Health Library
MK 677 Guide 2023 - A Comprehensive Overview
Author: Dr. George Shanlikian, M.D. | Last Updated: January 30th, 2024
- Home
- >
- Health Library
- >
- MK-677 (IBUTAMOREN)
Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Benefits of Ibutamoren
- Key Takeaways of Ibutamoren
- What is Ibutamoren (MK 677)?
- How Ibutamoren (MK 677) Works
- Chemical Structure of Ibutamoren
- Research on Ibutamoren (MK 677)
- Unlocking the Power of MK-677
- Muscle Rebuilding
- Unlocking the Power Bulking Stack
- Optimizing Muscle Growth
- Ibutamoren (MK 677)
- MK 677 vs Other Growth Hormone
- Ibutamoren (MK 677) Dosage
- MK-677 Before and After Results
- Ibutamoren (MK 677) Side Effects
- FAQ
- Blog
- Reference
Table of Contents
- Potential Benefits of Ibutamoren
- Key Takeaways of Ibutamoren
- What is Ibutamoren (MK 677)?
- How Ibutamoren (MK 677) Works
- Chemical Structure of Ibutamoren
- Research on Ibutamoren (MK 677)
- Unlocking the Power of MK-677
- Muscle Rebuilding
- Unlocking the Power Bulking Stack
- Optimizing Muscle Growth
- Ibutamoren (MK 677)
- MK 677 vs Other Growth Hormone
- Ibutamoren (MK 677) Dosage
- MK-677 Before and After Results
- Ibutamoren (MK 677) Side Effects
- FAQ
- Blog
- Reference
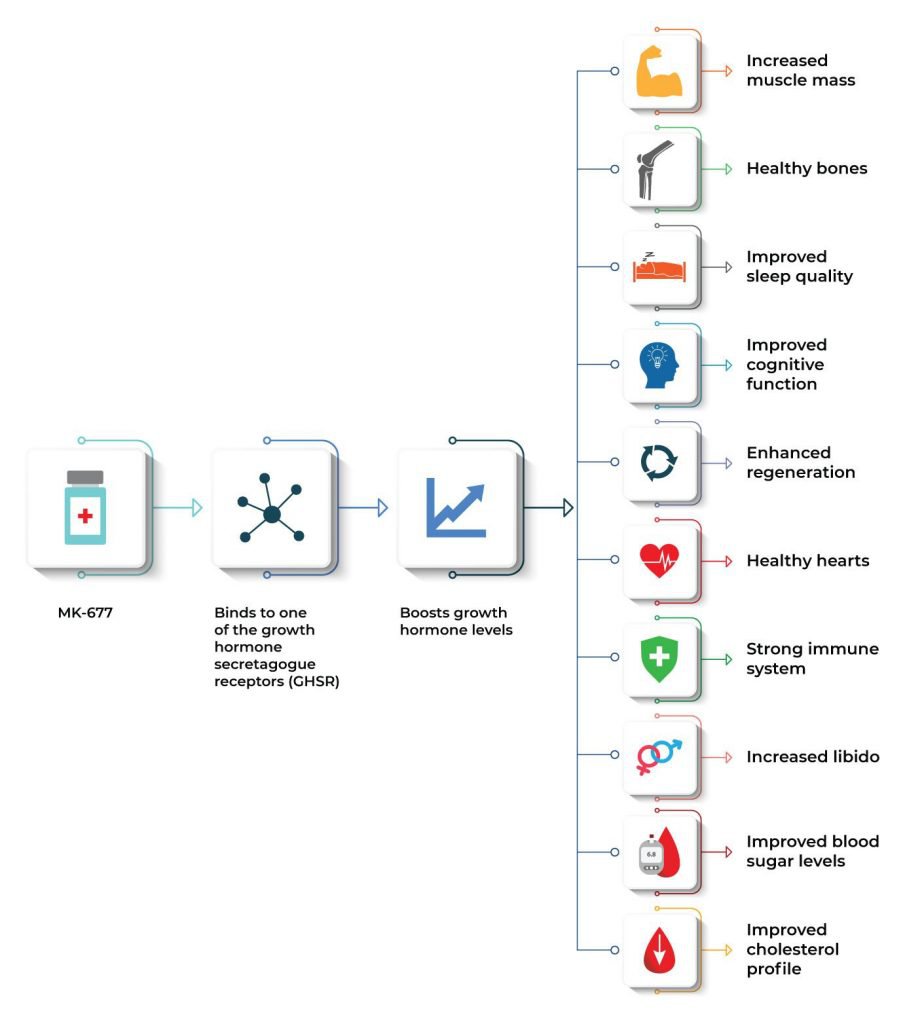
Potential Benefits of Ibutamoren (MK 677)
MK 677 offers a variety of benefits, including improving lean muscle mass, promoting fat loss, maintaining a healthy skeletal frame, enhancing sleep quality, and boosting cognitive function. Additionally, it accelerates wound healing, supports heart health, strengthens the immune system, enhances sexual function and drive, improves blood sugar levels, and positively influences cholesterol profiles.
- Improves lean muscle mass and promotes fat loss [3-26]
- Maintains a healthy skeletal frame [27-56]
- Improves sleep quality [57-81]
- Improves cognitive function [82-136]
- Accelerates wound healing and tissue regeneration [137-150]
- Maintains a healthy heart [151-202]
- Strengthens the immune system [203-240]
- Improves sex drive and sexual function [241-273]
- Improves blood sugar levels [273-308]
- Improves cholesterol profile [309-332]
Key Takeaways of Ibutamoren (MK 677)
- Ibutamoren, commonly known as MK-677, is a growth hormone secretagogue that stimulates the pituitary gland to release more growth hormone and insulin-like growth factor 1 (IGF-1).
- MK-677 benefits include improved muscle mass, fat loss, healthy bones, improved cognitive function, enhanced wound healing and tissue regeneration, healthy heart, strong immune system, improved sex drive and sexual function, improved blood sugar levels, and improved cholesterol profile.
- MK-677 has several potential advantages over anabolic steroids, as it promotes muscle growth, increases bone mineral density, and aids in recovery without the adverse side effects associated with traditional steroids. Unlike traditional anabolic steroids, MK-677 is considered safer and does not suppress natural hormone production, making it a promising option for muscle growth without major side effects.
- The usual recommended dosage range for MK-677 is between 10-25 mg per day, typically administered once daily. However, some individuals may prefer dividing the dosage into two or three smaller administrations. To ensure safety and effectiveness, it is advisable to commence with a lower dose and incrementally adjust until finding the optimal dosage that suits your needs.
- When considering purchasing MK-677, it is crucial to ensure that you obtain it from a legally accredited US pharmacy. It is recommended to do so only under the guidance and prescription of a qualified weight loss doctor who can tailor the dosage and treatment plan specifically for your individual needs and health condition. Buying MK-677 from reputable sources and following your doctor’s instructions will help ensure its quality, safety, and effectiveness in supporting your weight loss journey.
What is Ibutamoren (MK 677)?
MK-677, also known as MK 677, ibutamoren, or ibutamoren mesylate, belongs to a group called growth hormone secretagogues. They are substances that boost growth hormone production. MK-677 can also increase the production of insulin-like growth factor 1 (IGF-1), a hormone similar in molecular structure and function to insulin. The ability of MK-677 to boost the levels of GH and IGF-1 is associated with a wide array of health benefits.
Discover the transformative power of Growth Hormone Boosting Peptides for enhanced muscle growth and recovery. Explore our cutting-edge peptides collection to unlock your body’s full potential. Elevate your fitness journey with science-backed solutions to optimize your growth hormone levels today!
How Ibutamoren (MK 677) Works
The exact mechanism by which Ibutamoren (MK 677) exerts these effects is by mimicking the action of the hunger hormone ghrelin and binding to one of the growth hormone secretagogue receptors (GHSR) in the brain. [1] This in turn boosts growth hormone (GH) levels. Interestingly, GHSR is located in certain regions of the brain that regulate appetite, mood, pleasure, and cognitive function. [2] Because of this, researchers believe that MK-677 can have beneficial effects on these functions.
In addition, MK-677 is also classified as a selective androgen receptor modulator (SARM), a class of therapeutic compounds similar in function to anabolic agents, but with lesser side effects. This makes MK-677 a safe and effective form of GH and IGF-1 replacement therapy.
Chemical Structure of Ibutamoren (MK 677)
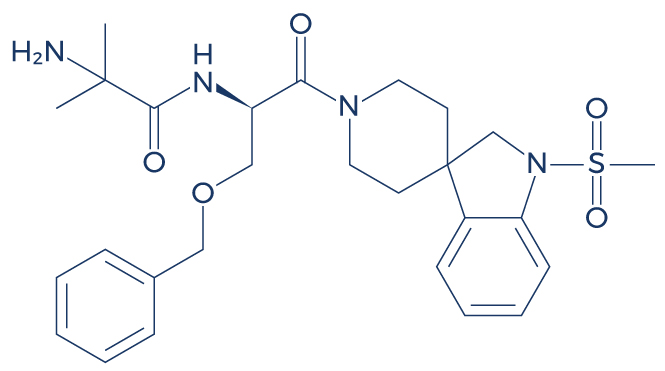
Research on Ibutamoren (MK 677)
A Improves Lean Muscle Mass and Promotes Fat Loss

Ibutamoren (MK 677) can produce a peak GH response by stimulating the release of growth hormone from the pituitary gland, leading to a sudden increase in circulating GH levels in the bloodstream. This peak response is observed shortly after ingestion and contributes to the various benefits associated with MK-677, such as enhanced muscle growth, fat loss, and improved body composition.
MK-677 is frequently used as an anabolic substance, which means that it can produce physical benefits such as increased muscle mass and strength as well as fat loss. Studies show that this powerful compound can help improve body composition and prevent muscle wasting related to old age and other medical conditions:
- In obese males, oral treatment with MK-677 increased fat-free mass by boosting basal metabolic rate (BMR), the rate at which the body uses energy while at rest. [3]
- Several high-quality studies showed that higher GH levels were strongly linked with increased muscle mass and strength in the elderly, suggesting that MK-677 supplementation may have positive effects. [4-13]
- Several lines of evidence also suggested that higher IGF-1 levels were associated with increased muscle mass and strength in the older population. [14-22]
- In patients with muscle wasting, MK-677 reversed diet-induced nitrogen wasting with higher tolerability and without clinically significant adverse experiences. Neither the serum cortisol response nor the PRL response showed a significant increase after 7 days of MK-677 dosing when compared with 7 days of placebo. There was no significant difference in IGF binding protein-2 observed between the MK-677 and placebo treatments. [23]
- In patients recovering from hip fracture, MK-677 treatment improved gait speed and stair climbing power, which is suggestive of improved muscle function. [24]
- In healthy older adults, 12 months of MK-677 treatment significantly increased fat-free mass without any adverse side effects. [25]
- In GH-deficient adults, MK-677 administration improved body composition by increasing IGF-1 levels. [26]
Discover the power of Peptides for Weight Loss – Explore more options now! Take the first step towards your weight loss goals today.
B. Maintains a Healthy Skeletal Frame

MK-677 belongs to a class of drugs known as growth hormone secretagogues, which work by stimulating the pituitary gland to produce and release more growth hormones. This enhanced production of growth hormone, along with the increased release of insulin-like growth factor 1 (IGF-1), plays a crucial role in promoting various physiological processes. One notable benefit of MK-677 is its ability to increase bone mineral density, which can be particularly beneficial for individuals looking to enhance bone health and strength.
Increased growth hormone and IGF-1 improve bone health by stimulating the cells responsible for bone formation, leading to higher bone density and strength. These hormones promote the synthesis of new bone tissue and help maintain bone mass, reducing the risk of fractures and promoting overall bone health. Clinical studies support MK-677’s ability to increase bone density and improve overall bone health:
- In healthy obese males, MK-677 treatment for 2 weeks improved bone turnover (total volume of bone formed and broken down). [27]
- In elderly adults (65 years or older), oral MK-677 treatment daily for 2 weeks improved bone building by increasing osteocalcin, a marker of bone turnover. [28]
- In postmenopausal women with osteoporosis, MK-677 treatment for 12 months improved bone mineral density in the femoral neck. [29]
- In patients with hip fracture (no more than 18 days post-fracture), daily treatment with MK-677 for 6 months resulted in greater improvement in three of four lower extremity functional performance measures and in the ability to live independently compared with placebo treatment. [30]
- A study reported that increased GH can stimulate the mobilization of endothelial progenitor cells (EPCs) from the bone marrow to the sites of injury, suggesting that MK-677 can help repair damaged bones. [31]
- A study found that MK-677 can improve bone health by increasing the activity of osteoblasts (bone cells). [32]
- By increasing GH levels, MK-677 can significantly lower the risk of osteoporosis, fractures, and other bone disorders. [33-45]
- By boosting IGF-1 levels, MK-677 can also help lower the prevalence of various bone disorders. [46-56]
C. Improves Sleep Quality

Enhanced release of growth hormone induced by MK-677 promotes good sleep because growth hormone plays a crucial role in regulating the sleep-wake cycle. It helps improve the quality of sleep by increasing the time spent in deep, restorative sleep stages. Additionally, growth hormone aids in muscle repair and recovery during sleep, leading to a more restful and rejuvenating sleep experience. As a result, individuals taking MK-677 often experience better sleep patterns and overall sleep quality.
The aging population is highly at risk for sleep problems and disorders that can significantly affect the quality of life. Whether it is age-related or caused by a certain medical condition, Ibutamoren (MK 677) supplementation may help improve sleep quality and quantity according to numerous clinical trials:
- In both younger and elderly subjects, MK-677 treatment for 14 days improved sleep quality by increasing rapid eye movement (REM) sleep duration by 50%. [57]
- In healthy older adults, oral administration of MK-677 (25 mg) once daily improved the Pittsburgh Sleep Quality Index, a measure of sleep. [58]
- In normal men, the administration of growth hormone secretagogue increases slow-wave sleep (SWS), which is often referred to as deep sleep. [59]
- In young healthy men, intravenous injections of growth hormone secretagogue during sleep consistently stimulated slow-wave sleep, suggesting that MK-677 may help improve sleep quality. [60]
- In healthy young adults, growth hormone secretagogue administration increases stage 2 sleep. [61]
- Higher GH levels are strongly associated with improved deep sleep stage, indicating that MK-677 supplementation may have positive effects on sleep. [62-67]
- Higher IGF-1 levels are also linked with better sleep, suggesting that MK-677 supplementation may have beneficial effects. [68-78]
- Studies on rats showed that growth hormone secretagogue administration activated sleep-regulatory neurons in the brain. [79]
- In obese patients, growth hormone secretagogue administration improved stage 2 sleep by reversing GH deficiency. [80-81]
D. Improves Cognitive Function
Doctors usually prescribe Ibutamoren (MK 677) for patients suffering from cognitive impairment because of its nootropic benefits. Nootropics are drugs, supplements, and other substances that have the ability to improve certain aspects of cognitive function such as learning, memory, creativity, and motivation. Several high-quality clinical studies support the beneficial effects of MK-677 on various cognitive functions and overall brain health:
- By increasing REM sleep duration and sleep quality, MK-677 can help improve cognitive function. [82-83]
- In older adults, the administration of growth hormone secretagogues improved short-term memory and active problem-solving skills, suggesting that MK-677 may also exert beneficial effects on cognitive function. [84]
- In adults with mild cognitive impairment and healthy older adults, 20 weeks of growth hormone secretagogue administration improved executive function and verbal memory. [85]
- MK-677 has also been found to improve various aspects of cognitive function by increasing IGF-1 levels. [86-90]
- With increased IGF-1 levels, the body activates intracellular pathways involved in the protection of nerve cells in the brain. [91-108]
- MK-677 also protects brain cells against programmed cell death (apoptosis) by increasing ghrelin levels, which in turn prevents cognitive decline. [109-114]
- MK-677 improves cognitive function and prevents age-related cognitive decline by increasing the levels of GH. [115-129]
- The administration of growth hormone secretagogues improves cognitive function by increasing the levels of brain chemicals such as gamma-Aminobutyric acid and N-acetyl-aspartyl-glutamate. [130]
- In healthy older adults, the administration of growth hormone secretagogues helped combat age-related cognitive decline and Alzheimer’s disease. [131]
- In rat models of Alzheimer’s disease, MK-677 administration reduced the formation of abnormal proteins known as amyloid beta (causative agent of AD). [132]
- SARMs such as MK-677 stimulate the growth and development of brain tissues, enhance communication of nerve cells (neurons), and promote brain cell survival. [133-136]
E. Accelerates Wound Healing and Tissue Regeneration
Growth hormone secretagogues such as Ibutamoren (MK 677) have the ability to accelerate the repair of damaged tissues caused by physical trauma or sports-related injuries. There is compelling evidence supporting the regenerative properties of MK-677:
- By boosting GH levels, MK-677 accelerates the migration of cells into the injured or damaged tissues, thus speeding up the wound healing process. [137-139]
- MK-677 can also speed up the wound healing process by increasing skin thickness. [140]
- MK-677 speeds up wound healing by increasing wound collagen content, granulation tissue, and wound tensile strength. [141-143]
- MK-677 increases IGF-1 levels, which in turn stimulates wound healing by increasing cell proliferation and collagen synthesis. [144-147]
- In mice, SARMs administration prevented damage to the central nervous system and improved regeneration by reducing inflammatory substances. [148]
- In rats, growth hormone secretagogue administration improved functional recovery after various injuries by stimulating the formation of new neuronal cells. [149]
- In mice, the administration of growth hormone secretagogue enhanced the healing of skin wounds resulting from trauma, surgery, or disease. [150]
Discover the power of advanced Wound Healing Peptides to accelerate recovery and promote tissue regeneration. Explore our range of cutting-edge products designed to optimize the wound healing process and enhance your body’s natural healing mechanisms. Take the first step towards faster and more effective wound healing today.
