a neurotrophic agent, in treating Alzheimer’s disease.
For more details https://www.ncbi.nlm.nih.gov/pubmed/12111446
Retrieved from http://www.wfsbp.org/doi/wfsbp2011-abstractscd/en/abstracts/10025.html.
Shabanov PD, Lebedev AA, Pavlenko VP, Ganapol’skiĭ VP. [Comparative study of behavioral effects of cortexin and cerebrolysine upon intraventricular and intraperitoneal administration in rats]. Eksperimental’naia i klinicheskaiafarmakologiia. ; 70(3):13-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17650626
Comparative study of behavioral effects of cortexin and cerebrolysine upon intraventricular and intraperitoneal administration in rats
The study by Shabanov, Lebedev, Pavlenko, and Ganapol’skiĭ, published in Eksperimental’naia i Klinicheskaia Farmakologiia, compares the behavioral effects of cortexin and cerebrolysin administered intraventricularly and intraperitoneally in rats. This research contributes to the understanding of the behavioral impacts of these substances in animal models.
For more details https://www.ncbi.nlm.nih.gov/pubmed/17650626
Krasnoperova MG, Bashina VM, Skvortsov IA, Simashkova NV. [The effect of cerebrolysin on cognitive functions in childhood autism and in Asperger syndrome]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2003; 103(6):15-8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12872620.
The effect of cerebrolysin on cognitive functions in childhood autism and in Asperger syndrome
The study by Krasnoperova, Bashina, Skvortsov, and Simashkova, published in 2003 in the Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova, investigated the effect of cerebrolysin on cognitive functions in children with autism and Asperger syndrome. The study aimed to assess the potential benefits of cerebrolysin in improving cognitive functions in these conditions.
For more details https://www.ncbi.nlm.nih.gov/pubmed/12872620
Chutko LS, Yakovenko EA, Surushkina SY, Kryukova EM, Palaieva SV. [The efficacy of cerebrolysin in the treatment of autism spectrum disorders]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2017; 117(9):71-75. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29053124.
The efficacy of cerebrolysin in the treatment of autism spectrum disorders
The 2017 study by Chutko, Yakovenko, Surushkina, Kryukova, and Palaieva, published in the Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova, explored the efficacy of cerebrolysin in the treatment of autism spectrum disorders. This study contributes to the growing body of research on potential treatments for autism spectrum disorders.
For more details https://www.ncbi.nlm.nih.gov/pubmed/29053124
Cuevas-Olguin R, Roychowdhury S, Banerjee A. Cerebrolysin prevents deficits in social behavior, repetitive conduct, and synaptic inhibition in a rat model of autism. Journal of neuroscience research. 2017; 95(12):2456-2468. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28609577.
Cerebrolysin prevents deficits in social behavior, repetitive conduct, and synaptic inhibition in a rat model of autism
The 2017 study by Cuevas-Olguin, Roychowdhury, and Banerjee, published in the Journal of Neuroscience Research, examined the effects of Cerebrolysin in a rat model of autism. The study focused on how Cerebrolysin impacts social behavior, repetitive conduct, and synaptic inhibition, offering insights into potential therapeutic approaches for autism.
For more details https://www.ncbi.nlm.nih.gov/pubmed/28609577
Sotnikova NY, Gromova OA, Novicova EA. Dual effect of cerebrolysin in children with attention deficit syndrome with hyperactivity: neuroprotection and immunomodulation. Russian journal of immunology : RJI : official journal of Russian Society of Immunology. 2002; 7(4):357-64. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12687248.
Dual effect of cerebrolysin in children with attention deficit syndrome with hyperactivity: neuroprotection and immunomodulation
The 2002 study by Sotnikova, Gromova, and Novicova, published in the Russian Journal of Immunology, investigated the dual effect of Cerebrolysin in children with attention deficit syndrome with hyperactivity. The study focused on both the neuroprotective and immunomodulatory effects of Cerebrolysin, providing insights into its potential therapeutic applications.
For more details https://www.ncbi.nlm.nih.gov/pubmed/12687248
Chutko LS, Yakovenko EA, Surushkina SY, Anisimova TI, Kropotov YD. [Clinical and neurophysiological heterogeneity of attention deficit hyperactivity disorder]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. ; 116(10):117-121. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27845323.
Clinical and neurophysiological heterogeneity of attention deficit hyperactivity disorder
The 2016 study by Chutko, Yakovenko, Surushkina, Anisimova, and Kropotov, published in the Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova, discusses the clinical and neurophysiological heterogeneity of attention deficit hyperactivity disorder (ADHD). This study contributes to the understanding of ADHD’s diverse clinical presentations and underlying neurophysiological mechanisms.
For more details https://europepmc.org/article/med/27845323
Nasiri J, Safavifar F. Effect of cerebrolysin on gross motor function of children with cerebral palsy: a clinical trial. ActaneurologicaBelgica. 2017; 117(2):501-505. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28074392.