F. Maintains a Healthy Heart
Heart disease ranks among the top in terms of mortality worldwide. Interestingly, Ibutamoren (MK 677) possesses potent cardioprotective properties that can help reduce the prevalence of heart disease and the rate of deaths associated with this condition. Recent research suggests that MK-677 and other growth hormone secretagogues can help preserve heart function through various important mechanisms:
- In obese patients, MK-677 treatment improved heart health by reducing low-density lipoprotein (bad cholesterol). [151]
- By increasing ghrelin levels, MK-677 prevents programmed cell death of cardiomyocytes (heart cells). [152-154]
- By increasing GH levels, MK-677 significantly reduces the risk of heart disease and related deaths. [155-164]
- By increasing IGF-1 levels, MK-677 also reduces the risk of heart disease and related deaths. [165-180]
- MK-677 and other growth hormone secretagogues protect against myocardial infarction by activating signaling pathways responsible for self-renewal and survival of cardiac cells. [181-188]
- MK-677 also induces cardiac repair after myocardial infarction. [189-191]
- In animal models, MK-677 and other growth hormone secretagogues stimulated the self-renewal of cardiac stem cells and promoted their survival. [192-195]
- In mice, growth hormone secretagogue administration restored the electrical activity of the heart. [196]
- In rats, growth hormone secretagogue administration protected against heart damage induced by reduced oxygen. [197-201]
- In mice, growth hormone secretagogue administration stimulated the formation of new cardiac tissues. [202]
G. Strengthens the Immune System
Ibutamoren (MK 677) and other growth hormone secretagogues have the ability to enhance the immune response. Research suggests that they play a role in the regulation of immune function through the following important mechanisms:
- In humans, growth hormone secretagogues modulate the immune function through stimulation of the GH/insulin-like growth factor-1 (IGF-I) axis. [203-206]
- In the elderly, both short- and long-term growth hormone secretagogue administration increased the numbers of lymphocytes, monocytes, B-cells, and T-cells. [207]
- Growth hormone secretagogues can also modulate the immune function through brain mechanisms involved in sleep regulation. [208]
- By increasing GH levels, MK-677 helps regulate T cell development, cytokine production, B cell development, antibody production, neutrophil adhesion, monocyte migration, and anti-apoptotic action. [209-210]
- By increasing GH levels, MK-677 also significantly lowers the risk of various diseases related to a compromised immune system. [211-222]
- By increasing IGF-1 levels, MK-677 also significantly lowers the risk of autoimmune diseases and other immune system-related disorders. [223-237]
- By boosting ghrelin levels, MK-677 strengthens the immune system by lowering inflammation. [238]
- In animal models, the administration of growth hormone secretagogues increased the numbers of CD2+ αβ T-cells, CD25+CD4+ cells, and CD4+CD45R+ cells in the immune system. [239-240]
H. Improves Sex Drive and Sexual Function
The ability of Ibutamoren (MK 677) to positively influence the levels of vital hormones such as GH and IGF-1 can help improve sex drive and sexual function. There is strong scientific evidence supporting the beneficial effects of MK-677 and other growth hormone secretagogues on the sexual health of both men and women:
- MK-677 indirectly improves sexual function by increasing GH and IGF-1 levels, which in turn boosts the levels of nitric oxide, a naturally occurring substance that induces harder and longer penile erections. [241-249]
- Another mechanism by which MK-677 improves libido is by increasing testosterone and estrogen levels, which are hormones that regulate sexual desire and sexual function. [250-251]
- By boosting ghrelin levels, MK-677 also helps improve sexual function. [252-255]
- A growing body of clinical evidence suggests that growth hormone deficiency is strongly linked with low libido and erectile dysfunction, suggesting that boosting GH levels through MK-677 supplementation may help ramp up sexual power. [256-264]
- Studies also showed that IGF-1 deficiency is strongly linked with low libido and erectile dysfunction, suggesting that MK-677 may have beneficial effects on sex drive and sexual function. [265-271]
- In healthy postmenopausal women, growth hormone secretagogue supplementation improved libido without any adverse side effects. [272]
- In age-advanced men and women, 4 months of growth hormone secretagogue supplementation improved general well-being and libido. [273]
I. Improves Blood Sugar Levels
Ibutamoren (MK 677) and other growth hormone secretagogues have potent anti-diabetic properties. There is compelling evidence supporting the ability of MK-677 to bring down elevated levels of blood sugar in diabetic patients and animal models:
- In diabetic patients, MK-677 administration improved the body’s response to the effects of insulin, which in turn lowers blood sugar levels. [274]
- In diabetic patients, MK-677 administration significantly reduced blood sugar levels by increasing GH secretion. [275]
- MK-677 boosts IGF-1 levels, which in turn lowers blood sugar levels. [276-287]
- MK-677 also boosts GH levels, which results in a significant reduction in blood sugar levels. [288-301]
- In diabetic rats, growth hormone secretagogue administration enhanced insulin secretion, which in turn lowered blood sugar levels. [302-303]
- In diabetic rats, pre-treatment with growth hormone secretagogue improved insulin secretion and insulin reserve. [304-308]
J. Improves Cholesterol Profile
Chronic elevation in cholesterol levels significantly increases one’s risk of stroke, heart disease, hypertension, and other deadly diseases. According to studies, one can greatly reduce their risk for these debilitating medical conditions by reducing cholesterol levels through Ibutamoren (MK 677) supplementation:
- In obese patients, MK-677 treatment reduced the levels of low-density lipoprotein, which is known as bad cholesterol. [309]
- In older men and women, growth hormone secretagogue administration significantly reduced total cholesterol levels. [310]
- In older adults, growth hormone secretagogue administration reduced low-density lipoprotein levels without any adverse side effects. [311]
- By increasing GH levels, MK-677 can help bring down elevated cholesterol levels. [312-321]
- By boosting IGF-1 levels, MK-677 can also help reduce low-density lipoprotein levels and increase high-density lipoprotein (good cholesterol). [322-331]
- In rats with abnormal cholesterol profiles, growth hormone secretagogue administration reduced cholesterol levels. [332]
Unlocking the Power of MK-677: How Nitrogen Balance Boosts Muscle Growth and Fat Loss
One of the significant benefits of MK-677 is that it promotes an increased nitrogen balance in the body. This refers to the balance between the amount of nitrogen taken in through dietary protein and the amount of nitrogen excreted from the body.
During a cutting phase, when someone is in a caloric deficit, there is an increased risk of muscle catabolism, where the body breaks down muscle tissue for energy. This process can hinder muscle growth and lead to a loss of muscle mass. However, MK-677’s ability to enhance nitrogen balance helps offset this diet-induced catabolism by preserving more muscle tissue.
When you have a positive nitrogen balance (more nitrogen retained than excreted), it signals an environment that promotes muscle growth and repair. By maintaining a positive nitrogen balance during cutting, MK-677 allows individuals to continue building muscle while simultaneously losing fat. This is considered a remarkable feat because typically, losing fat and gaining muscle are two separate and often challenging processes. However, with MK-677’s influence on nitrogen balance, it becomes more achievable to attain these goals simultaneously.
It is essential to note that individual responses to MK-677 can vary, and the effectiveness of this process depends on various factors, including diet, exercise, and overall health. If considering MK-677 or any other supplement, it’s crucial to consult with a healthcare professional to ensure it is safe and appropriate for individual circumstances.
Muscle Rebuilding: Unveiling the Synergistic Power of MK-677, Ghrelin, and Human Growth Hormone for Optimal Gains
MK-677, also known as Ibutamoren, stimulates the release of growth hormone (GH) and interacts with ghrelin receptors, which are the same receptors that the hunger hormone ghrelin binds to. By activating these receptors, MK-677 can mimic the effects of ghrelin in certain tissues. When ghrelin is released, it stimulates appetite and increases food consumption, which can lead to a surplus of nutrients available for muscle growth. Additionally, ghrelin has been found to stimulate the release of growth hormone.
The increase in growth hormone levels brought about by MK-677 and ghrelin can contribute to muscle rebuilding and growth as well as other key benefits through several mechanisms:
- Protein Synthesis: Growth hormone plays a crucial role in promoting protein synthesis in muscles. It enhances the uptake of amino acids into muscle cells, leading to increased protein production and muscle repair.
- Muscle Preservation: MK-677 can help preserve muscle mass during periods of caloric deficit or catabolic states. This is particularly beneficial during cutting phases or when the body is under stress, as it aids in preventing muscle breakdown.
- IGF-1 Production: Growth hormone stimulates the liver to produce insulin-like growth factor 1 (IGF-1). IGF-1 is an anabolic hormone that plays a key role in muscle growth and repair, promoting the proliferation of muscle cells.
- Enhanced Recovery: By promoting deeper and more restful sleep, MK-677 supports better recovery. Adequate rest and recovery are essential for muscle rebuilding and overall athletic performance.
- Energy Metabolism: By enhancing growth hormone and IGF-1, MK-677 encourages the utilization of nutrients like glucose and amino acids for energy production, leading to improved muscle function and performance during physical activities.
It’s important to note that while MK-677 can provide benefits for muscle rebuilding, its effects may vary from person to person, and it should be used responsibly and under proper medical supervision. Additionally, individual responses to MK-677 can be influenced by factors such as diet, exercise regimen, and overall health.
Unlocking the Power of MK-677 Bulking Stack: Amplifying Muscle Growth through Enhanced Nitrogen Balance and Blood Glucose Utilization
The MK-677 bulking stack has gained immense popularity among bodybuilders and fitness enthusiasts for its remarkable ability to unlock the true potential of muscle growth. At the heart of its effectiveness lies the unique combination of enhanced nitrogen balance and blood glucose utilization. MK-677, a growth hormone secretagogue, stimulates the production and release of growth hormone, leading to an increase in insulin-like growth factor 1 (IGF-1). This growth-promoting environment fosters heightened protein synthesis and cellular regeneration, creating the perfect foundation for substantial muscle gains.
One of the key mechanisms through which MK-677 supports muscle growth is by influencing nitrogen balance. Nitrogen, an essential component of proteins, is crucial for building and repairing muscle tissues. MK-677 enhances nitrogen retention in muscle cells, facilitating a positive nitrogen balance, where the body retains more nitrogen than it excretes. This anabolic state allows for increased muscle protein synthesis, promoting faster recovery from workouts and ultimately leading to more significant muscle growth.
Moreover, the bulking stack’s impact on blood glucose utilization plays a pivotal role in fueling muscle development. MK-677 has been shown to increase blood glucose levels, resulting in higher availability of energy for the muscles. As a result, muscle cells are better equipped to uptake glucose, converting it into glycogen, the primary energy source for intense workouts. The surplus energy enables athletes to train harder and longer, breaking through plateaus and experiencing accelerated muscle hypertrophy (growth).
In conclusion, the MK-677 bulking stack harnesses the power of enhanced nitrogen balance and blood glucose utilization to revolutionize muscle growth. By promoting a highly anabolic environment and providing ample energy for intense training, MK-677 paves the way for substantial gains in lean muscle mass. When combined with a well-structured training program and proper nutrition, this stack becomes a potent tool in the arsenal of those seeking to take their physique and performance to new heights.
Optimizing Muscle Growth: The Ideal MK-677 Cycle Length for Maximum Results
The typical MK-677 cycle length ranges from 8 to 12 weeks. However, some individuals may choose to extend it up to 16 weeks, but this should be approached with caution due to potential side effects and diminishing returns on muscle growth beyond a certain point. It is essential to follow the recommended dosage guidelines and cycle length to ensure safety and maximize the benefits of MK-677 without compromising long-term health.
After completing a cycle, it is advisable to take a break and allow the body to recover before considering another cycle. Always consult a healthcare professional or qualified expert before starting any supplement or cycle to ensure it aligns with individual health goals and needs.
Ibutamoren (MK 677): Unlocking the Advantages of a Safer Growth Hormone Secretagogue over Anabolic Steroids
MK-677, also known as Ibutamoren, offers several key advantages as a growth hormone secretagogue that distinguish it from traditional anabolic steroids. These advantages make it a popular choice among bodybuilders and athletes seeking enhanced performance and muscle growth without some of the drawbacks associated with steroids:
- Enhanced Muscle Growth: MK-677 stimulates the production of growth hormone, leading to increased muscle mass and strength. Unlike anabolic steroids, it promotes lean muscle gains without excess fat and unwanted side effects such as water retention and bloating.
- Fat Loss: By boosting growth hormone levels, MK-677 helps facilitate fat loss and improves body composition. It promotes the utilization of stored fat as an energy source, making it an ideal option for those aiming to achieve a leaner physique.
- Safety Profile: Unlike anabolic steroids, MK-677 does not suppress natural testosterone production, reducing the risk of hormone imbalances and post-cycle therapy. It is also non-toxic to the liver, which is a common concern with some oral steroids.
- No Estrogenic Effects: Estrogenic side effects, such as gynecomastia (development of breast tissue in males), are not observed with MK-677. This is a significant advantage over certain anabolic steroids that can lead to estrogen-related complications.
- Joint and Tissue Repair: MK-677 aids in collagen synthesis, benefiting joint and ligament health. This makes it valuable for athletes and bodybuilders who undergo rigorous training and may experience joint stress.
- Oral Administration: MK-677 is taken orally in the form of a pill, eliminating the need for injections common with some anabolic steroids. This ease of administration makes it a more convenient option for many users.
MK 677 vs Other Growth Hormone Boosting Peptides
A. MK 677 vs CJC-1295
MK-677 and CJC-1295 are both peptides that have been shown to increase growth hormone (GH) levels. However, they have different mechanisms of action and may have different effects on the body.
MK-677 is a non-peptide GH secretagogue, which means that it works by mimicking the action of the hormone ghrelin. Ghrelin is a hormone that is produced by the stomach and stimulates the release of GH from the pituitary gland. MK-677 has been shown to increase GH levels by up to 300%.
CJC-1295 is a synthetic growth hormone releasing hormone (GHRH). GHRH is a hormone that is produced by the hypothalamus and stimulates the pituitary gland to release GH. CJC-1295 has been shown to increase GH levels by up to 200%.
In addition to increasing GH levels, MK-677 has also been shown to increase insulin-like growth factor 1 (IGF-1) levels. IGF-1 is a hormone that is produced by the liver and acts on many tissues in the body, including muscle, bone, and fat. IGF-1 is responsible for many of the anabolic effects of GH, such as increased muscle growth and strength.
CJC-1295 does not appear to increase IGF-1 levels to the same extent as MK-677. However, CJC-1295 has been shown to have a longer half-life than MK-677, meaning that it stays in the body for longer and provides a more sustained release of GH.
Overall, MK-677 and CJC-1295 are both effective at increasing GH levels. However, MK-677 appears to have more anabolic effects than CJC-1295, while CJC-1295 has a longer half-life. The best peptide for you will depend on your individual goals and preferences.
B. MK 677 vs Ipamorelin
MK-677 and Ipamorelin are both growth hormone secretagogues (GHS), which means they stimulate the pituitary gland to release growth hormone (GH). However, they have different mechanisms of action and may have different effects on the body.
MK-677 is a non-peptide GHS, which means it is a small molecule that can be taken orally. It works by binding to the ghrelin receptor, which is a receptor that is also activated by the hormone ghrelin. Ghrelin is a hormone that is produced by the stomach and is involved in appetite regulation and growth hormone release. MK-677 has been shown to increase GH levels by up to 300%.
Ipamorelin is a peptide GHS, which means it is a chain of amino acids. It works by binding to the growth hormone secretagogue receptor (GHSR), which is a receptor that is also activated by the hormone GHRH. GHRH is a hormone that is produced by the hypothalamus and is involved in the release of GH from the pituitary gland. Ipamorelin has been shown to increase GH levels by up to 200%.
In addition to increasing GH levels, MK-677 has also been shown to increase insulin-like growth factor 1 (IGF-1) levels. IGF-1 is a hormone that is produced by the liver and acts on many tissues in the body, including muscle, bone, and fat. IGF-1 is responsible for many of the anabolic effects of GH, such as increased muscle growth and strength.
Ipamorelin does not appear to increase IGF-1 levels to the same extent as MK-677. However, Ipamorelin has been shown to have a longer half-life than MK-677, meaning that it stays in the body for longer and provides a more sustained release of GH.
Overall, MK-677 and Ipamorelin are both effective at increasing GH levels. However, MK-677 appears to have more anabolic effects than Ipamorelin, while Ipamorelin has a longer half-life. The best peptide for you will depend on your individual goals and preferences.
C. MK 677 vs Tesamorelin
MK-677 and Tesamorelin are both peptides that have been shown to increase growth hormone (GH) levels. However, they have different mechanisms of action and may have different effects on the body.
MK-677 is a non-peptide GH secretagogue, which means that it works by mimicking the action of the hormone ghrelin. Ghrelin is a hormone that is produced by the stomach and stimulates the release of GH from the pituitary gland. MK-677 has been shown to increase GH levels by up to 300%.
Tesamorelin is a synthetic growth hormone releasing hormone (GHRH). GHRH is a hormone that is produced by the hypothalamus and stimulates the pituitary gland to release GH. Tesamorelin has been shown to increase GH levels by up to 200%.
In addition to increasing GH levels, MK-677 has also been shown to increase insulin-like growth factor 1 (IGF-1) levels. IGF-1 is a hormone that is produced by the liver and acts on many tissues in the body, including muscle, bone, and fat. IGF-1 is responsible for many of the anabolic effects of GH, such as increased muscle growth and strength.
Tesamorelin does not appear to increase IGF-1 levels to the same extent as MK-677. However, Tesamorelin has been shown to have a longer half-life than MK-677, meaning that it stays in the body for longer and provides a more sustained release of GH.
Overall, MK-677 and Tesamorelin are both effective at increasing GH levels. However, MK-677 appears to have more anabolic effects than Tesamorelin, while Tesamorelin has a longer half-life. The best peptide for you will depend on your individual goals and preferences.
D. MK 677 vs Sermorelin
MK-677 and Sermorelin are both peptides that have been shown to increase growth hormone (GH) levels. However, they have different mechanisms of action and may have different effects on the body.
MK-677 is a non-peptide GH secretagogue, which means that it works by mimicking the action of the hormone ghrelin. Ghrelin is a hormone that is produced by the stomach and stimulates the release of GH from the pituitary gland. MK-677 has been shown to increase GH levels by up to 300%.
Sermorelin is a synthetic growth hormone releasing hormone (GHRH). GHRH is a hormone that is produced by the hypothalamus and stimulates the pituitary gland to release GH. Sermorelin has been shown to increase GH levels by up to 200%.
In addition to increasing GH levels, MK-677 has also been shown to increase insulin-like growth factor 1 (IGF-1) levels. IGF-1 is a hormone that is produced by the liver and acts on many tissues in the body, including muscle, bone and fat. IGF-1 is responsible for many of the anabolic effects of GH, such as increased muscle growth and strength.
Sermorelin does not appear to increase IGF-1 levels to the same extent as MK-677. However, Sermorelin has been shown to have a longer half-life than MK-677, meaning that it stays in the body for longer and provides a more sustained release of GH.
Overall, MK-677 and Sermorelin are both effective at increasing GH levels. However, MK-677 appears to have more anabolic effects than Sermorelin, while Sermorelin has a longer half-life. The best peptide for you will depend on your individual goals and preferences.
Ibutamoren (MK 677) Dosage
The typical dosage of MK-677 is 10-25 mg per day. It is typically taken once per day, but some people may choose to split the dose into two or three smaller doses. It is important to start with a low dose and gradually increase it to find the best dosage for you.
There is no one-size-fits-all answer to the question of how much MK-677 you should take. The best way to determine the right dosage for you is to talk to your doctor or a qualified healthcare professional. They can help you assess your individual needs and recommend a dosage that is safe and effective for you.
Here are some things to keep in mind when determining your MK-677 dosage:
- Your weight: The dosage of MK-677 may need to be adjusted based on your weight.
- Your age: Older adults may need a lower dosage than younger adults.
- Your health status: If you have any health conditions, such as diabetes or high blood pressure, you may need to start with a lower dosage and increase it more slowly.
- Your goals: If you are taking MK-677 for muscle growth, you may need a higher dosage than if you are taking it for other purposes, such as increasing your appetite or improving your sleep.
MK-677 Before and After Results
About Dr. George Shanlikian
Dr. George Shanlikian, renowned as the world’s best hormone therapy doctor, possesses expertise in various medical domains. These include Bio-Identical Hormone Replacement Therapy, Peptide Replacement Therapy, Anti-Aging Medicine, Regenerative Medicine, Stress Management, Nutrition Consulting, Nutritional Supplement Consulting, and Exercise Consulting.
Read more about him here: https://www.genemedics.com/dr-george-shanlikian-md-best-hormone-therapy-doctor
Read more success stories here:
Men’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/mens-success-stories/
Women’s Success Stories: https://www.genemedics.com/about-ghi/ghi-success-stories/womens-success-stories/
Ibutamoren (MK 677) Side Effects
MK-677 side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on MK-677. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of MK-677. Despite this, it was listed as a side effect associated with MK-677 even though these associated side effects are very uncommon.
Side effects associated with MK-677 may include the following:
- Headaches
- Increased appetite
- Increased blood sugar levels
- Increased insulin levels
- Increased levels of the stress hormone cortisol
- Sleepiness
- Mild lower extremity edema
- Muscle pain
FAQ
How long does it take to see the effects of MK-677?
The time it takes to see the effects of MK-677 can vary from person to person. Some individuals may start noticing certain effects, such as improved sleep patterns, within the first two weeks of taking MK-677. However, for other benefits like increased muscle mass, fat loss, increased bone density, or body weight gain due to muscle growth, it may take several weeks or even months to become noticeable.
What is the most effective way to take MK-677?
The time it takes to see the effects of MK-677 can vary from person to person. Some individuals may start noticing certain effects, such as improved sleep pattens, within the first two weeks of taking MK-677. However, for other benefits like increased muscle mass or fat loss, it may take several weeks or even months to become noticeable.
MK-677 works by stimulating the release of growth hormones, which can lead to various positive effects on the body. These effects may occur gradually over time as the body responds to the increased growth hormone levels. Patience is important when taking MK-677, as the full benefits may take some time to manifest.
What to know when taking MK-677?
When taking MK-677, there are several important things to know:
- Dosage: Take MK-677 as directed by the product label or your healthcare provider. Typically, it is taken once daily, usually at night.
- Patience: MK-677’s effects may take time to become noticeable. Be patient and consistent with its use to see potential benefits.
- Potential Side Effects: Like any supplement, MK-677 may have side effects. Common ones include increased appetite and blood sugar levels. Talk to your doctor if you experience any unusual symptoms.
- Interactions: Inform your healthcare provider about any other medications or supplements you are taking to check for potential interactions with MK-677.
- Not a Magic Pill: MK-677 can be helpful for some, but it is not a magic solution for fitness or health goals. It works best when combined with a healthy diet and exercise routine.
- Consult a Healthcare Provider: Before starting MK-677 or any new supplement, consult with your healthcare provider to ensure it is safe and appropriate for your specific health needs.
- Individual Responses Vary: Results from MK-677 can differ from person to person. What works for one may not work the same for another.
How much muscle can I gain with MK-677?
The amount of muscle gain you can achieve with MK-677 can vary from person to person. MK-677 is not a muscle-building steroid, but it may help support muscle growth indirectly by increasing growth hormone levels in the body. The extent of muscle gain depends on several factors, including:
- Diet: Consuming enough protein and calories to support muscle growth is essential.
- Exercise: Regular strength training exercises can promote muscle development.
- Individual Response: People may respond differently to MK-677, and genetics play a role in muscle-building potential.
- Duration of Use: The length of time you use MK-677 may influence the results.
Does MK-677 affect sleep?
Yes, MK-677 can affect sleep in some individuals. One of the potential benefits of MK-677 is improved sleep quality. Many people who take MK-677 report experiencing better and deeper sleep, which can contribute to better overall rest and recovery.
Is MK-677 good for cutting?
MK-677 is not specifically designed as a cutting supplement, but it may have some benefits that could be helpful during a cutting phase. Cutting is a term used in bodybuilding and fitness to describe a phase where individuals aim to reduce body fat while maintaining muscle mass.
MK-677, although more commonly known for its bulking properties, offers a unique advantage during cutting phases due to its ability to boost nitrogen balance. This means it effectively counteracts diet-induced protein breakdown and nitrogen loss, preserving valuable muscle mass. In simpler terms, when combined with the right diet, this stack aids in fat shredding without sacrificing muscle, with a focus on preventing diet-induced protein catabolism.
MK-677 may indirectly support a cutting phase in the following ways:
- Muscle Preservation: MK-677 may help preserve lean muscle, which is important during cutting to avoid excessive muscle loss.
- Improved Recovery: Better sleep and potential benefits on muscle recovery could be helpful during intense workouts.
- Increased Metabolism: Some users report increased food intake with MK-677, which could potentially aid in meeting caloric needs during a cutting diet.
Does MK-677 need PCT?
No, MK-677 does not typically require Post Cycle Therapy (PCT). Post Cycle Therapy (PCT) is a process used by some people who have taken anabolic steroids or other performance-enhancing substances. In simple terms, it’s like a “recovery plan” for the body after using these substances. When someone takes steroids, their body’s natural hormone production gets disrupted, and PCT helps the body get back to normal.
PCT usually involves taking different medications or supplements to support the body in restoring its natural hormone balance. The goal of PCT is to minimize side effects, maintain the gains made during steroid use, and help the body recover safely and efficiently.
MK-677 is not a traditional anabolic steroid or hormone. It is a growth hormone secretagogue that stimulates the release of growth hormone and insulin-like growth factor 1 (IGF-1) in the body. Unlike anabolic steroids, MK-677 does not directly interfere with the body’s natural growth hormone production itself, and it does not typically require Post Cycle Therapy (PCT).
Does MK-677 make you heal faster?
MK-677 may potentially help with faster healing indirectly. MK-677 is a growth hormone secretagogue, which means it can increase the body’s production of growth hormones. Growth hormone is known to play a role in the process of tissue repair and regeneration.
By increasing growth hormone levels, MK-677 may support the body’s natural healing processes and contribute to faster recovery from injuries or workouts. It could aid in repairing damaged tissues and promoting overall healing.
Does MK-677 burn fat?
MK-677 is not a fat-burning supplement itself, but it may indirectly support fat loss in some individuals. MK-677 is a growth hormone secretagogue, which means it increases the body’s production of growth hormones. Growth hormone plays a role in metabolism and can help the body break down fats for energy.
By boosting growth hormone levels, MK-677 may assist in maintaining lean muscle during weight loss efforts. More muscle can lead to a higher metabolic rate, which can help burn calories and potentially support fat loss.
Does MK-677 speed up recovery?
Yes, MK-677 may potentially help speed up recovery after workouts or physical activities. MK-677 is a growth hormone secretagogue, which means it increases the body’s production of growth hormones. Growth hormone plays a crucial role in the recovery and repair of tissues and muscles after exercise or injury.
By increasing growth hormone levels, MK-677 may aid in faster recovery from intense workouts, reduce muscle soreness, and enhance overall healing processes. This can allow individuals to bounce back quicker and be ready for their next training session.
How does MK-677 make you feel?
MK-677 can make people feel differently based on their individual responses. Some people may experience positive effects, while others may not notice any significant changes.
The most commonly reported effects of MK-677 include:
- Improved Sleep: Some individuals may experience better sleep quality and feel more rested.
- Increased Appetite: MK-677 can sometimes lead to a greater appetite, which can be beneficial for those looking to gain weight or increase muscle mass.
- Enhanced Recovery: Some users may feel that their body recovers faster after exercise or physical activity.
- Positive Mood: Some individuals may experience an improved sense of well-being and mood since MK-677 interacts with ghrelin receptors, which are primarily found in the brain and play a role in mood and pleasure.
Does MK-677 make you look younger?
MK-677 is not specifically designed to make you look younger, but some people may notice certain skin-related benefits when using it. MK-677 is a growth hormone secretagogue, which means it can increase the body’s production of growth hormones. Growth hormone is known to play a role in collagen production, which is essential for maintaining skin elasticity and firmness.
By boosting growth hormone levels, MK-677 may potentially improve the appearance of the skin, making it look healthier and more youthful. This can include reduced wrinkles and improved skin texture.
Does MK-677 mess with testosterone?
MK-677 does not directly affect testosterone levels. It is a growth hormone secretagogue, which means it promotes the production of growth hormones in the body. Growth hormone and testosterone are separate hormones with different functions.
What is a safe alternative to MK-677?
A safe alternative to MK-677 is to focus on natural methods to support overall health and fitness goals. Some alternatives include:
- Balanced Diet: Eating a nutritious diet that includes a variety of whole foods, lean proteins, healthy fats, and plenty of fruits and vegetables can support your body’s natural functions.
- Regular Exercise: Engaging in regular physical activity, such as cardiovascular exercises, strength training, or other forms of exercise, can help improve overall health and fitness.
- Proper Sleep: Getting enough quality sleep is essential for overall well-being, including muscle recovery and hormonal balance.
- Stress Management: Reducing chronic stress through relaxation techniques, meditation, or hobbies can positively impact health.
- Consult with Healthcare Provider: If you have specific health goals or concerns, talking to a healthcare provider can provide personalized guidance and advice on safe and effective approaches.
Does MK-677 increase bone size?
Yes, MK-677 may potentially increase bone size indirectly. MK-677 is a growth hormone secretagogue, which means it promotes the production of growth hormones in the body. Growth hormone plays a crucial role in the development and maintenance of bones and tissues.
By increasing growth hormone levels, MK-677 may support bone health and increase bone mineral density, which can lead to a potential increase in bone size and strength over time. However, the effects can vary among individuals, and MK-677 is not specifically intended for bone size enhancement.It is important to note that while some studies suggest potential benefits of MK-677 for immune function, more research is needed to understand its effects fully. Additionally, it is important to consult with a healthcare professional before taking any supplement or medication for immune system support.
Does MK-677 raise blood sugar?
Yes, MK-677 may raise blood sugar levels. MK-677 is a growth hormone secretagogue, which means it can increase the production of growth hormones in the body. One of the potential side effects of increased growth hormone levels is the elevation of blood sugar or glucose.
Is MK-677 anti-aging?
MK-677 is often considered to have potential anti-aging benefits. As a growth hormone secretagogue, it can increase the production of growth hormones in the body. Growth hormones are involved in various processes, including tissue repair, muscle growth, and metabolism. Some of these functions are associated with the aging process.
Does MK-677 affect cholesterol?
MK-677 may have some impact on cholesterol levels, but the effects can vary among individuals. MK-677 is a growth hormone secretagogue, which means it can increase the production of growth hormones in the body. Growth hormone is known to influence certain aspects of cholesterol metabolism.
In some cases, MK-677 has been reported to increase high-density lipoprotein cholesterol (HDL-C), which is often referred to as “good cholesterol” because it helps remove cholesterol from the blood vessels. However, it may not significantly affect total cholesterol and low-density lipoprotein cholesterol (LDL-C), often referred to as “bad cholesterol.”
Can a doctor prescribe MK-677?
Yes, a doctor can prescribe MK-677, but it’s essential to understand that MK-677 is not a typical medication or drug approved for most medical conditions. MK-677 is classified as a growth hormone secretagogue, which means it can increase the production of growth hormones in the body.
Does MK-677 lower insulin?
MK-677 may have the potential to influence insulin levels, but the effects can vary among individuals. MK-677 is a growth hormone secretagogue, which means it can increase the production of growth hormones in the body. Growth hormones can interact with insulin and affect how the body uses glucose (sugar).
In some cases, MK-677 has been reported to reduce insulin sensitivity, which means the body may require more insulin to regulate blood sugar levels. This can lead to higher insulin levels in the blood.
What happens if you take too much MK-677?
Taking too much MK-677 can lead to potential side effects and health risks. Since MK-677 is a growth hormone secretagogue, excessive intake may cause unnaturally high levels of growth hormone in the body, which can have various adverse effects.
Some of the possible side effects of taking too much MK-677 may include lethargy, joint pain, numbness, swelling, anxiety, and changes in blood sugar levels. Additionally, elevated levels of growth hormone for prolonged periods may have long-term effects on the body, including potential impacts on bone health, cardiovascular health, and hormone regulation.
Is MK-677 safer than steroids?
MK-677 is generally considered to be safer than traditional anabolic steroids. While both MK-677 and steroids may have an impact on muscle growth, they work through different mechanisms in the body.
MK-677 is a growth hormone secretagogue, which means it stimulates the production of growth hormones in the body. Growth hormone is essential for various physiological processes, including muscle growth, metabolism, and tissue repair. MK-677 does not directly introduce synthetic hormones into the body, and it does not significantly interfere with the body’s natural hormonal balance.
On the other hand, anabolic steroids are synthetic variations of testosterone, and their use can lead to a range of potential side effects, including hormonal imbalances, liver damage, cardiovascular issues, mood swings, and fertility problems, among others. Misuse or abuse of steroids can significantly increase these risks.
Overall, MK-677 is considered to be safer than steroids. However, it is important to note that both substances can have side effects, and it is important to talk to your doctor before taking either one.
How much muscle will I gain on MK-677?
The amount of muscle gained on MK-677 varies from person to person and depends on factors like diet, exercise, and individual response to the compound.
How much MK-677 should I take a day?
The recommended dosage of MK-677 is typically around 10-25mg per day, but it’s essential to follow the specific instructions and guidelines provided by the manufacturer or a healthcare professional.
Does MK-677 make you lean?
MK-677 can potentially help with reducing body fat and increasing muscle mass, which may contribute to a leaner physique in some individuals. MK-677 increases daily nitrogen balance in muscles by enhancing the body’s ability to retain and utilize nitrogen. This leads to a positive nitrogen balance, where the amount of nitrogen taken in exceeds the amount excreted. This favorable nitrogen balance promotes muscle protein synthesis and growth, supporting the development of lean muscle mass over time.
What are the results of MK-677 within 2 months?
Within 2 months of using MK-677, some users may experience improved recovery and increased muscle fullness. However, individual responses may vary. It’s essential to consult a healthcare professional before trying this new diet and study drug.
How long should you stay on MK-677?
The duration of MK-677 use varies based on individual goals and responses. Some people may use it for several months in cycles with breaks in between, while others may follow different protocols.
Can you eat after taking MK-677?
Yes, you can eat after taking MK-677. It is generally taken orally, and there are no specific restrictions on food intake after consumption.
Should I take MK-677 on rest days?
Taking MK-677 on rest days is a personal preference. It can be taken daily for consistent effects, but the timing can be adjusted to suit individual schedules.
Can MK-677 increase height?
MK-677 has been associated with an increase in growth hormone levels, but its impact on height in adults is not well-established. It’s not recommended for height increase in adults as the growth plates have typically closed.
Does MK-677 raise blood sugar?
Yes, MK-677 may increase blood sugar levels in some individuals. Diabetic individuals should use caution and consult with a healthcare professional before using MK-677.
How effective is MK-677?
MK-677 can be effective in increasing growth hormone levels and may have benefits such as improved muscle mass and recovery. However, its effectiveness can vary based on individual response and lifestyle factors.
Does ibutamoren affect testosterone?
Ibutamoren (MK-677) does not significantly affect testosterone levels, but it may increase other growth factors and hormones like IGF-1.
Can you mix MK-677 with juice?
Yes, MK-677 can be mixed with juice or other beverages for consumption, but it’s essential to follow the recommended dosage and instructions for accurate dosing.
Does MK-677 increase energy?
Some individuals may experience increased energy levels as a result of improved recovery and potential effects on growth hormones, but responses may vary.
What is the best stack for MK-677?
The ideal stack for MK-677 depends on individual goals, but it is often combined with other SARMs or compounds to enhance specific benefits like muscle growth or fat loss. Consulting a healthcare professional is advised for personalized advice.
How to prevent insulin resistance with MK-677?
To prevent or minimize the risk of insulin resistance while using MK-677, maintaining a balanced diet, regular exercise, and monitoring blood sugar levels are essential.
What is the alternative to MK-677?
MK-677 is a specific compound, and alternatives may not have identical effects. Other SARMs or compounds with different mechanisms of action might be considered alternatives for specific goals.
Does MK-677 stop testosterone?
MK-677 does not typically suppress testosterone levels. However, it’s essential to use it responsibly and consult a healthcare professional if you have concerns.
Is MK-677 a hormone?
MK-677 is not a hormone itself; it is a growth hormone secretagogue, meaning it stimulates the secretion of growth hormones in the body.
Why does MK-677 make you hungry?
MK-677 can increase appetite as a side effect, which is attributed to its influence on certain hormones that regulate hunger and satiety.
Can I eat after taking MK-677?
Yes, you can eat after taking MK-677. It is generally taken orally, and there are no specific restrictions on food intake after consumption.
Do you lose gains after MK-677?
The gains achieved while using MK-677 may be maintained with proper diet and training even after stopping the use of the compound, though individual results may vary.
Does MK-677 make you bulk?
MK-677 can contribute to increased muscle mass, but its effects may vary based on individual response and overall training and diet plan.
Is MK-677 bulk or cut?
MK-677 can be used for both bulking and cutting phases, depending on individual goals and how it is combined with other compounds.
How long is the MK 677 cycle?
MK-677 cycles can vary in length, but they are typically several weeks long, often ranging from 8 to 12 weeks. Consulting with a healthcare professional is recommended for personalized cycle planning.
What are the benefits of MK 677?
The potential benefits of MK-677 include increased muscle mass, improved recovery, enhanced bone density, and potential fat loss. Individual results may vary.
How to take MK-677 safely?
To take MK-677 safely, follow the recommended dosage and cycle guidelines provided by the manufacturer or a healthcare professional. Regular health monitoring is also essential.
Does MK-677 increase cortisol?
There is some evidence that MK-677 can increase cortisol levels.
Should you take MK-677 on an empty stomach?
Taking MK-677 on an empty stomach may enhance its absorption and effectiveness, but it’s essential to follow the specific instructions provided with the product.
How do I know if MK-677 is working?
While individual responses may vary, signs that MK-677 is working may include improved recovery, better sleep, increased food intake, and potential changes in muscle fullness.
What happens when you take MK-677?
When you take MK-677, it stimulates the secretion of growth hormone and insulin-like growth factor 1 (IGF-1), which may contribute to various potential physical benefits like muscle growth and recovery.
How long does it take for MK-677 to start working?
The effects of MK-677 may become noticeable within a few weeks, but individual response times can vary.
Does MK-677 affect fertility?
The impact of MK-677 on fertility is not well-studied in humans. It’s best to consult with a healthcare professional if you have concerns.
Does MK-677 need PCT?
MK-677 typically does not require post-cycle therapy (PCT) as it does not significantly suppress testosterone production.
How much HGH does MK-677 produce?
MK-677 does not directly produce HGH (human growth hormone), but it stimulates the release of growth hormone, which, in turn, may lead to increased levels of HGH.
When was MK-677 discovered?
MK-677, also known as Ibutamoren, was first developed in the mid-1990s.
Does MK-677 grow bones?
MK-677 has been associated with improved bone density and may have a positive impact on bone health in some individuals.
Does MK-677 increase stamina?
Improved stamina is one of the potential benefits reported by some users of MK-677, but individual responses can vary.
How much strength do you gain on MK-677?
MK-677 can contribute to increased strength, but the extent of strength gains can vary based on individual factors, training, and diet.
Should MK-677 be taken on an empty stomach?
Taking MK-677 on an empty stomach may enhance its absorption and effectiveness, but it’s essential to follow the specific instructions provided with the product.
Can you bulk with MK-677?
Yes, MK-677 can be used for bulking phases to support muscle growth and recovery when combined with appropriate training and nutrition.
What is best to stack with MK-677?
When should I start cutting?
The timing for starting a cutting phase depends on individual goals, current body composition, and desired target weight or physique.
Can you cut fat in 2 weeks?
A significant decrease in fat in just 2 weeks may be challenging and may not be sustainable. A healthy and gradual approach to fat loss is generally recommended for long-term results.
Can you gain muscle while cutting?
Gaining muscle while cutting (body recomposition) is possible, especially for beginners or those with higher body fat levels. However, it becomes more challenging as one gets closer to their genetic potential.
How fast do you see results with MK-677?
Individual responses to MK-677 can vary, but some users may start noticing certain effects within a few weeks of use.
Does MK-677 work immediately?
MK-677 does not work immediately, and it may take some time for its effects to become noticeable.
Does MK-677 speed healing?
MK-677 may potentially aid in the recovery process due to its impact on growth hormone levels, but it should not be used as a replacement for proper medical care for injuries.
How long is the MK677 cycle?
MK-677 cycles are typically several weeks long, often ranging from 8 to 12 weeks. It’s important to follow the specific guidelines provided by the manufacturer or a healthcare professional.
Blog

MK-677 for Muscle Growth and Strength
MK-677, also known as Ibutamoren, is a supplement that has gained popularity for its potential to promote muscle growth and enhance strength. By stimulating the release of growth hormone (GH) and insulin-like growth factor 1 (IGF-1), MK-677 may support anabolic processes and muscle hypertrophy.
MK-677, also known as Ibutamoren, is a supplement that has gained popularity for its potential to promote muscle growth and enhance strength. By stimulating the release of growth hormone (GH) and insulin-like growth factor 1 (IGF-1), MK-677 may support anabolic processes and muscle hypertrophy.
Effects on Muscle Growth and Strength:
Research suggests that MK-677 supplementation can significantly improve muscle mass, lean body mass, and strength. When combined with resistance training, the effects of MK-677 on muscle development may be further enhanced.
Safety Considerations:
Prioritize safety when considering MK-677. Consult with a healthcare professional before use to evaluate individual circumstances and discuss dosage, potential side effects, and any contraindications.
Conclusion: MK-677 shows promise as a supplement for promoting muscle growth and enhancing strength. By stimulating the release of growth hormone and insulin-like growth factor 1, it may support anabolic processes and muscle hypertrophy. Seek professional advice and combine it with proper nutrition, exercise, and rest for optimal results.