Effect of cerebrolysin on gross motor function of children with cerebral palsy: a clinical trial
The 2017 clinical trial by Nasiri and Safavifar, published in Acta Neurologica Belgica, evaluated the effect of cerebrolysin on the gross motor function of children with cerebral palsy. This study contributes to the understanding of potential therapeutic interventions for improving motor function in children with this condition.
For more details https://www.ncbi.nlm.nih.gov/pubmed/28074392
Gershman RN, Vasilenko MA. [Use of cerebrolysin and ATP in treating infantile cerebral paralysis]. Pediatriiaakusherstvo i ginekologiia.. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1228606.
Retrieved from https://cerebralpalsynewstoday.com/2017/06/07/cerebrolysin-can-help-improve-motor-skills-in-cerebral-palsy-patients/.
Retrieved from https://clinicaltrials.gov/ct2/show/NCT02116348
Biesenbach G, Grafinger P, Eichbauer-Sturm G, Zazgornik J. [Cerebrolysin in treatment of painful diabetic neuropathy]. Wiener medizinischeWochenschrift (1946). 1997; 147(3):63-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9173675.
Cerebrolysin in treatment of painful diabetic neuropathy
The 1997 study by Biesenbach, Grafinger, Eichbauer-Sturm, and Zazgornik, published in Wiener Medizinische Wochenschrift, investigated the use of Cerebrolysin in the treatment of painful diabetic neuropathy. This study contributes to the understanding of potential treatment options for managing neuropathic pain in individuals with diabetes.
For more details https://europepmc.org/article/med/9173675
Dong H, Jiang X, Niu C, Du L, Feng J, Jia F. Cerebrolysin improves sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy. Neural Regeneration Research. 2016;11(1):156-162. doi:10.4103/1673-5374.175063. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4774211/
The study by Dong, Jiang, Niu, Du, Feng, and Jia, published in Neural Regeneration Research in 2016, investigated the effects of Cerebrolysin on sciatic nerve dysfunction in a mouse model of diabetic peripheral neuropathy. This research explored the potential benefits of Cerebrolysin in improving nerve function in the context of diabetic peripheral neuropathy.
For more details https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4774211/
-
Masliah E, Armasolo F, Veinbergs I, Mallory M, Samuel W. Cerebrolysin ameliorates performance deficits, and neuronal damage in apolipoprotein E-deficient mice. Pharmacology, biochemistry, and behavior. 1999; 62(2):239-45. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9972690.
Cerebrolysin ameliorates performance deficits, and neuronal damage in apolipoprotein E-deficient mice
The 1999 study by Masliah, Armasolo, Veinbergs, Mallory, and Samuel, published in Pharmacology, Biochemistry, and Behavior, investigated the effects of Cerebrolysin in apolipoprotein E-deficient mice. The study aimed to assess the impact of Cerebrolysin on performance deficits and neuronal damage in this mouse model.
For more details https://www.ncbi.nlm.nih.gov/pubmed/9972690
Keilhoff G, Lucas B, Pinkernelle J, Steiner M, Fansa H. Effects of cerebrolysin on motor-neuron-like NSC-34 cells. Experimental cell research. 2014; 327(2):234-55. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24997385.
Effects of cerebrolysin on motor-neuron-like NSC-34 cells
The 2014 study by Keilhoff, Lucas, Pinkernelle, Steiner, and Fansa, published in Experimental Cell Research, examined the effects of Cerebrolysin on motor-neuron-like NSC-34 cells. The research aimed to investigate the impact of Cerebrolysin on these cells, providing insights into its potential effects on motor neuron function.
For more details https://www.ncbi.nlm.nih.gov/pubmed/24997385
Shchudlo NA, Shchudlo MM, Borisova IV. [The effect of cerebrolysin on the regeneration of the peripheral nerve depending on the scheme of paraneural administration]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2013; 113(12):76-80. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24430040.
The effect of cerebrolysin on the regeneration of the peripheral nerve depending on the scheme of paraneural administration
The 2013 study by Shchudlo, Shchudlo, and Borisova, published in Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova, examined the effect of Cerebrolysin on the regeneration of peripheral nerves, depending on the scheme of paraneural administration. This research contributes to our understanding of how the administration of Cerebrolysin may impact peripheral nerve regeneration.
For more details https://www.ncbi.nlm.nih.gov/pubmed/24430040
Available from https://journals.lww.com/nrronline/Abstract/2011/06180/Cerebrolysin_as_a_nerve_growth_factor_for.10.aspx.
Sharma HS, Sharma A, Mössler H, Muresanu DF. Neuroprotective effects of cerebrolysin, a combination of different active fragments of neurotrophic factors and peptides on the whole body hyperthermia-induced neurotoxicity: modulatory roles of co-morbidity factors and nanoparticle intoxication. International review of neurobiology. 2012; 102:249-76. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22748833.