MK-677 for Improved Sleep
MK-677, also known as Ibutamoren, is a supplement that has shown potential benefits in promoting improved sleep. Adequate sleep is essential for overall health and well-being, and MK-677 may offer a solution for individuals experiencing sleep difficulties.
MK-677, also known as Ibutamoren, is a supplement that has shown potential benefits in promoting improved sleep quality. Adequate sleep is crucial for overall health and well-being, and MK-677 may offer a solution for individuals experiencing sleep difficulties.
Enhancing Sleep Quality:
Studies have explored the effects of MK-677 for sleep patterns, and findings indicate that supplementation with MK-677 may contribute to enhanced sleep quality. MK-677 has been associated with increased duration of sleep, as well as improvements in rapid eye movement (REM) sleep and deep sleep stages. By positively influencing sleep architecture, MK-677 has the potential to support individuals in achieving more restful and rejuvenating sleep.
Benefits for Sleep Disorders:
Individuals suffering from sleep disorders, such as insomnia or sleep disturbances linked to medical conditions, may find relief with MK-677. The supplement’s ability to regulate sleep patterns and promote better sleep quality can be particularly beneficial in addressing sleep-related issues. While further research is necessary to establish MK-677 as a primary treatment for sleep disorders, initial findings are promising.
Considerations and Precautions:
Before incorporating MK-677 into your routine for sleep improvement, it is crucial to consult with a healthcare professional. They can provide personalized guidance regarding appropriate dosage, potential interactions with other medications, and address any underlying health concerns that may impact sleep quality. It’s important to note that MK-677 should not be used as a substitute for proper sleep hygiene practices, which include maintaining a consistent sleep schedule, creating a comfortable sleep environment, and practicing relaxation techniques before bedtime.
Conclusion: MK-677 shows promise in promoting improved sleep quality and addressing sleep-related issues. By potentially enhancing sleep duration and regulating sleep patterns, MK-677 may contribute to overall well-being and daytime functioning. However, it’s essential to consult with a healthcare professional and prioritize healthy sleep practices to optimize the benefits of MK-677 for sleep quality.

Maximize Muscle Growth with MK-677
When it comes to maximizing muscle growth, fitness enthusiasts are constantly on the lookout for innovative approaches. One compound that has gained significant attention in recent years is MK-677, a growth hormone secretagogue. In this blog, We delve into something into the potential benefits of MK-677 and explore how it can be utilized to optimize muscle growth.
Unlocking the Potential :
MK-677, also known as Ibutamoren, works by stimulating the release of growth hormone and insulin-like growth factor 1 (IGF-1) in the body. These two hormones play a vital role in muscle development and repair. By increasing the levels of these hormones, MK-677 can create an ideal environment for muscle growth. Additionally, this compound enhances nitrogen retention and protein synthesis, facilitating the building of lean muscle mass.
Improved Recovery :
One of the most remarkable benefits of MK-677 is its ability to accelerate recovery. Intense workouts can lead to muscle damage, but MK-677 aids in the repair process. By promoting collagen synthesis and increasing the production of key growth factors, it reduces downtime between workouts. This means you can hit the gym more frequently, leading to increased training volume and ultimately, enhanced muscle growth.
Enhanced Fat Loss:
MK-677’s benefits extend beyond muscle growth. It has been shown to increase basal metabolic rate (BMR), resulting in improved fat oxidation. This means that while you’re packing on muscle, MK-677 also helps shed unwanted body fat. It’s a win-win situation, as a leaner physique not only showcases your hard-earned muscle but also improves overall aesthetics.
Safety and Considerations:
Before incorporating MK-677 into your fitness regimen, it’s important to consult with a healthcare professional. While it generally has a good safety profile, side effects such as increased appetite, water retention, and numbness may occur. Additionally, it’s worth noting that MK-677 is not a substitute for proper nutrition and training. It should be used as an adjunct to a well-rounded fitness program.
Conclusion:
MK-677 presents an exciting opportunity for individuals looking to maximize muscle growth. By stimulating the release of growth hormone and IGF-1, enhancing recovery, and promoting fat loss, it offers a multi-faceted approach to achieving your fitness goals. However, it’s essential to approach its usage responsibly and in consultation with a healthcare professional.

MK-677: Boost Your Fat Burning
If you’re on a mission to shed excess body fat, you may have heard about MK-677. In this blog, we explore how MK-677 can enhance your fat-burning journey and help you achieve your weight loss goals.
Unleashing the Fat-Burning Potential:
MK-677, also known as Ibutamoren, is gaining recognition for its ability to boost fat burning. By increasing growth hormone levels and improving metabolic rate, MK-677 creates an environment conducive to efficient fat metabolism. This compound stimulates lipolysis, the breakdown of stored fat, while also promoting the use of fat for energy during exercise. In combination with a balanced diet and regular exercise, MK-677 can amplify your fat-burning efforts.
Enhanced Energy Expenditure:
One of the key benefits of MK-677 is its ability to increase basal metabolic rate (BMR). With a higher metabolic rate, your body naturally burns more calories throughout the day, even at rest. This elevated energy expenditure supports greater fat loss over time. By incorporating MK-677 into your weight loss journey, you can optimize your body’s calorie-burning potential and accelerate fat loss.
Considerations and Safety:
As with any supplement, it’s important to consult with a healthcare professional before using MK-677. While generally well-tolerated, it may have side effects such as increased appetite or water retention. Stick to recommended dosages and prioritise a balanced lifestyle for best results.
Conclusion:
MK-677 can be a valuable addition to your fat-burning journey. Its ability to boost fat metabolism and increase energy expenditure can accelerate your progress towards achieving a leaner and healthier body composition.

Unveiling the Impact: Side Effects of MK-677
MK-677 is a compound that has gained popularity in the fitness and bodybuilding community due to its potential to promote muscle growth and enhance bone density. However, like any other medication or supplement, it’s important to be aware of potential side effects. In this article, we will explore the possible side effects of MK-677 and provide you with valuable information to make informed decisions about its usage.
Common Side Effects of MK-677
While MK-677 is generally well-tolerated, some individuals may experience certain side effects. It’s important to note that these effects can vary from person to person. Here are some common side effects associated with MK-677:
Increased appetite: MK-677 may stimulate your hunger, leading to an increase in appetite. It’s essential to maintain a balanced diet and make healthy food choices to prevent excessive calorie intake.
Water retention: Some users may experience mild water retention while taking MK-677. It’s crucial to stay adequately hydrated and monitor your fluid intake.
Fatigue and lethargy: MK-677 may cause temporary feelings of fatigue and lethargy. Ensure you prioritise proper rest and recovery to combat these symptoms.
Mild muscle pain: In some cases, individuals may experience mild muscle pain as a side effect of MK-677. Incorporating stretching exercises and applying heat to affected areas can help alleviate discomfort.
Tingling or numbness: Tingling sensations or numbness in certain body parts may occur. If you experience these symptoms, it is advisable to consult a healthcare professional.
Rare Side Effects of MK-677
While rare, some individuals may experience the following side effects with MK-677:
Joint pain: MK-677 can occasionally cause joint pain. Proper warm-up exercises and maintaining joint health are essential to mitigate this discomfort.
Elevated blood sugar levels: MK-677 might affect blood sugar levels, so individuals with diabetes or insulin resistance should closely monitor their blood glucose levels.
Insomnia: Difficulty falling asleep or disrupted sleep patterns may occur in some cases. Practicing good sleep hygiene and relaxation techniques can aid in improving sleep quality.
Changes in mood: MK-677 may lead to mood swings or emotional changes in certain individuals. Open communication with a healthcare professional is crucial if such symptoms arise.
Acne and oily skin: MK-677 has the potential to increase acne and skin oiliness. Adopting a proper skincare routine and using suitable cleansing techniques can help manage these effects.
Conclusion
While MK-677 holds promise for muscle growth and performance enhancement, it’s essential to understand the potential side effects associated with its usage. By being aware of these effects, you can make informed decisions and take necessary precautions. Remember to consult with a healthcare professional before starting any new supplement regimen, especially if you have pre-existing medical conditions. Responsible use and adherence to recommended dosages are key to ensuring a safe and productive experience with MK-677.

Ibutamoren MK-677: A Comprehensive Guide to Its Potential Benefits
Ibutamoren MK-677, also referred to as Nutrobal, is a type of SARM that has gained popularity due to its various potential benefits. In this article, we will review its benefits in detail.
Muscle growth:
Ibutamoren MK-677 can stimulate the release of growth hormone (GH) and insulin-like growth factor 1 (IGF-1), which may lead to increased muscle growth and improved body composition.
Recovery:
Ibutamoren MK-677 has the potential to speed up recovery and reduce muscle soreness after intense workouts, making it a favourite among athletes and bodybuilders.
Bone density:
Ibutamoren MK-677 has been shown to have a positive impact on bone health by increasing bone density, which may benefit people with conditions such as osteoporosis.
Anti-aging effects:
Ibutamoren MK-677 may have potential anti-aging effects such as improved skin elasticity, increased collagen production, and better sleep quality.
Metabolism:
Ibutamoren MK-677 has shown promise in increasing metabolism, which may aid in weight loss and improved energy levels.
Cognitive benefits:
Some studies suggest that Ibutamoren MK-677 may have cognitive-enhancing effects, including improved memory and cognitive function.
Minimal side effects:
Ibutamoren MK-677 is generally considered safe and has minimal side effects compared to traditional growth hormone therapies.
In conclusion, Ibutamoren MK-677 has potential benefits in areas such as muscle growth, recovery, bone health, anti-aging effects, metabolism, and cognitive function. However, it is essential to consult with a healthcare professional and follow recommended dosages for safe and effective use.
MK-677: Unveiling Benefits & Side Effects!
MK-677, also known as Ibutamoren, is a synthetic compound classified as a growth hormone secretagogue. It has garnered attention for its potential as a performance-enhancing drug, boasting remarkable benefits in muscle growth, fat loss, and anti-aging properties.
Discover the Benefits:
Muscle Growth Amplified:
MK-677 stimulates protein synthesis, fostering lean muscle mass development and enhancing strength. It has shown effectiveness in both young and elderly individuals.
Shredding Fat:
By revving up the body’s metabolic rate and promoting fat breakdown, MK-677 aids in shedding excess fat, leading to notable reductions in body fat percentage.
Sleep Soundly:
Experience improved sleep quality and duration with MK-677, which enhances the crucial slow-wave sleep phase, essential for rest and recovery.
Stronger Bones:
MK-677 contributes to increased bone density and strength, benefiting older adults at risk of osteoporosis.
Anti-Aging Potential:
Elevating GH and IGF-1 levels, MK-677 exhibits anti-aging properties by preserving muscle mass, reducing body fat, and promoting youthful skin and hair.
Navigating the Side Effects:
Water Retention Woes: MK-677 may lead to water retention, resulting in temporary bloating and weight gain due to heightened aldosterone levels regulating fluid balance.
Cravings and Appetite:
Some individuals experience increased appetite when using MK-677, potentially posing a challenge for weight loss goals.
Blood Sugar Spikes:
MK-677 can elevate blood sugar levels, necessitating caution for individuals with diabetes or insulin resistance.
Natural Hormone Suppression:
Long-term use of MK-677 may suppress the body’s own GH and IGF-1 production, potentially requiring higher doses to maintain its effects.
In conclusion, MK-677 holds great promise for athletes, bodybuilders, and those seeking overall health enhancement. However, it is vital to acknowledge potential side effects and the limited knowledge surrounding its long-term safety. Remember, individual experiences may vary, and prioritizing safety and well-being remains paramount when considering the use of MK-677 or any supplement.
18. Reference Sources and Summaries
- Meyer RM, Burgos-Robles A, Liu E, Correia SS, Goosens KA. A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fear. Molecular psychiatry. 2014;19(12):1284-1294. doi:10.1038/mp.2013.135.
A ghrelin-growth hormone axis drives stress-induced vulnerability to enhanced fearThe hypothalamus-pituitary-adrenal (HPA) axis is involved in the body’s response to stressors, but their levels do not appear to be
directly linked to stress-related mental illnesses such as posttraumatic stress disorder (PTSD). This suggests that other hormones may be involved in stress-related mental health problems. The researchers investigated the role
of the peptide hormone ghrelin in stress-related vulnerability to fear learning in a rat model of PTSD. The study revealed that stress-related increases in ghrelin levels are both necessary and sufficient for the exacerbation of
fear learning and that these effects occur in the amygdala independently of the HPA axis. The rats were subjected to a stressor that increased ghrelin levels and improved fear memory without increasing corticotropin-releasing factor
(CRF) or corticosterone. Without affecting corticosterone release, blocking ghrelin receptor activity prevented the stress-related enhancement of fear memory. The researchers also looked at the connection between ghrelin and growth
hormone (GH), which is a major downstream effector of the ghrelin receptor. Chronic stress increased GH protein levels in the amygdala and ghrelin receptor stimulation increased GH protein release from amygdala neurons. The fear-inducing
effects of repeated ghrelin receptor stimulation were blocked by overexpression of GH in the amygdala. These findings suggest that ghrelin mediates a novel branch of the stress response and highlights a previously unrecognized
role for ghrelin and growth hormone in maladaptive changes following prolonged stress. By identifying the involvement of ghrelin and GH in the amygdala in stress-induced fear learning, this study provides new insights into the
mechanisms underlying stress-related mental health problems. These findings could lead to the development of new treatments for PTSD and other stress-related disorders by targeting the ghrelin-GH pathway in the amygdala.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3988273/. - Sun Y, Wang P, Zheng H, Smith RG. Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptor. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(13):4679-4684.
doi:10.1073/pnas.0305930101.Ghrelin stimulation of growth hormone release and appetite is mediated through the growth hormone secretagogue receptorThe effects of synthetic agonists of the growth hormone secretagogue receptor (GHSR) on growth
hormone release and body composition in elderly and obese subjects were investigated in this study. The authors discussed ghrelin, a natural agonist of the GHSR that was initially linked to obesity but has yet to be proven to be
a physiologically relevant ghrelin receptor. To further investigate this, the author created Ghsr-null mice, which proved that the GHSR is a biologically relevant ghrelin receptor. The absence of Ghsr, on the other hand, had no
effect on the mice’s appetite or body composition. Furthermore, because ghrelin is assumed to play a role in establishing an insulin-like growth factor 1 set-point for anabolic metabolism, chronic treatment with ghrelin antagonists
may have little effect. The first portion of the study discussed how synthetic GHSR agonists can revitalize the pulsatile pattern of growth hormone release in elderly people and increase lean mass but not fat mass in obese people.
These agonists were discovered by screening tissue extracts in a GHSR-overexpressed cell line. This screening resulted in the discovery of ghrelin, a natural agonist of the GHSR that was initially linked to obesity. However, ghrelin’s
structure differs significantly from the synthetic agonist used to clone the GHSR, and it has yet to be confirmed that the GHSR is a physiologically relevant ghrelin receptor. To investigate this question, the author created Ghsr-null
mice and discovered that acute ghrelin treatment did not stimulate growth hormone release or food intake in these mice, indicating that the GHSR is a biologically relevant ghrelin receptor. However, the absence of Ghsr had no impact
on the mice’s appetite or body composition, implying that ghrelin is not a major regulator of these factors. Furthermore, the finding revealed that ghrelin may play a role in establishing an insulin-like growth factor 1 set-point
for anabolic metabolism. Overall, the study suggests that chronic treatment with ghrelin antagonists may have little effect on growth or appetite. However, the author notes that further research is needed to fully understand the
role of ghrelin and the GHSR in regulating these and other physiological processes. You can read the full article athttps://www.pnas.org/doi/10.1073/pnas.0305930101. - Svensson J, Lönn L, Jansson JO, et al. Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditure. J Clin Endocrinol Metab. 1998;83(2):362-9.
Two-month treatment of obese subjects with the oral growth hormone (GH) secretagogue MK-677 increases GH secretion, fat-free mass, and energy expenditureA study evaluated how the GH secretagogue MK-677 affects GH secretion
and body composition in healthy obese men. The study was double-blind, randomized, parallel, and placebo-controlled. It included 24 obese males between the ages of 18 and 50 with body mass index (BMI) greater than 30 kg/m2 and
waist/hip ratios greater than 0.95. For eight weeks, the participants were given MK-677 25 mg or a placebo. Results showed that MK-677 treatment increased serum insulin-like growth factor I (IGF-I) levels by approximately 40% compared
with placebo. Serum IGF-binding protein-3 levels were also significantly higher. The initial dose of MK-677 significantly increased GH and PRL (peak and area under the curve values). Significant increases in GH and PRL managed
to remain after 2 and 8 weeks of treatment. On the other hand, the increases in GH and PRL after the initial dose were significantly greater than the increases seen after multiple doses. Cortisol levels in the blood and urine did
not increase after 2 or 8 weeks. When measured with dual-energy X-ray absorptiometry, the subjects treated with MK-677 had a significant increase in fat-free mass. Active therapy had no discernible effect on total or visceral fat.
The basal metabolic rate also increased significantly after 2 weeks of MK-677 treatment but not after 8 weeks. Fasting glucose and insulin concentrations remained unchanged, but an oral glucose tolerance test demonstrated impaired
glucose homeostasis (blood sugar balance) at 2 and 8 weeks. The study concluded that a two-month treatment with MK-677 in healthy obese males caused a sustained increase in serum levels of GH,IGF-I, and IGF-binding protein-3. The treatment also caused a sustained increase in fat-free mass and basal metabolic rate. Further studies are still needed to determine whether a higher dose of MK-677 or a
more prolonged treatment period can reduce body fat in people with obesity. You can read the full article athttps://academic.oup.com/jcem/article/83/2/362/2865156?login=false.. - Welle S, Thornton C, Statt M, Mchenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81(9):3239-43.
Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years oldMyofibrillar protein production in the muscles of healthy adults over 60
years old is slower than in younger adults. Previous research has suggested that the slowing of protein synthesis may be prompted by a decrease in the activity of the GH/insulin-like growth factor-I system. A study was conducted
on healthy individuals over 60 years old who were given a single injection of recombinant human GH or a placebo, or three months of either GH or placebo treatment, to see if GH can increase the rate of myofibrillar protein synthesis.
The tracer L-[1-13C]leucine was used to measure the rate of myofibrillar protein synthesis and whole-body protein metabolism. The results showed that GH reduced the whole-body leucine oxidation by 36%, but had no effect on whole-body
protein breakdown or synthesis or myofibrillar protein synthesis in the quadriceps. However, GH treatment for three months led to an increase in lean body mass, muscle mass, and thigh strength in healthy men over 60 years old.
These findings suggest that GH can increase muscle mass and strength in older individuals but it does not restore a youthful rate of myofibrillar protein synthesis. In conclusion, this research reveals that GH may be beneficial
in increasing muscle mass and strength in healthy men over the age of 60. However, it does not appear to be effective in restoring myofibrillar protein synthesis rates that are observed in younger adults. More research is needed
to determine whether GH has similar effects in other populations and whether other interventions can be developed to address the slowing of protein synthesis in older people. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/8784075/.. - Kim KR, Nam SY, Song YD, Lim SK, Lee HC, Huh KB. Low-dose growth hormone treatment with diet restriction accelerates body fat loss, exerts anabolic effect and improves growth hormone secretory dysfunction in obese adults. Horm Res. 1999;51(2):78-84.
Low-dose growth hormone treatment with diet restriction accelerates body fat loss, exerts anabolic effect and improves growth hormone secretory dysfunction in obese adultsThe growth hormone (GH) can speed up fat breakdown
but obese people may have reduced GH secretion, resulting in a loss of lipolytic (fat-burning) effects. While dietary restriction is a popular obesity treatment, it has drawbacks such as poor adherence, protein breakdown, and slow
weight loss. GH tends to increase insulin-like growth factor-I (IGF-I), which has an anabolic (muscle-building) effect. A 12-week study was conducted on 24 obese participants, 12 of whom received recombinant human GH and the other
12 a placebo, to better understand the effects of GH treatment and dietary restriction on fat-burning and muscle-building actions, as well as the effects on insulin and GH secretion. When compared to the placebo group, GH treatment
resulted in a 1.6-fold increase in the percentage of weight loss as fat and a greater reduction in visceral fat (fat around the organs). The placebo group had a negative nitrogen balance and lost lean body mass, whereas the GH
group gained lean body mass and had a positive nitrogen balance. Despite the caloric restriction, GH injections increased IGF-I by 1.6-fold. While all participants’ responses to L-dopa stimulation (a test for GH secretion) were
initially reduced, they improved in both groups after treatment. GH treatment did not lead to a further increase in insulin levels during an oral glucose tolerance test (OGTT) but significantly reduced free fatty acid (FFA) levels
during OGTT. The reduction in FFA levels was positively correlated with visceral fat loss. The study concluded that a low-dose GH with caloric restriction could be a potential therapeutic approach to treat obesity as it accelerates
fat loss, promotes muscle growth, and improves GH secretion. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/10352397/. - Welle S, Thornton C, Statt M, Mchenry B. Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years old. J Clin Endocrinol Metab. 1996;81(9):3239-43.
Growth hormone increases muscle mass and strength but does not rejuvenate myofibrillar protein synthesis in healthy subjects over 60 years oldA study was conducted to determine if using growth hormone (GH) could boost muscle
protein synthesis in healthy adults over the age of 60. The researchers conducted two experiments: one in which they gave the subjects a single injection of GH, and the other in which they gave them GH or a placebo for three months. Previous
studies had suggested that a reduction in the GH/insulin-like growth factor-I system activity could contribute to slower muscle protein synthesis in healthy individuals over the age of 60. Therefore, the researchers hypothesized that GH
could potentially boost protein synthesis rates. The rate of myofibrillar protein synthesis was found to be slower in healthy people over 60 than in young adults. However, neither the immediate nor the three-month use of GH resulted in
any significant increase in myofibrillar protein synthesis rates. The study did discover, however, that using GH for three months resulted in an increase in muscle mass, lean body mass, and thigh strength in healthy men over the age of
60. The GH also decreased whole-body leucine oxidation, implying that it may have some favorable effects on overall protein metabolism. Overall, the study concluded that while GH may not restore a youthful rate of myofibrillar protein
synthesis, it may still have some benefits for improving muscle mass and strength in healthy adults over the age of 60. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/8784075/. - Tavares ABW, Micmacher E, Biesek S, et al. Effects of Growth Hormone Administration on Muscle Strength in Men over 50 Years Old. International Journal of Endocrinology. 2013;2013:942030. doi:10.1155/2013/942030.
Effects of Growth Hormone Administration on Muscle Strength in Men over 50 Years OldGrowth hormone (GH) supplementation is thought to improve physical performance in people who do not have GH deficiency (GHD) by increasing
collagen synthesis in the muscles and tendons, resulting in better exercise training and muscle strength. This suggests that GH therapy could be used to improve muscle strength in healthy elderly people. A study looked into the effect
of GH therapy on muscle strength in healthy men over the age of 50. It included 14 healthy men between the ages of 50 and 70 who were evaluated for body composition and muscle strength using leg press and bench press exercises, which target
the lower and upper body muscles. The subjects were randomly assigned to either GH therapy or a placebo and were reevaluated six months later. The study included thirteen subjects, six in the placebo group and seven in the GH group. Results
showed that both groups were similar at baseline, but after six months of therapy, there was a statistically significant increase in muscle strength in the leg press responsive muscles of the GH group. However, there was no significant
increase in muscle strength in the bench press responsive muscles of either group. Therefore, the study suggests that GH therapy can improve muscle strength in the lower body muscles of healthy men over 50 years old but further research
is needed to evaluate its effectiveness in frail older populations who may experience physical incapacity primarily due to proximal muscle weakness. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC3870652/. - Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. British Journal of Pharmacology. 2008;154(3):557-568. doi:10.1038/bjp.2008.153.
Regulation of muscle mass by growth hormone and IGF-IGrowth hormone (GH) is a popular performance-enhancing drug. One of its main functions is to raise blood levels of insulin-like growth factor I (IGF-I), which is primarily
produced by the liver. GH also stimulates IGF-I synthesis in most non-liver tissues. The effects of GH on promoting body growth after birth are largely dependent on IGF-I but new research suggests that IGF-I-independent functions may exist.
While GH has been shown to help people with GH deficiency, there is currently little evidence to support its anabolic role in increasing muscle mass in healthy people, even when used at supraphysiological levels. However, there could be
other ways that GH improves performance. In contrast, the effects of muscle-specific IGF-I infusion on muscle hypertrophy (an increase in size) have been well-established in animal models and muscle cell culture systems. Many studies examining
the molecular responses to hypertrophic stimuli in both animals and humans have cited an increase in IGF-I messenger RNA or immunoreactivity. Both the circulatory/systemic (endocrine) and local (autocrine/paracrine) effects of GH and IGF-I
may have different impacts on muscle mass regulation. In conclusion, because of its ability to increase IGF-I levels in circulation and stimulate IGF-I synthesis in non-liver tissues, GH is widely used as a performance-enhancing drug.
While GH has been shown to benefit those with GH deficiency, evidence for its anabolic effects on muscle mass in healthy individuals is lacking. The effects of muscle-specific IGF-I infusion on muscle hypertrophy, on the other hand, have
been well documented in animal models and cell culture systems. More research is needed to fully understand how GH and IGF-I affect muscle mass regulation. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC2439518/.. - Weber MM. Effects of growth hormone on skeletal muscle. Horm Res. 2002;58 Suppl 3:43-8.
Effects of growth hormone on skeletal muscleHuman growth hormone (GH) is a common performance-enhancing drug used by athletes and bodybuilders. Its effects on skeletal muscle mass, strength, and fiber composition, however,
is currently unclear. The goal of this study is to provide an overview of the current knowledge about GH’s role in regulating muscle growth and function, as well as to assess its potential as a muscle anabolic hormone. Decreased muscle
mass and strength are common symptoms of GH deficiency that can be effectively treated with GH supplementation. However, the available evidence suggests that GH administration, alone or in combination with strength training, has little
to no effect on muscle volume, strength, or fiber composition in healthy young people who do not have GH deficiency. This compares the lack of evidence for GH’s significant performance-enhancing effects in athletes. Despite this, more
research is needed to identify populations that might benefit from GH treatment, such as frail elderly individuals. In conclusion, while GH supplementation may be useful for treating GH deficiency-related muscle weakness and atrophy (decrease
in size), its effectiveness as a performance-enhancing drug for healthy young individuals is questionable. Further studies are necessary to determine the potential therapeutic benefits of GH for muscle anabolism in specific patient populations.
You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/12435897/. - Rennie M. Claims for the anabolic effects of growth hormone: a case of the Emperor’s new clothes? British Journal of Sports Medicine. 2003;37(2):100-105. doi:10.1136/bjsm.37.2.100.
Claims for the anabolic effects of growth hormone: a case of the emperor’s new clothes?This study looked at how growth hormone impacts adult human metabolism. After careful consideration of the available evidence, it was found
that growth hormone has a significant impact on how the body processes fat and carbohydrates. Growth hormone, in particular, encourages the use of stored fat as an energy source. Except in the case of connective tissue, there is no conclusive
evidence that growth hormone promotes protein retention in the body. Regardless, the perceived benefits of growth hormone for muscle building have been greatly exaggerated, leading to its abuse by athletes and elderly men. This misuse
poses significant health risks for little gain. According to the findings, growth hormone does have a significant impact on adult metabolism. It has been revealed to play a role in fat and carbohydrate processing, leading to an increase
in the use of adipose tissue triacylglycerol as an energy source. Except for connective tissue, the evidence does not support the notion that growth hormone promotes protein retention in the body. As a result, the benefits of growth hormone
for muscle building have been affected, leading to the hormone’s inappropriate use by athletes and elderly men. This misuse poses significant health risks while providing little benefit. In conclusion, the research indicates that growth
hormone does have powerful effects on fat and carbohydrate metabolism in adult humans. However, the idea that it promotes muscle building has been greatly exaggerated, leading to its misuse by athletes and elderly men. This misuse comes
with significant risks to health for little gain. It is important that the true effects of growth hormone are understood to prevent further misuse and ensure that individuals do not put themselves at risk unnecessarily. You can read the
full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC1724606/pdf/v037p00100.pdf. - Johannsson G, Grimby G, Sunnerhagen KS, Bengtsson BA. Two years of growth hormone (GH) treatment increase isometric and isokinetic muscle strength in GH-deficient adults. J Clin Endocrinol Metab. 1997;82(9):2877-84.
Two Years of Growth Hormone (GH) Treatment Increase Isometric and Isokinetic Muscle Strength in GH-Deficient AdultsA lack of growth hormone (GH) in adults has been linked to a loss of muscle mass and strength. The purpose
of this study was to see how two years ofGH treatmentin GH-deficient adults affected muscle performance in comparison to a control group. Using a Kin-Com
dynamometer, the researchers assessed knee extensor and flexor strength for isometric and isokinetic concentric muscle strength. The fatigue index was calculated as the percent reduction in peak torque after 50 repeated isokinetic knee
extensions. In addition, electrical stimulation with a single twitch was used to evaluate its impact on isometric contraction and hand-grip strength. The findings revealed that GH-deficient people had lower isometric knee extensor, knee
flexor, and hand-grip strength than the control group. The mean isometric knee extensor and flexor strengths increased and normalized after two years of GH treatment. Concentric knee flexor and extensor strength increased as well, especially
in younger patients and those with lower baseline muscle strength. Quadriceps endurance, on the other hand, decreased. The effect of GH treatment on isometric contraction and hand-grip strength was unchanged by superimposing single twitches.
Furthermore, the dynamic local muscle fatigue index decreased while hand-grip strength and the degree of lack of maximal motor unit activation on voluntary isometric knee extensor force did not change. In conclusion, the study suggests
that two years ofGH therapyin GH-deficient adults can increase and normalize isokinetic and isometric muscle strength in proximal muscle groups. However,
it may lead to a decrease in quadriceps endurance. Additionally, GH treatment did not affect hand-grip strength or maximal motor unit activation on voluntary isometric knee extensor force. The findings of this study may contribute to the
development of effective GH treatment strategies for adults with GH deficiency. You can read the full article athttps://academic.oup.com/jcem/article/82/9/2877/2865950?login=false.. - Harman SM, Blackman MR. The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and men. Horm Res. 2003;60(Suppl 1):121-4.
The effects of growth hormone and sex steroid on lean body mass, fat mass, muscle strength, cardiovascular endurance and adverse events in healthy elderly women and menGrowth hormone (GH) and insulin-like growth factor 1 (IGF-1)
levels, as well as testosterone in men and estrogen in women, decline as people age. These hormonal changes can reduce muscle strength, lean body mass, and cardiac endurance. However, hormone replacement therapy such as GH, testosterone,
andestrogen/progestin can partially reverse these negative effects. Despite their potential benefits, these treatments can have side effects,
so the safety of hormonal therapy in the elderly population must be evaluated. A research investigation was carried out to assess the effects of GH and sex steroids on body composition, muscle strength, cardiac endurance, and the rate
of adverse events in healthy elderly people. The study found that while GH and sex steroids had positive effects, there was also a high percentage of adverse events after 26 weeks of treatment. It was found that blood levels of IGF-1 correlated
with the total number of GH-related adverse effects. With regards to body weight or blood pressure, there were no changes observed. In women, there was a non-significant onset of blood sugar intolerance but diabetes mellitus did not occur.
In men, 6 developed diabetes. There was a significant increase in blood sugar levels in both men and women at 26 weeks. However, there were no significant increases in hematocrit values and prostate symptom scores. This highlights the
importance of additional research into the safety of hormonal therapy in the elderly population. In conclusion, hormonal changes in the elderly population can lead to reduced muscle strength, lean body mass, and cardiac endurance. Hormone
replacement therapy may offer a solution but the potential adverse effects need to be considered. Further research is required to determine the safety of such treatments in the elderly. You can read the abstract of this article athttps://karger.com/hrp/article/60/Suppl.%201/121/372368/The-Effects-of-Growth-Hormone-and-Sex-Steroid-on.. - Norrelund H, Nair KS, Jorgensen JO, Christiansen JS, Moller N. The protein-retaining effects of growth hormone during fasting involve inhibition of muscle-protein breakdown. Diabetes. 2001;50(1):96-104.
The protein-retaining effects of growth hormone during fasting involve inhibition of muscle-protein breakdownFasting causes the body to go through a series of hormonal and metabolic changes in order to conserve protein. One
such change is an increase in growth hormone serum levels (GH). The purpose of this study was to determine whether GH plays a role in protein conservation during fasting and to identify the mechanisms involved. Eight healthy individuals
were studied under four conditions: 1) in the postabsorptive state, 2) after 40 hours of fasting, 3) after 40 hours of fasting with suppression of GH using somatostatin, and 4) after 40 hours of fasting with suppression of GH and replacement
with exogenous GH. The latter two conditions involved the administration of somatostatin, insulin, glucagon, and GH for 28 hours, followed by an investigation of substrate metabolism during the final 4 hours. Fasting without GH suppression
resulted in a 35% and 70% decrease in IGF-I and free IGF-I, respectively, when compared to the condition with GH replacement. Urinary and serum urea levels increased without GH during fasting, indicating increased protein breakdown. Fasting
with GH suppression resulted in a higher negative phenylalanine balance, indicating greater protein breakdown. The muscle-protein breakdown was also increased during fasting without GH, as shown by a rise in the rate of appearance of phenylalanine.
During fasting without GH, levels of free fatty acids and lipid oxidation decreased. Overall, the study discovered that suppressing GH during fasting increases urea-nitrogen excretion as well as the rate and appearance of phenylalanine
across the forearm. These findings suggest that GH is an important component of protein conservation during fasting, possibly by maintaining circulating concentrations of free IGF-I. A decrease in muscle protein breakdown is the underlying
mechanism. You can read the full article athttps://diabetesjournals.org/diabetes/article/50/1/96/10905/The-Protein-Retaining-Effects-of-Growth-Hormone.. - Adams GR.Insulin-like growth factorin muscle growth and its potential abuse by athletes. Western Journal of Medicine. 2001;175(1):7-9.
Insulin-like growth factor in muscle growth and its potential abuse by athletesSkeletal muscle is a dynamic tissue that constantly adapts to changing physical activity demands. Growth factors and hormones, particularly the
growth hormone/insulin-like growth factor-I (GH/IGF-I) system, orchestrate these adaptations at the local level. Simple notions that exogenous anabolic agents can safely and effectively stimulate or augment muscle mass are, however, incorrect.
Anabolic substances are non-specific in that they affect not only muscle cells but also other cells and tissues. Furthermore, muscle is made up of different cell types that must work together, so a treatment that stimulates muscle cell
hypertrophy (an increase in size) must also strengthen connective tissues, increase angiogenesis (formation of new blood vessels), and improve mitochondrial function. Animal studies have shown that administering GH or IGF-I to mitigate
muscle atrophy (muscle wasting) during unloading conserves the mass of normally weight-bearing muscles but the overall body weight of treated animals increases, resulting in less-normalized muscle mass than untreated animals. In humans,
attempts to augment muscle mass using IGF-I have had less dramatic impacts, and its supplementation has been associated with moderate to severe hypoglycemia (low blood sugar levels), decreased growth hormone secretion, a shift from lipid
to carbohydrate oxidation for energy, and a general disruption of the insulin/glucagon system. Additionally, the biological activity of IGF-I in the body is substantially influenced by the family of IGF binding proteins, which complicates
the issue of augmenting IGF-I. Furthermore, IGF-I is capable of stimulating the proliferation and differentiation of muscle stem cells but it has also been linked to cellular transformation in prostate, colorectal, and lung cancers. As
a result, exogenous IGF-I augmentation is not a particularly appealing or effective method of increasing muscle mass or function. Instead, a more comprehensive approach that takes into account the integrated nature of physiological systems
and the coordinated functioning of various cell types is required. You can read the abstract of this article athttps://bjsm.bmj.com/content/34/6/412.. - Velloso CP. Regulation of muscle mass by growth hormone and IGF-I. British Journal of Pharmacology. 2008;154(3):557-568. doi:10.1038/bjp.2008.153.
Regulation of muscle mass by growth hormone and IGF-IGrowth hormone (GH) is widely used as a performance-enhancing drug. One of the most important effects of GH is an increase in the level of insulin-like growth factor I (IGF-I)
in the blood, which is primarily produced by the liver. GH also promotes IGF-I synthesis in most non-hepatic tissues. GH promotes postnatal body growth through IGF-I but IGF-I-independent functions of GH are beginning to be understood.
Although GH administration has been shown to benefit individuals with GH deficiency, there is currently insufficient evidence to suggest that supraphysiological levels of systemic GH or IGF-I in healthy people’s skeletal muscle play an
anabolic role. It is possible that GH has other performance-enhancing impacts. In contrast, the hypertrophic effects of muscle-specific IGF-I infusion have been well-documented in animal models and muscle cell culture systems. Research
examining the molecular responses to hypertrophic stimuli in animals and humans often refers to upregulation of IGF-I messenger RNA or immunoreactivity. The circulatory/systemic (endocrine) and local (autocrine/paracrine) effects of GH
and IGF-I may have distinct effects on muscle mass regulation. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC2439518/.. - Christoffolete MA, Silva WJ, Ramos GV, et al. Muscle IGF-1-Induced Skeletal Muscle Hypertrophy Evokes Higher Insulin Sensitivity and Carbohydrate Use as Preferential Energy Substrate. BioMed Research International. 2015;2015:282984. doi:10.1155/2015/282984.
Muscle IGF-1-induced skeletal muscle hypertrophy evokes higher insulin sensitivity and carbohydrate use as preferential energy substrateThe purpose of this study was to look at the metabolic profile of mice that had been genetically
modified to have increased muscle mass due to increased mIGF-1 expression (MLC/mIGF-1). The researchers discovered that 6-month-old MLC/mIGF-1 mice weighed more than 6-month-old WT mice and had a higher respiratory quotient, indicating
that they preferred carbohydrates as a fuel source. MLC/mIGF-1 mice also had a faster rate of glucose disposal than WT mice, which was associated with a higher GLUT4 content in the extensor digitorum longus (EDL) muscle. The researchers
observed a threefold upregulation of mRNA content for the glycolysis-related gene PFK-1 in MLC/mIGF-1 animals. Interestingly, the researchers also found a 50% downregulation of PGC1α mRNA levels in the MLC/mIGF-1 mouse EDL muscle, indicating
a lower abundance of mitochondria in this tissue. However, there was no difference in the expression of PPARα and PPARβ/δ, suggesting that key elements of oxidative metabolism were not modulated in these mice. Overall, these findings suggest
that the MLC/mIGF-1 mice rely more heavily on carbohydrates for energy and have increased insulin sensitivity in their hypertrophied skeletal muscle due to a shift in metabolism towards higher carbohydrate utilization. You can read the
full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC4334619/.. - Hamarneh SR, Murphy CA, Shih CW, et al. Relationship Between Serum IGF-1 and Skeletal Muscle IGF-1 mRNA Expression to Phosphocreatine Recovery After Exercise in Obese Men With Reduced GH. The Journal of Clinical Endocrinology and Metabolism. 2015;100(2):617-625.
doi:10.1210/jc.2014-2711. doi:10.1210/jc.2014-2711.Relationship between serum IGF-1 and skeletal muscle IGF-1 mRNA expression to phosphocreatine recovery after exercise in obese men with reduced GHThe purpose of this study was to look into the connection between growth hormone
(GH) and insulin-like growth factor-1 (IGF-1) and skeletal muscle mitochondria in obese subjects with low GH production. The study included fifteen men who were abdominally obese and had low GH secretion. For 12 weeks, these participants
were given recombinant human GH. The researchers used (31)P-magnetic resonance spectroscopy to assess the recovery of phosphocreatine (PCr) as a method of measuring mitochondrial function in skeletal muscle. They also performed percutaneous
muscle biopsies at the beginning and end of the 12-week period to assess mRNA expression of IGF-1 and mitochondrial-related genes. The researchers found an important correlation between skeletal muscle IGF-1 mRNA expression and PCr recovery,
as well as the expression of nuclear respiratory factor-1, mitochondrial transcription factor A, peroxisome proliferator-activated receptor (PPAR) and PPAR mRNA, at the start of the study. However, there was no association between serum
IGF-1 concentration and PCr recovery or any mitochondrial gene expression. The participants’ serum IGF-1 concentrations and muscle IGF-1 mRNA increased after receiving recombinant human GH. An increase in serum IGF-1 was linked to gains
in body fat and lean mass, but not to PCr recovery. However, an increase in muscle IGF-1 mRNA was associated with an improvement in PCr recovery but not body composition parameters. This study demonstrates a new association between skeletal
muscle mitochondria and muscle IGF-1 mRNA expression. The findings also show that this association is independent of serum IGF-1 concentrations. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC4318910/.. - Heszele MF, Price SR. Insulin-like growth factor I: the yin and yang of muscle atrophy. Endocrinology. 2004;145(11):4803-5.