Neuroprotective effects of cerebrolysin, a combination of different active fragments of neurotrophic factors and peptides on the whole body hyperthermia-induced neurotoxicity: modulatory roles of co-morbidity factors and nanoparticle intoxication
The 2012 study by Sharma, Sharma, Mössler, and Muresanu, published in the International Review of Neurobiology, investigated the neuroprotective effects of Cerebrolysin on whole-body hyperthermia-induced neurotoxicity. The study explored the potential benefits of Cerebrolysin and its modulation by co-morbidity factors and nanoparticle intoxication.
For more details https://www.ncbi.nlm.nih.gov/pubmed/22748833
Sharma A, Muresanu DF, Mössler H, Sharma HS. Superior neuroprotective effects of cerebrolysin in nanoparticle-induced exacerbation of hyperthermia-induced brain pathology. CNS & neurological disorders drug targets. 2012; 11(1):7-25. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22229316.
Superior neuroprotective effects of cerebrolysin in nanoparticle-induced exacerbation of hyperthermia-induced brain pathology
The 2012 study by Sharma, Muresanu, Mössler, and Sharma, published in CNS & Neurological Disorders Drug Targets, investigated the superior neuroprotective effects of Cerebrolysin in the context of nanoparticle-induced exacerbation of hyperthermia-induced brain pathology. The study explored how Cerebrolysin may provide enhanced protection in this scenario.
For more details https://www.ncbi.nlm.nih.gov/pubmed/22229316
Martínez-Laorden E, Hurle MA, Milanés MV, Laorden ML, Almela P. Morphine withdrawal activates hypothalamic-pituitary-adrenal axis and heat shock protein 27 in the left ventricle: the role of extracellular signal-regulated kinase. The Journal of pharmacology and experimental therapeutics. 2012; 342(3):665-75. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22647273.
Morphine withdrawal activates hypothalamic-pituitary-adrenal axis and heat shock protein 27 in the left ventricle
The 2012 study by Martínez-Laorden, Hurle, Milanés, Laorden, and Almela, published in The Journal of Pharmacology and Experimental Therapeutics, examined the effects of morphine withdrawal on the activation of the hypothalamic-pituitary-adrenal (HPA) axis and heat shock protein 27 (HSP27) in the left ventricle of the heart. The study also explored the role of extracellular signal-regulated kinase (ERK) in this process
For more details https://www.ncbi.nlm.nih.gov/pubmed/22647273
Sharma HS, Ali SF, Patnaik R, Zimmermann-Meinzingen S, Sharma A, Muresanu DF. Cerebrolysin Attenuates Heat Shock Protein (HSP 72 KD) Expression in the Rat Spinal Cord Following Morphine Dependence and Withdrawal: Possible New Therapy for Pain Management. Current neuropharmacology. 2011; 9(1):223-35. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21886595.
Cerebrolysin Attenuates Heat Shock Protein (HSP 72 KD) Expression in the Rat Spinal Cord Following Morphine Dependence and Withdrawal: Possible New Therapy for Pain Management
The 2011 study by Sharma, Ali, Patnaik, Zimmermann-Meinzingen, Sharma, and Muresanu, published in Current Neuropharmacology, investigated the effects of Cerebrolysin on the expression of heat shock protein (HSP 72 KD) in the rat spinal cord following morphine dependence and withdrawal. The study explored the potential therapeutic use of Cerebrolysin for pain management.
For more details https://www.ncbi.nlm.nih.gov/pubmed/21886595
Belokrylov GA, Malchanova IV. [Levamin and cerebrolysin as immunostimulants]. Biulleten’ eksperimental’noibiologii i meditsiny. 1992; 113(2):165-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1611065.
Levamin and cerebrolysin as immunostimulants
The 1992 study by Belokrylov and Malchanova, published in Biulleten’ Eksperimental’noi Biologii i Meditsiny, explored the immunostimulant effects of Levamin and Cerebrolysin. The study investigated their potential as immunostimulants, which are substances that enhance the immune system’s activity.
For more details https://www.ncbi.nlm.nih.gov/pubmed/1611065
Govorin NV, Zlova TP, Akhmetova VV, Tarasova OA. [The pathophysiological analysis of cerebrolysin therapy of children with mental developmental delay caused by ecological factors]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 2008; 108(5):51-5. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18577958.
The pathophysiological analysis of cerebrolysin therapy of children with mental developmental delay caused by ecological factors
The 2008 study by Govorin, Zlova, Akhmetova, and Tarasova, published in Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova, conducted a pathophysiological analysis of Cerebrolysin therapy in children with mental developmental delay caused by ecological factors. The study aimed to assess the effects of Cerebrolysin treatment in this specific group of children.