Insulin-Like Growth Factor I: The Yin and Yang of Muscle AtrophySkeletal muscle is the human body’s largest protein reservoir. To maintain muscle mass, a delicate balance of protein synthesis and degradation must be maintained.
A healthy adult generates and diminishes about 1.0-1.5 kg of protein per day, and a small, sustained change in either process can have a significant impact on muscle mass if not balanced by the opposite process. Muscle atrophy, defined
as the unintentional loss of 5-10% of muscle mass, is common in catabolic conditions such as diabetes, cancer, and sepsis (blood infection), and it can lead to a decreased quality of life as well as increased morbidity and mortality. Recent
research has shed light on the molecular mechanisms that underpin muscle wasting. Insulin-like growth factor 1 (IGF-I) and insulin are important muscle mass regulators because they promote growth while inhibiting protein degradation. IGF-I
promotes muscle growth by stimulating muscle satellite cells and their differentiation, and IGF-I and insulin stimulate protein translation in mature muscle cells by activating the mammalian target of rapamycin (mTOR). Muscle wasting can
be caused by low levels of IGF-I and insulin, as well as increased catabolic signals such as glucocorticoids and cytokines. In summary, IGF-I and insulin are crucial regulators of muscle mass and they help maintain muscle mass in healthy
individuals by enhancing protein synthesis and suppressing proteolysis (protein breakdown). In conditions where IGF-I and insulin levels are reduced or their effectiveness is compromised, various proteolytic responses occur, leading to
the destruction of muscle proteins. Studies have indicated that IGF-I may have the potential as an agent to combat muscle atrophy, but more research is needed to understand the mechanisms underlying muscle wasting and to develop effective
treatments. You can read the abstract of this article athttps://academic.oup.com/endo/article/145/11/4803/2499798?login=false. - Shavlakadze T, Chai J, Maley K, et al. A growth stimulus is needed for IGF-1 to induce skeletal muscle hypertrophy in vivo. J Cell Sci. 2010;123(Pt 6):960-71.
A growth stimulus is needed for IGF-1 to induce skeletal muscle hypertrophy in vivoThe researchers investigated the effects of overexpressing Class 2 IGF-1 Ea in skeletal myofibers of both normal and dystrophic (mdx) mice
in this study. Findings revealed that transgenic mice had significantly higher levels of IGF-1 in their muscles, resulting in a significant increase in muscle mass (between 24-56%). However, the hypertrophic effect (an increase in muscle
mass) of IGF-1 was only seen in muscles that were growing or regenerating as a result of endogenous necrosis (tissue death). Normal adult muscles were found to be resistant to the elevated IGF-1, with no increase in muscle mass from 3
to 12 months. In contrast, the dystrophic muscles from mdx/IGF-1(C2:Ea) mice continued to increase in mass during adulthood. This suggests that elevated IGF-1 has a hypertrophic effect on skeletal muscle only in growth situations. Furthermore,
increased Akt phosphorylation at Ser473 was discovered in fasted normal young transgenic muscles but not in fasted normal adult transgenic muscles. The level of phosphorylation in young transgenic muscles was 1.9-fold higher than in age-matched
wild-type controls, and four-fold higher in adult mdx/IGF-1(C2:Ea) muscles than in mdx muscles. This suggests that increased IGF-1 has an effect on muscle growth via the Akt pathway. In addition, the level of mRNA encoding myogenin was
found to be increased in normal young (but not adult) transgenic muscles, indicating enhanced myogenic differentiation. Overall, the results demonstrate that elevated IGF-1 has a hypertrophic effect on skeletal muscle only in growth situations,
and the effect is mediated through the Akt pathway. You can read the full article athttps://journals.biologists.com/jcs/article/123/6/960/31470/A-growth-stimulus-is-needed-for-IGF-1-to-induce. - Zou Y, Dong Y, Meng Q, Zhao Y, Li N. Incorporation of a skeletal muscle-specific enhancer in the regulatory region of Igf1 upregulates IGF1 expression and induces skeletal muscle hypertrophy. Scientific Reports. 2018;8:2781. doi:10.1038/s41598-018-21122-5.
Incorporation of a skeletal muscle-specific enhancer in the regulatory region of Igf1 upregulates IGF1 expression and induces skeletal muscle hypertrophyThe research team accomplished this by engineering an enhancer into the
non-coding regions of the IGF1 gene, specifically in the skeletal muscle. They then verified that the expected changes occurred in a mouse model. The researchers identified three candidate sites with the least evolutionary conservation
to find the best location for introducing the skeletal muscle-specific myosin light chain (MLC) enhancer. They evaluated these sites in C2C12 single-cell colonies that contained the MLC enhancer at each location. They found that IGF1 was
significantly upregulated only in the site 2 single-cell colony series, leading to the formation of extra myotubes. Based on these findings, the researchers decided to use CRISPR/Cas9 methods to develop a genetically modified (GM) mouse
model with the MLC enhancer incorporated into site 2. The IGF1 levels in the GM mice were significantly higher, stimulating downstream pathways in skeletal muscle. Female GM mice exhibited more muscle hypertrophy (an increase in muscle
mass) than male GM mice. The GM mice had comparable circulating IGF1 levels and tibia length to their WT littermates and showed no evidence of heart abnormalities. The researchers concluded that modifying a non-coding region of a gene
is a practical approach to upregulating gene expression and creating animals with desirable traits. This study demonstrates the potential of genetic modification to enhance desired traits in animals, particularly for agriculture or medical
research. You can read the full article athttps://www.nature.com/articles/s41598-018-21122-5.. - Available athttps://eurapa.biomedcentral.com/articles/10.1007/s11556-007-0022-1#:~:text=IGF%2DI%20has%20been%20implicated%20in%20the%20loss%20of%20muscle,than%20noncarriers%20with%20strength%20training..
Alterations in IGF-I affect elderly: role of physical activityThe growth hormone-insulin-like growth factor I (IGF-I) axis is a key regulator in muscle development. The individuals’ ability to synthesize IGF-I may decline
as they age, and exercise may lose its ability to induce the isoform of IGF-I that promotes satellite cell proliferation. Enhanced IGF-I expression in the muscle, on the other hand, can protect against sarcopenia or muscle loss associated
with aging. Strength training is the preferred intervention for sarcopenia prevention and treatment. In older adults, IGF-I expression levels change as a result of strength training, but advancing age, rather than declining serum IGF-I
levels, is the main factor in lifetime changes in body composition in both men and women. It has been concluded that resistive exercise significantly influences muscle mass and function. Physically active individuals have higher levels
of IGF-I than sedentary individuals. Recent research suggests that IGF-I plays an important role as a mediator in muscle hypertrophy (an increase in size) and angiogenesis (the formation of new blood vessels), both of which are characteristics
of the anabolic adaptation of muscles to exercise. You can read the full article athttps://eurapa.biomedcentral.com/articles/10.1007/s11556-007-0022-1#:~:text=IGF%2DI%20has%20been%20implicated%20in%20the%20loss%20of%20muscle,than%20noncarriers%20with%20strength%20training. - Ye F, Mathur S, Liu M, et al. Overexpression of insulin-like growth factor-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuse. Exp Physiol. 2013;98(5):1038-52.
Overexpression of insulin-like growth factor-1 attenuates skeletal muscle damage and accelerates muscle regeneration and functional recovery after disuseSkeletal muscle is a dynamic tissue that reacts to both internal and
external factors such as mechanical loading changes and growth factors. The soleus muscle is especially vulnerable to atrophy (muscle wasting) and damage during periods of inactivity. Previous research has suggested that insulin-like growth
factor-1 (IGF-1) is important in muscle repair and size regulation. Researchers tested the effect of IGF-1 on the soleus muscle by injecting a recombinant IGF-1 cDNA virus into the posterior hindlimbs of mice, with the contralateral limb
serving as a control. After that, the mice were immobilized for two weeks in order to cause muscle atrophy in the soleus and ankle plantarflexor muscle groups. Following this, the mice were allowed to re-ambulate, and the researchers monitored
muscle damage and recovery over a period of 2-21 days. The study’s primary finding was that IGF-1 overexpression reduced reloading-induced muscle damage in the soleus muscle and accelerated muscle regeneration and force recovery. The researchers
used magnetic resonance imaging to assess muscle damage and found that muscle T2, a non-specific marker of muscle damage, was significantly lower in the IGF-1-injected soleus muscles compared to the contralateral muscles at 2 and 5 days
re-ambulation. This reduction in muscle damage was confirmed by histology, which showed a lower percentage of abnormal muscle tissue in the IGF-1-injected muscles at 2 days re-ambulation. The researchers also found evidence of the effect
of IGF-1 on muscle regeneration, including an increase in the number of central nuclei, paired-box transcription factor 7, embryonic myosin, and elevated MyoD mRNA in the IGF-1-injected limbs. These results suggest that IGF-1 may protect
unloaded skeletal muscles from damage while also accelerating muscle repair and regeneration. This research could lead to the development of therapies for people with muscle atrophy or damage, particularly in populations like astronauts
or people with conditions that necessitate prolonged bed rest. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC4937435/.. - Murphy MG, Plunkett LM, Gertz BJ, et al. MK-677, an orally active growth hormone secretagogue, reverses diet-induced catabolism. J Clin Endocrinol Metab. 1998;83(2):320-5.
MK-677, an orally active growth hormone secretagogue, reverses diet-induced catabolismThe study aimed to investigate the potential of MK-677, a nonpeptide mimic of GH-releasing peptide, to reverse protein catabolism induced
by diet. The research used a double-blind, randomized, placebo-controlled, two-period crossover design on eight healthy volunteers aged 24 to 39 years. The subjects were calorically restricted (18 kcal/kg.day) for two 14-day periods, with
either MK-677 (25 mg) or placebo given orally once daily during the last 7 days of each diet period. A 14- to 21-day washout interval between the two periods was implemented. Both groups experienced equivalent daily nitrogen losses during
the first week of caloric restriction. However, when compared to the placebo group, the MK-677 group showed a significant improvement in nitrogen balance during the second week. MK-677 also improved the integrated nitrogen balance compared
to the placebo group over the 7-day treatment period. The study found that MK-677 produced a peak GH response of 55.9 +/- 31.7 micrograms/L after a single dose and 22.6 +/- 9.3 micrograms/L after a week of dosing, while the placebo treatment
peak GH values were approximately 9 and 7 micrograms/L for day 1 and day 7 of treatment, respectively. Following the initial 7-day caloric restriction, IGF-I levels declined in both groups, but the MK-677 group showed a significant increase
in mean IGF-I concentrations during treatment compared to the placebo group. Additionally, MK-677 significantly increased the mean IGF binding protein-3 levels during the last 5 days of treatment compared to the placebo. The study concluded
that MK-677 reverses diet-induced nitrogen wasting and may have therapeutic potential for catabolic patients if the short-term anabolic effects are sustained in patients with acute or chronic disease states that lead to catabolism (muscle
wasting). Furthermore, MK-677 (25 mg) was well tolerated with no clinically significant adverse events. You can read the full article athttps://academic.oup.com/jcem/article/83/2/320/2865101.. - Adunsky A, Chandler J, Heyden N, et al. MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fracture: a multicenter, randomized, placebo-controlled phase IIb study. Arch Gerontol Geriatr. 2011;53(2):183-9.
MK-0677 (ibutamoren mesylate) for the treatment of patients recovering from hip fracture: a multicenter, randomized, placebo-controlled phase IIb studyThe majority of elderly patients hospitalized for hip fractures have functional
decline. Previous research has suggested that MK-0677 may help people with hip fractures recover essentially. In this double-blind study, 123 elderly hip fracture patients were randomly assigned to receive either a daily dose of 25mg of
MK-0677 or a placebo. Changes in objective functional performance measurements and blood insulin-like growth factor-1 (IGF-1) levels were the primary outcomes assessed. After 24 weeks, the MK-0677 group showed a significant increase in
gait speed compared to the placebo group. However, there was no improvement in several other functional performance measures in the MK-0677 group. The MK-0677 group also had fewer falls during the study compared to the placebo group, although
the difference was not statistically significant. Furthermore, IGF-1 levels in the MK-0677 group increased significantly by 51.4 ng/ml compared to the placebo group. The trial, however, was halted early due to safety concerns regarding
congestive heart failure in a small number of patients. As a result, the increase in IGF-1 levels in the MK-0677 group did not translate into an overall improvement in most functional performance measures, and MK-0677 had an unfavorable
safety profile in hip fracture patients. In summary, this study suggests that MK-0677 may have a limited benefit in improving gait speed in elderly hip fracture patients, but the safety concerns associated with this drug in this population
outweigh any potential benefits. Further research is needed to better understand the safety and efficacy of MK-0677 in hip fracture patients. You can read the full article athttps://www.sciencedirect.com/science/article/abs/pii/S0167494310002530?via%3Dihub.. - Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149(9):601-11.
Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trialGrowth hormone secretion begins to decline after puberty, resulting in a loss of muscle mass, which can
eventually lead to sarcopenia (muscle wasting), frailty, loss of function, and decreased quality of life. The role of growth hormone in the development of sarcopenia requires further investigation. The purpose of this study was to see
if the oral ghrelin mimic, MK-677, could increase growth hormone secretion into the range seen in young adults, prevent fat-free mass loss, and reduce abdominal visceral fat in healthy older adults. The study included 65 healthy adults
aged 60 to 81 years old, including men, women onhormone replacement therapy, and women who were not on hormone replacement therapy. The study’s primary endpoints were growth hormone
and insulin-like growth factor I levels, fat-free mass, and abdominal visceral fat after one year of MK-677 treatment. Body weight, fat mass, insulin sensitivity, lipid and cortisol levels, bone mineral density, limb lean and fat mass,
isokinetic strength, function, and quality of life were also measured. All endpoints were assessed at the start and every six months. Results showed that the daily administration of MK-677 significantly increased growth hormone and insulin-like
growth factor I levels to those observed in healthy young adults without any serious adverse effects. The placebo group showed a decrease in mean fat-free mass while the MK-677 group showed an increase in fat-free mass. The increase in
limb fat was also greater in the MK-677 group than in the placebo group while there were no significant differences in abdominal visceral fat or total fat mass. Body weight increased in the MK-677 group compared to the placebo group. The
most common side effects of MK-677 were a temporary increase in appetite and transient, mild lower-extremity edema (swelling), and muscle pain. In addition, fasting blood glucose (blood sugar) levels increased by 0.3 mmol/L (5 mg/dL) on
average in the MK-677 group while insulin sensitivity decreased. Cortisol levels and pulsatile growth hormone secretion were increased in MK-677 recipients and bone mineral density changed, indicating increased bone remodeling. Increased
fat-free mass had no effect on strength or function. Overall, MK-677 treatment was generally well tolerated over a 12-month period. In conclusion, MK-677 can boost pulsatile growth hormone secretion and increase fat-free mass with higher
tolerability. Long-term studies are required to assess the functional and economic benefits of MK-677 in the elderly. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC2757071/.. - Chapman IM, Pescovitz OH, Murphy G, et al. Oral administration of growth hormone (GH) releasing peptide-mimetic MK-677 stimulates the GH/insulin-like growth factor-I axis in selected GH-deficient adults. J Clin Endocrinol Metab. 1997;82(10):3455-63.
Oral administration of growth hormone (GH) releasing peptide-mimetic MK-677 stimulates the GH/insulin-like growth factor-I axis in selected GH-deficient adultsA study was conducted to investigate the impact of the GH releasing
peptide (GHRP)-mimetic, MK-677, on the GH/insulin-like growth factor-I (IGF-I) axis in a selected group of severely GH-deficient men who had been treated for GH deficiency during childhood. The study involved nine men aged between 17 and
34 years, with a peak serum GH concentration in response to insulin-induced hypoglycemia (low blood sugar levels). The subjects received once-daily oral doses of 10 or 50 mg MK-677 or placebo for four days separated by at least 28 days.
Blood samples were taken every 20 minutes for 24 hours prior to treatment and at the end of each period to measure GH using an ultrasensitive assay. The study found that the drug was generally well-tolerated with no significant changes
from baseline in circulating concentrations of cortisol, PRL, andthyroid hormones.The serum IGF-I and 24-H mean GH concentrations increased in all subjects after
treatment with both 10 and 50 mg/day MK-677 vs. baseline. After treatment with 10 mg MK-677, the IGF-I and GH concentrations increased. Serum IGF binding protein-3 concentrations also increased with both 10 mg and 50 mg MK-677. The GH
response to MK-677 was greater in subjects who were the least GH/IGF-I deficient at baseline. Fasting and postprandial insulin and postprandial glucose increased significantly after MK-677 treatment. According to the findings, oral administration
of GHRP-mimetic compounds like MK-677 may play a role in the treatment of GH deficiency of childhood onset. Longer-term studies are needed, however, to determine the clinical significance of the changes observed in this study. You can
read the full article athttps://academic.oup.com/jcem/article/82/10/3455/2823475?login=false. - Svensson J, Ohlsson C, Jansson JO, et al. Treatment with the oral growth hormone secretagogue MK-677 increases markers of bone formation and bone resorption in obese young males. J Bone Miner Res. 1998;13(7):1158-66.
Treatment with the oral growth hormone secretagogue MK-677 increases markers of bone formation and bone resorption in obese young malesThis study aimed to determine the effects of an oral growth hormone secretagogue called
MK-677 on markers of bone metabolism in healthy obese males. The study was conducted on 24 males, aged 19-49 years, with a body mass index (BMI)https://www.nhlbi.nih.gov/health/educational/lose_wt/BMI/bmicalc.htmgreater
than 30 kg/m2. The participants were randomly assigned to either receive MK-677 (25mg/day) or a placebo for 8 weeks. The findings revealed that MK-677 treatment had a significant effect on markers of bone formation and resorption (bone
breakdown). MK-677 increased carboxy-terminal propeptide levels of type I procollagen by 23% and procollagen III peptide levels by 28% in just two weeks compared to the placebo group. However, it took 8 weeks of treatment to detect a 15%
increase in the blood levels of osteocalcin (maintains and regenerates bone tissue). In addition to this, markers of bone resorption were also induced within 2 weeks of treatment with MK-677. The serum levels of carboxy-terminal cross-linked
telopeptide of type I collagen were increased by 26% at 8 weeks compared to the placebo group. Moreover, urine hydroxyproline/creatinine and calcium/creatinine ratios at 8 weeks were increased by 23% and 46% compared to the placebo group.
The study also found that MK-677 increased the serum insulin-like growth factor binding protein-5 (IGFBP-5) by 43-44% after 2-8 weeks of treatment compared to the placebo group. The serum IGFBP-4 was increased by 25% after 2 weeks of treatment
compared to the placebo group, but there was no significant change from baseline after 8 weeks of treatment. Plasma interleukin-6, however, was not significantly changed by active treatment. In conclusion, short-term MK-677 treatment can
increase markers of bone resorption and formation. The treatment can also produce significant increases in the serum levels of IGF-1 and IGFBP-5, and a transient increase in serum IGFBP-4. More research is needed to determine whether long-term
MK-677 treatment can result in an increase in bone mass. You can read the full article athttps://asbmr.onlinelibrary.wiley.com/doi/10.1359/jbmr.1998.13.7.1158.. - Murphy MG, Bach MA, Plotkin D, et al. Oral administration of the growth hormone secretagogue MK-677 increases markers of bone turnover in healthy and functionally impaired elderly adults. The MK-677 Study Group. J Bone Miner Res. 1999;14(7):1182-8.
Oral administration of the growth hormone secretagogue MK-677 increases markers of bone turnover in healthy and functionally impaired elderly adults. The MK-677 Study GroupGrowth hormone (GH) has been shown to stimulate osteoblasts
(bone-building cells) and increase bone turnover in elderly subjects. The primary effect of GH on bone is thought to be mediated by the stimulation of insulin-like growth factor I (IGF-I), which can be produced locally or circulated in
the body. Three clinical studies were conducted on 187 subjects aged 65 and older to test the effect of MK-677, a GH secretagogue, on bone turnover. The studies lasted between 2 and 9 weeks and were randomized, double-blind, and placebo-controlled.
The studies measured the levels of N-telopeptide cross-links (NTXs), a marker of bone resorption, as well as blood levels of osteocalcin (maintains and regenerates bone tissue) and bone-specific alkaline phosphatase (BSAP), which are markers
of bone formation, and serum IGF-I levels before and after treatment. The dose response of MK-677 was first tested in 50 healthy elderly subjects who received oral doses of 10mg or 25mg of MK-677 or placebo for 2 weeks. Treatment with
MK-677 resulted in increased mean urine NTXs by 10-17% and increased mean blood levels of osteocalcin by 8%, as well as significantly increased serum IGF-I levels. In subsequent studies, 105 elderly people with functional impairment were
randomly assigned to receive either a placebo or 5, 10, or 25mg of MK-677 orally every day for 9 weeks. After the treatment period, MK-677 treatment increased blood levels of osteocalcin by 29.4%, BSAP by 10.4%, and urinary NTX excretion
by 22.6%. In the MK-677 group, changes in the blood levels of osteocalcin correlated with changes in IGF-I levels. Overall, once daily dosing with MK-677 was found to stimulate bone turnover in elderly subjects based on elevations in biochemical
markers of bone resorption and formation. These results suggest that MK-677 may be a useful treatment for age-related bone loss in elderly individuals. You can read the full article athttps://asbmr.onlinelibrary.wiley.com/doi/10.1359/jbmr.1999.14.7.1182.. - Murphy MG, Weiss S, Mcclung M, et al. Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab.
2001;86(3):1116-25.Effect of alendronate and MK-677 (a growth hormone secretagogue), individually and in combination, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic womenThe study aimed to investigate the
effects of combining MK-677, an oral GH secretagogue, with alendronate, a potent inhibitor of bone resorption (breakdown), on bone mineral density (BMD) in postmenopausal women with osteoporosis. The researchers hypothesized that the combination
therapy would increase bone formation and BMD more than alendronate alone. A randomized, double-blind, placebo-controlled study of 292 women aged 64-85 years with low femoral neck BMD was conducted. The participants were divided into four
groups and given either MK-677 plus alendronate, alendronate alone, MK-677 alone, or a placebo every day for 12 months. Except for BMD, the primary outcomes were measured at 12 months while BMD was measured at 18 months. In comparison
to the placebo, MK-677, with or without alendronate, increased insulin-like growth factor I levels and biochemical markers of bone formation but not bone resorption. Furthermore, MK-677 and alendronate both mitigated and reduced the effect
of alendronate alone on bone formation and resorption. However, when compared to alendronate alone, the combination therapy only showed a significant increase in BMD at the femoral neck, but not at other sites. The researchers noted side
effects associated with GH secretion in the groups receiving MK-677, although adverse events resulting in discontinuation from the study were relatively infrequent. The researchers concluded that the anabolic effect of GH, as produced
through MK-677, attenuated the suppressive effect of alendronate on bone formation but did not translate into significant increases in BMD at sites other than the femoral neck. The potential side effects of enhanced GH secretion should
also be weighed against the benefits of combination therapy. You can read the full article athttps://academic.oup.com/jcem/article/86/3/1116/2847590?login=false.. - Bach, M. A., Rockwood, K., Zetterberg, C., Thamsborg, G., Hébert, R., Devogelaer, J. P., Christiansen, J. S., Rizzoli, R., Ochsner, J. L., Beisaw, N., Gluck, O., Yu, L., Schwab, T., Farrington, J., Taylor, A. M., Ng, J., Fuh, V., & MK 0677 Hip Fracture
Study Group (2004). The effects of MK-0677, an oral growth hormone secretagogue, in patients with hip fracture. Journal of the American Geriatrics Society, 52(4), 516–523.https://doi.org/10.1111/j.1532-5415.2004.52156.x..The effects of MK-0677, an oral growth hormone secretagogue, in patients with hip fractureA group of researchers evaluated how MK-0677, an orally active growth hormone secretagogue, can affect the functional recovery of previously
mobile older people who had a hip fracture. The study included 161 hip-fracture patients aged 65 and up who were ambulatory prior to the fracture, medically stable after surgery, and mentally competent. Patients with multiple fractures,
severe trauma, diabetes, cancer, uncontrolled hypertension, congestive heart failure, or total hip replacement in the involved extremity were excluded. The participants were randomly assigned to receive either MK-0677 or a placebo for
six months, after which they were followed up for another six months. The results of the study showed that MK-0677 treatment increased serum IGF-I levels by 84% compared to an increase of 17% on the placebo. However, there were no significant
differences between MK-0677 and placebo in terms of improvement in functional performance measures or in the overall SIP-NH score. Although MK-0677 patients showed greater improvement in three out of four lower extremity functional performance
measures, in the physical domain of the SIP, and in the ability to live independently, these differences were not statistically significant. Therefore, it is uncertain whether clinically significant effects on physical function were achieved.
The researchers also mentioned that measuring function in clinical trials in hip fracture patients is difficult due to a lack of validated outcome measures, high variability, and the absence of a baseline assessment. They suggested that
current functional performance measures might not be responsive enough to be used as the primary endpoint of small intervention studies. In contrast, GH stimulation may not result in significant functional improvement. In summary, the
study found that MK-0677 treatment increased serum IGF-I levels but did not result in significant improvement in functional performance measures or in the overall SIP-NH score. The researchers highlighted the challenges of measuring function
in clinical trials in hip-fracture patients and suggested the need for more sensitive outcome measures. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/15066065/.. - Devin, J. K., Vaughan, D. E., Blevins, L. S., Jr, Chen, Q., Covington, J., Verity, D. K., & Young, P. P. (2008). Low-dose growth hormone administration mobilizes endothelial progenitor cells in healthy adults. Growth hormone & IGF research : official
journal of the Growth Hormone Research Society and the International IGF Research Society, 18(3), 253–263.https://doi.org/10.1016/j.ghir.2007.11.001..Low-dose growth hormone administration mobilizes endothelial progenitor cells in healthy adultsThe body contains a type of cell called Endothelial Progenitor Cells (EPCs), which are produced in the bone marrow and travel to
damaged blood vessels to help with the repair process. A group of researchers investigated whether administering growth hormone (GH) to healthy adults could increase the number of EPCs in the bloodstream, thus promoting better vascular
health. A trial was conducted on 10 healthy adults ranging from 26 to 65 years old. The subjects received a low dose of GH for up to eight weeks. The number of EPCs in the bloodstream was determined using two methods: a colony-forming
unit (CFU) assay and flow cytometry. The presence of other mediators of EPC mobilization as well as the level of nitric oxide in the plasma were also monitored. Results showed that GH administration increased the level of Insulin-like
growth factor 1 (IGF-1) in the serum (blood), which is known to play a role in the production of EPCs. The trial found a correlation between the peak IGF-1 level and an increase in the number of early-outgrowth EPCs, which are a specific
type of EPCs. However, the number of other types of EPCs did not significantly change. The trial also found that other mediators of EPC mobilization remained stable while nitrite levels in the plasma increased. The researchers concluded
that GH administration selectively increased the number of early-outgrowth EPCs in healthy individuals, which could have implications for future regenerative cell-based therapies. Furthermore, the findings suggest that the decline in EPCs
seen with aging may be due in part to a deteriorating somatotropic axis, which may contribute to cardiovascular senescence. These findings support the use ofGH replacement therapyto
maintain vascular health in people with GH deficiency. You can read the full article athttps://www.sciencedirect.com/science/article/abs/pii/S1096637407001505?via%3Dihub.. - Eriksen EF, Kassem M, Langdahl B. Growth hormone, insulin-like growth factors and bone remodelling. Eur J Clin Invest. 1996;26(7):525-34.
Growth hormone, insulin‐like growth factors and bone remodelingGrowth hormone (GH) stimulates precursor cells in the epiphyseal cartilage and thus plays an important role in bone growth after birth. This stimulation is accomplished
through both direct interactions with GH receptors on the cell membrane and indirect effects such as increased insulin-like growth factor production (IGF-I and -II). In addition to influencing skeletal size, these hormones also have an
impact on the mature skeleton by influencing bone remodeling in adults. A review of several studies on the effects of these growth-promoting peptides on adult bone remodeling was conducted. Results showed that GH had significant effects
on the growth of bones after birth by promoting the activity of precursor cells in the epiphyseal cartilage. The hormone’s effects are mediated by both direct interactions with GH receptors on the cell membrane and indirect effects through
the production of IGF-I and -II. GH and IGFs also impact bone remodeling in adults. In conclusion, GH and IGF-I both play a role in bone remodeling in adult individuals. You can read the abstract of this article athttps://onlinelibrary.wiley.com/doi/abs/10.1046/j.1365-2362.1996.00292.x?sid=nlm%3Apubmed.. - Ohlsson C, Bengtsson BA, Isaksson OG, Andreassen TT, Slootweg MC. Growth hormone and bone. Endocr Rev. 1998;19(1):55-79.
Growth hormone and boneGrowth hormone deficiency (GHD) is linked to low bone mass in both children and adults, in addition to its known effects on growth. While growth hormone (GH) and insulin-like growth factor I have direct
effects on bones, it’s possible that abnormal secretion or function of parathyroid hormone may also play a role in the negative effects of GHD on bones. Several studies have shown thatGH replacement therapyimproves bone mineral density, but it’s unclear if this benefit is consistent across different groups of patients. Furthermore, it’s not yet established if GH replacement therapy reduces the risk
of fractures. GH replacement therapy has been shown to improve growth in children with GHD and other pediatric conditions. There is also emerging evidence that GH may play a role in bone physiology and other disease states other than GHD.
Although the beneficial effects ofGH replacement therapyon bone growth and density are well established, more research is needed to determine whether GH
replacement therapy reduces the risk of fractures and provides benefits in osteoporosis. In summary, while GH replacement therapy has been shown to improve bone density, further research is needed to determine its impact on fracture risk
and potential benefits in other bone-related conditions. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/19770650/.. - Olney RC. Regulation of bone mass by growth hormone. Med Pediatr Oncol. 2003;41(3):228-34.
Regulation of bone mass by growth hormoneThe pituitary gland secretes growth hormone (GH), a peptide hormone regulated by the hypothalamus. GH performs a variety of functions in the body, including the regulation of metabolic
pathways. Insulin-like growth factor-I mediates some of its effects (IGF-I). Both GH and IGF-I play important roles in bone metabolism and growth, making them important regulators of bone mass. Bone mass is built up during childhood as
a result of bone growth and remodeling, with bone remodeling involving the formation of new bone by cells called osteoblasts and bone resorption by cells called osteoclasts. GH directly and indirectly through IGF-I stimulates osteoblast
proliferation and activity, thus promoting bone formation. It also stimulates osteoclast differentiation and activity, which in turn promotes bone resorption. These effects increase the overall rate of bone remodeling, resulting in a net
effect of bone accumulation. The absence of GH results in a reduced rate of bone remodeling and a gradual loss of bone mineral density. Bone growth occurs primarily at the epiphyseal growth plates and is the result of the proliferation
and differentiation of chondrocytes (produce the cartilage matrix). GH has direct effects on these chondrocytes but primarily regulates this function through IGF-I, which stimulates the proliferation and matrix production by these cells.
A lack of GH can severely limit bone growth and mass accumulation. This deficiency is common in oncology and it has long-term consequences for bone health. Bone mass rises steadily throughout childhood and peaks in the mid-20s, followed
by a slow decline that accelerates in old age. Both GH and IGF-I play important roles in the regulation of bone mass and metabolism, making them important bone health regulators. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/12868124/.. - Bex M, Bouillon R. Growth hormone and bone health. Horm Res. 2003;60 Suppl 3:80-6.
Growth hormone and bone healthGrowth hormone (GH) and insulin-like growth factor-I both influence bone cell and growth plate chondrocyte growth. Childhood-onset GH deficiency (GHD) can have a severe impact on linear growth
and three-dimensional bone size if left untreated, resulting in adult peak bone mass that is only around 50% of what is considered normal. This has the most impact on bone volume, while true bone mineral density (BMD) remains mostly normal.
A large cohort of untreated Russian adults with childhood-onset GHD, on the other hand, demonstrated an increased prevalence of fractures, indicating that bone size and mass are more important than true bone density. Treatment with GH
can significantly correct bone size and mass over a prolonged period of more than 5 years, but it has only shown modest increases in bone mineral density (BMD) of the lumbar spine and femoral neck in male adults with adult-onset GHD. No
significant changes were observed in women. Untreated adult-onset GHD results in a mild deficit in bone mineral content and BMD of the lumbar spine, radius, and femoral neck, and increases the incidence of fractures. GHD affects bone mass
and strength in both children and adults. If given for an extended period of time, adequate substitution therapy with GH can largely correct these deficiencies. GH therapy for other bone disorders that are not associated with primary GHD
requires more research but it may have beneficial effects on the bone remodelling cycle. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/14671403/.. - Tritos NA, Klibanski A. Effects of Growth Hormone on Bone. Prog Mol Biol Transl Sci. 2016;138:193-211.
Effects of Growth Hormone on BoneThe GH and IGF-1 axis is important in bone formation and resorption, affecting the skeleton throughout life. In adults, GH deficiency causes decreased bone turnover, stunted growth, low bone
mass, and an increased risk of fractures. However, GH replacement can improve adult height in GH-deficient children, increase bone mineral density in adults, and optimize peak bone acquisition in patients with persistent GH deficiency
transitioning from adolescence to adulthood. GH replacement therapy also appears to reduce the risk of GH-related fractures. Acromegaly, a condition characterized by an excess of GH and IGF-1, is linked to increased bone turnover and decreased
bone mineral density in the lumbar spine, particularly in patients with hypogonadism (low sex hormones). Patients with acromegaly are also at a higher risk of vertebral fractures, especially if the disease is active or there is concurrent
hypogonadism. Chronic renal failure, Turner syndrome, Prader-Willi syndrome, postnatal growth delay in patients with intrauterine growth retardation who do not demonstrate catchup growth, idiopathic short stature, short stature homeobox-containing
gene mutations, and Noonan syndrome all benefit from GH therapy for statural growth. Overall, GH and IGF-1 play essential roles in skeletal physiology, and GH replacement therapy is beneficial in both GH deficiency and insensitivity states.
GH therapy helps to improve bone health, increase height, and optimize peak bone acquisition in patients transitioning from adolescence to adulthood. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/26940392/.. - Kuzma M, Payer J. [Growth hormone deficiency, its influence on bone mineral density and risk of osteoporotic fractures]. Cas Lek Cesk. 2010;149(5):211-6.
Growth hormone deficiency, its influence on bone mineral density and risk of osteoporotic fracturesThe pituitary gland produces a significant amount of growth hormone (GH). It promotes linear bone growth by stimulating the
production of insulin-like growth factor I (IGF I), which regulates bone remodeling. The GH/IGF axis is critical for achieving peak bone mass (PBM) during childhood and adolescence, which is an important predictor of osteoporotic fractures
later in life. While the growth plates eventually close, the effects of GH/IGF on bone density, strength, and turnover are still regulated by bone remodeling. Studies have shown that low bone mineral density (BMD) is common in hypopituitarism
(low pituitary hormones), particularly in patients with GH deficiency (GHD). Initially, a reduction in BMD is observed after 6-12 months of GH therapy due to enhanced activation of bone turnover. However, with continued therapy, BMD levels
tend to normalize or even increase compared to baseline. The decrease in bone growth and PBM also influences the incidence of fractures in older patients. Several studies have suggested that GH therapy may increase BMD in adult men with
adult-onset GHD (AO-GHD) over a period of 18-24 months. However, this effect is not significant in women. Additionally, there is evidence that GHD is associated with a higher risk of fractures, particularly in women with childhood-onset
GHD (CO-GHD). Men, on the other hand, have a lower incidence of fractures compared to the control group. The influence of sex hormones on GH secretion may explain sexual differences in response to GH therapy. Despite the fact that women
have higher levels of GH secretion, the normal range for serum IGF-I is the same in men and women. Men and women with GHD received the same dose of GH per body surface area in a placebo-controlled study, but men had a greater increase
in serum IGF-I levels. In conclusion, the GH/IGF axis plays a crucial role in bone growth, density, and strength. GH therapy can lead to a temporary reduction in BMD due to increased bone turnover but can eventually normalize or increase
BMD levels. There are sexual differences in the response to GH therapy and patients with childhood-onset GHD may be at higher risk of fractures. More studies with larger sample sizes and appropriate control groups are necessary to estimate
the risk of fractures in patients with GHD. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/20629339/.. - Lindsey RC, Mohan S. Skeletal Effects of Growth Hormone and Insulin-like Growth Factor-I Therapy. Molecular and cellular endocrinology. 2016;432:44-55. doi:10.1016/j.mce.2015.09.017.
Skeletal effects of growth hormone and insulin-like growth factor-I therapyThe control of bone development is greatly influenced by the GH/IGF axis. Osteoporosis and other conditions linked to poor bone mass may occur if this
mechanism isn’t functioning properly. In order to control the GH/IGF axis, a complex interplay of hormonal and local variables, including ligands, receptors, IGFBPs, and IGFBP proteases, must be present. The importance of both GH and IGF-I
in skeletal growth and maintenance is underlined by a variety of evidence from in vitro research, transgenic animal models, and clinical human investigations. These two GH/IGF axis components have crucial local and endocrine effects. Osteoporosis
and related conditions can be prevented and treated with GH- and IGF-based therapy. To break it down further, the GH/IGF axis is a vital component of the body’s regulatory mechanisms for bone formation. It operates through a complex network
of hormones and local factors that can affect its various components. In vitro studies, transgenic animal models, and clinical human investigations have confirmed the importance of GH and IGF-I in maintaining skeletal health. GH and IGF-I
act both locally and systemically to ensure proper skeletal growth and maintenance. This makes GH- and IGF-based therapies a promising avenue for treating conditions like osteoporosis that are linked to low bone mass. You can read the
full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC4808510/.. - Bouillon R. Growth hormone and bone. Horm Res. 1991;36 Suppl 1:49-55.
Growth hormone and boneGrowth hormone deficiency has been linked to low bone mass in both children and adults, in addition to its already-known impact on growth. While growth hormone (GH) and insulin-like growth factor I (IGF-I)
have direct effects on bones, it is possible that the secretion or effect of parathyroid hormone may also play a role in the negative effects of GH deficiency on bone health. Many studies have demonstrated thatGH replacement therapycan increase bone mineral density, but it is uncertain if these benefits are consistent across different patient subgroups. Additionally, it is still unclear whether GH replacement therapy can
reduce the risk of fractures. GH administration has been shown to improve growth in children with GH deficiency and other pediatric conditions. GH is also being studied for research purposes. Beyond GH deficiency, GH may play an important
role in bone physiology and a variety of disease states. While the benefits of GH replacement therapy on bone growth and density are well known, more research is needed to investigate its impact on fracture risk and potential benefits
in osteoporosis. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/19770650/.. - Capozzi A, Casa SD, Altieri B, Pontecorvi A. Low bone mineral density in a growth hormone deficient (GHD) adolescent. Clinical Cases in Mineral and Bone Metabolism. 2013;10(3):203-205.