For more details https://www.ncbi.nlm.nih.gov/pubmed/18577958
Sotnikova NY, Gromova OA, Novikova EA, Burtsev EM. Immunoactive Properties of Cerebrolysin. Russian journal of immunology : RJI : official journal of Russian Society of Immunology. 2000; 5(1):63-70. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12687163.
Immunoactive Properties of Cerebrolysin
The 2000 study by Sotnikova, Gromova, Novikova, and Burtsev, published in the Russian Journal of Immunology, focused on the immunoactive properties of Cerebrolysin. The study aimed to investigate how Cerebrolysin affects the immune system, particularly its immunoactive properties.
For more details https://www.ncbi.nlm.nih.gov/pubmed/12687163
Garmanchuk LV, Perepelitsyna EM, SidorenkoMv, Makarenko AN, Kul’chikov AE. [Cytoprotective effect of neuropeptides on immunocompetent cells (in vitro study)]. Eksperimental’naia i klinicheskaiafarmakologiia. ; 72(4):28-32. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19803367
Cytoprotective effect of neuropeptides on immunocompetent cells (in vitro study)
The study conducted by Garmanchuk, Perepelitsyna, Sidorenko, Makarenko, and Kul’chikov, published in Eksperimental’naia i Klinicheskaia Farmakologiia, investigated the cytoprotective effect of neuropeptides on immunocompetent cells in an in vitro study. This research focused on how neuropeptides, which include substances like Cerebrolysin, impact the protection of immunocompetent cells.
For more details https://www.ncbi.nlm.nih.gov/pubmed/19803367
González ME, Francis L, Castellano O. Antioxidant systemic effect of short-term Cerebrolysin administration. Journal of neural transmission. Supplementum. 1998; 53:333-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9700669.
Antioxidant systemic effect of short-term Cerebrolysin administration
The study conducted by González, Francis, and Castellano, published in the Journal of Neural Transmission – Supplementum in 1998, focused on the antioxidant systemic effect of short-term Cerebrolysin administration. The research aimed to investigate how short-term administration of Cerebrolysin affects the antioxidant system in the body.
For more details https://www.ncbi.nlm.nih.gov/pubmed/9700669
Gromova OA, Avdeenko TV, Burtsev EM, Skal’nyĭ AV, Solov’ev OI. [Effects of cerebrolysin on the oxidant homeostasis, the content of microelements and electrolytes in children with minimal brain dysfunction]. Zhurnalnevrologii i psikhiatriiimeni S.S. Korsakova. 1998; 98(1):27-30. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9505400.
Effects of cerebrolysin on the oxidant homeostasis, the content of microelements and electrolytes in children with minimal brain dysfunction
The study conducted by Gromova, Avdeenko, Burtsev, Skal’nyĭ, and Solov’ev, published in Zhurnal Nevrologii i Psikhiatrii imeni S.S. Korsakova in 1998, investigated the effects of Cerebrolysin on the oxidant homeostasis, the content of microelements, and electrolytes in children with minimal brain dysfunction. The research aimed to assess how Cerebrolysin impacts these parameters in children with minimal brain dysfunction.
For more details https://www.ncbi.nlm.nih.gov/pubmed/9505400
-
González ME, Francis L, Castellano O. Antioxidant systemic effect of short-term Cerebrolysin administration. Journal of neural transmission. Supplementum. 1998; 53:333-41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9700669.
Antioxidant systemic effect of short-term Cerebrolysin administration. Journal of neural transmission
The study titled “Antioxidant systemic effect of short-term Cerebrolysin administration” was published in the Journal of Neural Transmission – Supplementum in 1998. Unfortunately, I’m unable to access the specific content of the linked article directly. However, based on the title, it suggests that the study investigated the antioxidant effects of short-term Cerebrolysin administration.
For more details https://link.springer.com/chapter/10.1007/978-3-7091-6467-9_29
Retrieved from http://europepmc.org/abstract/med/9505400.
Huang TL, Huang SP, Chang CH, Lin KH, Sheu MM, Tsai RK. Protective effects of cerebrolysin in a rat model of optic nerve crush. The Kaohsiung journal of medical sciences. 2014; 30(7):331-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24924838.
Protective effects of cerebrolysin in a rat model of optic nerve crush
The study titled “Protective effects of cerebrolysin in a rat model of optic nerve crush” was published in The Kaohsiung Journal of Medical Sciences in 2014. This study investigated the potential protective effects of Cerebrolysin in a rat model of optic nerve crush, a condition that can lead to optic nerve damage and vision impairment.
For more details https://www.sciencedirect.com/science/article/pii/S1607551X1400062X
Retrieved from http://www.roneurosurgery.eu/atdoc/16CostinDCombined.pdf.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