Low bone mineral density in a growth hormone deficient (GHD) adolescentThe importance of Growth Hormone (GH) in promoting linear growth during childhood and achieving appropriate height in early adulthood is well recognized.
In addition, GH has significant effects on various metabolic processes, cardiovascular function, and quality of life (QoL) in adults. GH also plays a crucial role in achieving optimal Bone Mineral Density (BMD), which is the most crucial
predictor of osteoporotic fractures, during the transition from childhood to adulthood. This period is crucial to determine the need for continuing recombinant human Growth Hormone (rhGH) treatment in patients with childhood-onset GHD
(COGHD) who may have persisting GHD. GHD has been linked to an increased risk of fractures in COGHD patients. Therefore, GH therapy can help reduce, if not prevent, osteoporosis in adults. Clinicians should assess the persistence of GHD
in COGHD patients during the critical period between childhood and adulthood to determine the need for continued rhGH treatment after the growth plates have closed. The goal of GH therapy is to promote linear growth while also preventing
osteoporosis and lowering the risk of fractures. As a result, monitoring bone health and performing regular bone density assessments is critical in COGHD patients receiving GH therapy. A group of researchers presented a case study of a
young man with COGHD who was treated with rhGH until he reached his final height but then suffered a wrist fracture and premature bone density loss. The subject was an 18-year-old boy with significant unexpected decalcification and no
history of secondary osteoporosis. Physical examination revealed that the subject was healthy and obese. The subject received rhGH treatment for 1 year. After the treatment period, a significant increase in the BMD at the lumbar spine
and femoral neck was observed. In addition, the treatment was also associated with an increase in bone turnover markers such as alkaline phosphatase (ALP) and osteocalcin (OCN). In summary, GH plays a vital role in promoting linear growth
during childhood and maintaining bone health in adulthood. It is crucial to monitor bone health and assess the persistence of GHD in COGHD patients during the transition to adulthood to ensure that they receive appropriate treatment to
prevent the development of osteoporosis and reduce the risk of fractures. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC3917585/.. - Gillberg P, Mallmin H, Petrén-mallmin M, Ljunghall S, Nilsson AG. Two years of treatment with recombinant human growth hormone increases bone mineral density in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 2002;87(11):4900-6.
Two Years of Treatment with Recombinant Human Growth Hormone Increases Bone Mineral Density in Men with Idiopathic OsteoporosisA study was performed to find out how growth hormone (GH) treatment can influence the health of
the bones in males who had idiopathic osteoporosis. Twenty-nine males between the ages of 27 and 62 participated in the study. They were divided into two groups at random: group A (n = 14) received a daily dose of 0.4 mg of growth hormone,
while group B (n = 15) received an intermittent dose of 0.8 mg of growth hormone for 14 days every three months. With a 12-month follow-up period, both groups underwent GH therapy for a total of 24 months. IGF-I levels, bone markers, and
other laboratory tests were determined during the course of the trial by collecting urine and blood samples at regular intervals. Dual-energy x-ray absorptiometry was used to assess body composition, bone mineral content (BMC), and bone
mineral density (BMD) at baseline and every six months. The results of the study showed that both continuous and intermittent treatment with GH led to an increase in BMD and BMC in the lumbar spine and total body. Group A experienced a
4.1% increase in BMD in the lumbar spine while both groups experienced a 2.6% increase in BMD in the total body. BMC of the total body and lean body mass increased while fat mass decreased in both treatment groups. After 36 months, the
BMD and BMC in the lumbar spine and total body continued to increase in both groups. The study concluded that two years of GH treatment in men with idiopathic osteoporosis resulted in sustained increases in BMD and BMC for at least one
year after treatment. These findings suggest that GH treatment may be a viable option for improving bone health in men with osteoporosis. You can read the full article athttps://academic.oup.com/jcem/article/87/11/4900/2823079.. - Aloia JF, Zanzi I, Ellis K, et al. Effects of growth hormone in osteoporosis. J Clin Endocrinol Metab. 1976;43(5):992-9.
Effects of growth hormone in osteoporosisA study investigated the effects of growth hormone (GH) on osteoporosis patients. The researchers utilized a number of methods, including whole-body counts, photon absorptiometry, calcium
tracer kinetics, quantitative microradiography, and urine hydroxyproline. Two alternative dosage regimens — 2 units per day and 0.2 w3/4 units of GH per day (where W is the patient’s body weight in kg) — were applied for a total of six
months each. Results showed that the indicators did not significantly change with the decreased dosage of 2 units. The majority of patients did not, however, experience any anabolic effects from the larger dosage, therefore there was no
rise in the amount of Ca, Na, K, P, or Cl in the body as a whole. Instead, there were rises in the rate of hydroxyproline and calcium excretion in the urine. Additionally, the mineral content of bone reduced. The number of surfaces for
bone production and resorption increased in response to therapy in bone biopsies, although these changes were not statistically significant. Therefore, it can be said that in this study, GH administration did not result in an increase
in skeletal mass. Furthermore, the researchers observed several side effects characteristic of acromegaly (abnormal bone growth), including hyperglycemia (increased blood sugar levels), hypertension, arthralgia (joint pain), and carpal
tunnel syndrome. Because the therapy did not demonstrate any benefits and was associated with complications, GH administration does not appear to be a useful treatment for osteoporosis.https://pubmed.ncbi.nlm.nih.gov/993324/. - Colao A, Di somma C, Pivonello R, et al. Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarism. J Clin Endocrinol Metab. 1999;84(6):1919-24.
Bone loss is correlated to the severity of growth hormone deficiency in adult patients with hypopituitarismThe study investigated the relationship between bone mineral density (BMD) and the severity of growth hormone deficiency
(GHD) in hypopituitary patients. BMD at the lumbar spine and femoral neck, insulin-like growth factor I (IGF-I), IGF-binding protein-3 (IGFBP-3), osteocalcin levels, and urinary cross-linked N-telopeptides of type I collagen (Ntx) levels
were evaluated in 101 adult hypopituitary patients and 35 healthy individuals matched for sex and age. Based on their GH response to arginine plus GHRH (ARG+/-GHRH), the patients were categorized into four groups: group 1 had very severe
GHD, group 2 had severe GHD, group 3 had partial GHD, and group 4 had non-GHD. Group 5 was given the assignment of controls. In comparison to patients in groups 3-5, patients in group 1 showed lower levels of circulating IGF-I, IGFBP-3,
osteocalcin, and urinary Ntx as well as lower t scores at the lumbar spine and femoral neck. Compared to patients in groups 1 and 5, patients in group 2 exhibited significantly lower t values at the femoral neck and higher t scores in
the lumbar spine. Serum osteocalcin and urinary Ntx levels were markedly higher in group 2 and lower in groups 3-5 than in group 1 respectively. To investigate the effect of isolated GHD vs. multiple pituitary hormone deficiencies (MPHD),
patients were subdivided according to the number of hormonal deficits. The t score at the lumbar spine and femoral neck and the biochemical parameters of bone turnover were not significantly different among the different subgroups with
similar GH secretions. A significant correlation was found between the GH peak after ARG+GHRH and IGF-I, osteocalcin, urinary Ntx levels, and the t score at the lumbar spine, but not that at the femoral neck level. A significant correlation
was also found between plasma IGF-I levels and the t score at the lumbar spine and femoral neck, serum osteocalcin, and urinary Ntx.Plasma IGF-I levels were found to be a stronger predictor of the t score at the lumbar spine than the GH
peak following ARG+GHRH, but not that at the femoral neck, according to multiple correlation analysis. The study discovered that whereas non-GHD hypopituitary individuals had normal BMD values, only patients with very severe or severe
GHD showed a significant drop in BMD linked with abnormalities of bone turnover parameters. Regardless of the presence of additional hormone deficits, these anomalies were continuously found in all patients with GHD, indicating that GHD
is a major factor in the onset of osteopenia in hypopituitary individuals.https://academic.oup.com/jcem/article/84/6/1919/2864471?login=false. - Krantz E, Trimpou P, Landin-wilhelmsen K. Effect of Growth Hormone Treatment on Fractures and Quality of Life in Postmenopausal Osteoporosis: A 10-Year Follow-Up Study. J Clin Endocrinol Metab. 2015;100(9):3251-9.
Effect of Growth Hormone Treatment on Fractures and Quality of Life in Postmenopausal Osteoporosis: A 10-Year Follow-Up StudyA study investigated the effects of growth hormone (GH) on fractures and the quality of life of postmenopausal
women with osteoporosis. In this study, women who took GH for three years and were tracked for ten years were compared to a control group in terms of bone data, fractures, and quality of life (QoL). Eighty women between the ages of 50
and 70 who needed replacement for osteoporosis were enrolled in the trial. They were randomized to receive either GH 1.0 U, GH 2.5 U, or placebo sc daily for three years. All subjects got 750 mg of calcium and 400 IU of vitamin D, and
they were monitored for ten years. Then, they were contrasted with a sample of 120 women drawn at random from an age-matched group from the World Health Organization’s Monitoring of Trends and Determinants in Cardiovascular Disease. The
results showed that GH increased bone mineral density (BMD) and bone mineral content in all regions in a dose-dependent manner, compared to the placebo group. After ten years, the number of fractures decreased from 56% to 28% in the GH
treatment group, while in the control group, the fractures increased from 8% to 32%. Quality of life did not change during GH treatment or the 10-year follow-up and did not differ compared with controls. Therefore, GH treatment was found
to be beneficial for bone and fracture outcomes after ten years but did not affect the quality of life of women with postmenopausal osteoporosis. In summary, this study demonstrated the long-term benefits of GH treatment for women with
postmenopausal osteoporosis, as it increased bone mineral density and decreased the number of fractures over a ten-year period. However, it is important to note that there was no significant improvement in the quality of life of these
patients.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4570174/. - Fall C, Hindmarsh P, Dennison E, Kellingray S, Barker D, Cooper C. Programming of growth hormone secretion and bone mineral density in elderly men: a hypothesis. J Clin Endocrinol Metab. 1998;83(1):135-9.
Programming of growth hormone secretion and bone mineral density in elderly men: a hypothesisEpidemiological studies have identified a correlation between low bone mass in adults and stunted growth during childhood, although
the cause of this connection is uncertain. Thirty-seven healthy men between the ages of 63 and 73 who had their weight increase from infancy tracked were the subject of the study. The researchers collected blood samples over a 24-hour
period and assessed GH secretory profile, insulin-like growth factor I (IGF-I), IGF-binding protein-1 and -3, and GH-binding protein, among other growth hormone-related variables. Using dual-energy x-ray absorption, they also assessed
bone mineral density in the lumbar spine and femoral neck. The study discovered an important connection between femoral neck bone density and peak GH and fasting IGF-I concentrations. After taking into account the peak GH level, median
GH was found to be inversely correlated with bone mineral density. Although peak GH was not correlated with weight at 1 year of age, the median GH concentration was. According to these results, GH secretion affects bone density in two
different ways: high peak GH levels promote the creation of IGF-I and maintain bone mineralization in adulthood while integrated GH secretion that has been corrected for pulse amplitude is associated with lower bone density. This specific
GH secretory profile trait is associated with infant growth and may be programmed by environmental influences during intrauterine or early postnatal life. In summary, the study provides evidence for a link between early growth and adult
bone density through the GH secretory profile. These findings suggest that growth during infancy may have long-term effects on bone health in adulthood and that environmental factors during early development may play a role in the programming
of GH secretion.https://academic.oup.com/jcem/article/83/1/135/2865098?login=false. - Rosen CJ. IGF-I and osteoporosis. Clin Lab Med. 2000;20(3):591-602.
IGF-I and osteoporosisThe hormone IGF-I, which is important for bone development and maintenance, is regulated by several different regulatory factors. The adult level of IGF-I is made up of both newly synthesized IGF-I from
tissues like the liver, heart, kidney, and bone as well as IGF-I that has left the circulation as a result of processes like receptor internalization and IGFBP proteolysis. The circulating depot of IGF-I is inert. IGF-I levels and bone
mass or fracture risk are correlated numerically, although it is not known if this correlation is causative. For instance, prolonged malnutrition inhibits hepatic IGF-I expression, which can result in musculoskeletal instability and fractures,
but it is unclear whether low levels of circulating IGF-I truly cause osteoporosis. Additionally, serum levels of IGF-I may not always reflect tissue concentrations, and therefore caution is required when interpreting low, normal, or high
IGF-I levels in relation to osteoporosis. Future studies should help to define the possible pathogenic relationship between IGF-I and bone mass more clearly. In conclusion, although serum IGF-I levels are influenced by several regulatory
factors, the exact relationship between IGF-I levels and bone health remains to be fully understood, and more research is needed to better elucidate this relationship.https://pubmed.ncbi.nlm.nih.gov/10986623/. - Kawai M, Rosen CJ. The Insulin-Like Growth Factor System in Bone: Basic and Clinical Implications. Endocrinology and metabolism clinics of North America. 2012;41(2):323-vi. doi:10.1016/j.ecl.2012.04.013.
The insulin-like growth factor system in bone: basic and clinical implicationsIGFs (IGF-I and IGF-II), the IGF receptor, and regulatory proteins including IGF-binding proteins (IGFBP-1 to IGFBP-6) and the acid-labile subunit
(ALS) make up the insulin-like growth factor (IGF) regulatory system. IGFs play a variety of biological roles in the system, which make them effective mitogens (stimulate cell division) and differentiation agents. IGFs are often bound
in binary or ternary complexes, and extracellular fluid or circulation rarely includes free IGF-I or IGF-II. IGF bioavailability is regulated by their interactions at the receptor level, and modifications to any part of the system have
a significant impact on the biological activity of the ligands. The IGFBPs play an important role in regulating IGF-I access to its receptor because their binding affinity is higher than that of the IGF receptors. The IGF system is unique
because the IGFBPs are regulated in a cell-specific manner in the pericellular microenvironment, which means that small changes in their concentrations can have a significant impact on the mitogenic activity of IGF-I. In the skeleton,
IGF-I and IGF-II are abundant and stored in their inactive form, bound to several IGFBPs within the matrix. The release of the IGFs in their active form during bone modeling and remodeling is a crucial aspect of skeletal homeostasis (balance).
Due to the abundant blood supply of the cortical and trabecular skeleton, these growth factors circulate in high quantities and can reach bone cells. It was thought that the recombinant IGFs may be employed clinically for a variety of
illnesses ranging from short stature to fracture repair and osteoporosis because the IGF regulatory system is essential for skeletal growth and maintenance. The potential of this family of growth factors in skeletal homeostasis and the
pathogenesis of various bone illnesses, however, was not realized, and basic and translational investigations have continued to offer important new information.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3576021/. - Niu T, Rosen CJ. The insulin-like growth factor-I gene and osteoporosis: a critical appraisal. Gene. 2005;361:38-56.
The insulin-like growth factor-I gene and osteoporosis: a critical appraisalOsteoporosis is a common condition among the elderly that has been defined by weak and fragile bones. The incidence of osteoporosis rises along with
the number of people who have poor bone mineral density, which is the most important predictor of fracture risk. Recent research indicates that bone mineral density is significantly influenced by insulin-like growth factor-I (IGF-I). IGF-I
serum levels, bone mineral density, and fracture risk have all been linked to genetic polymorphisms in the P1 promoter region of the human IGF-I gene. Numerous quantitative trait loci (QTLs) have been found to influence the levels of blood
IGF-I in mice. On mouse chromosome 6, the most promising QTL has revealed information on the molecular pathways controlling osteoblast (cells that form bones) differentiation. The crucial role of IGF-I in bone growth during both embryonic
and postnatal stages has been confirmed by studies employing genetically modified mice that either lack or overexpress IGF-I. The IGF-I gene’s role in bone remodeling is determined by several distinct mechanisms: the skeletal IGF regulatory
system, the systemic growth hormone/IGF-I axis, parathyroid hormone signaling, sex steroids, and the OPG/RANKL/RANK cytokine system. Further molecular dissection of the IGF regulatory system and its signaling pathway may reveal novel therapeutic
targets for the treatment of osteoporosis.https://pubmed.ncbi.nlm.nih.gov/16183214/. - Chen Y-C, Zhang L, Li E-N, et al. Association of the insulin-like growth factor-1 single nucleotide polymorphisms rs35767, rs2288377, and rs5742612 with osteoporosis risk: A meta-analysis. Pany. S, ed. Medicine. 2017;96(51):e9231. doi:10.1097/MD.0000000000009231.
Association of the insulin-like growth factor-1 single nucleotide polymorphisms rs35767, rs2288377, and rs5742612 with osteoporosis risk: A meta-analysisIt is generally accepted that the hormone insulin-like growth factor-1
(IGF-1) is essential for controlling the mineralization and development of bones. A meta-analysis was performed to examine the association between three particular polymorphisms in the IGF-1 gene (rs35767, rs2288377, and rs5742612) with
the risk of osteoporosis. Four separate case-control studies involving a total of 2807 Chinese participants were included in the meta-analysis. The results indicated that one of the genetic variations, rs35767, was associated with an increased
risk of osteoporosis across all study subjects (both men and women) in various models including dominant, recessive, homozygote, and allelic. However, there was no evidence of any association between the other two genetic variations, rs2288377
and rs5742612, and osteoporosis risk. The results of additional subgroup analysis based on gender showed that rs35767 was specifically linked to a higher risk of osteoporosis in females. Most importantly, the meta-analysis did not detect
any heterogeneity or publication bias between the included studies. Taken together, these results suggest that rs35767 may be a relevant genetic risk factor for osteoporosis in Chinese individuals, particularly women. However, further
research will be needed to validate these findings and explore potential underlying mechanisms. Furthermore, the meta-analysis has potential limitations such as the included studies were conducted in the Chinese population, the sample
size was relatively small, and 3 studies were performed on postmenopausal female subjects.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5758177/. - Vittorio Locatelli and Vittorio E. Bianchi, “Effect of GH/IGF-1 on Bone Metabolism and Osteoporsosis,” International Journal of Endocrinology, vol. 2014, Article ID 235060, 25 pages, 2014.https://doi.org/10.1155/2014/235060
Effect of GH/IGF-1 on Bone Metabolism and OsteoporsosisGrowth hormone (GH) and (insulin-like growth factor) IGF-1 increase tissue formation by acting directly and indirectly on target cells, with IGF-1 being a critical mediator
of bone growth. An article discussed the importance of GH and IGF-1 in skeletal growth during puberty and bone health throughout life. The article search found 39 clinical studies investigating the effects of GH and IGF-1 administration
on bone metabolism in osteopenic and osteoporotic human subjects and on bone healing in operated patients with normal GH secretion. Of the 39 studies, 18 focused on the effect of GH treatment, 14 reported on the clinical effects of IGF-1
administration, and 7 related to the GH/IGF-1 effect on bone healing. Results showed that, in the majority of cases, injection of GH and IGF-1 considerably boosted both bone resorption and bone formation. The administration of GH/IGF-1
to individuals with hip or tibial fractures promoted faster clinical recovery and enhanced bone repair. However, some contradictory findings were noted. According to the article, GH administration for the treatment of osteoporosis and
bone fractures may significantly improve clinical outcomes because GH and IGF-1 therapy have a considerable anabolic effect. It is stated that the anabolic process in which GH and sex steroids interact also takes into account the GH resistance
mechanism. Overall, the investigations point to the crucial functions that GH and IGF-1 play in bone health and their potential therapeutic value for people with osteoporosis and bone fractures.https://www.hindawi.com/journals/ije/2014/235060/?utm_source=google&utm_medium=cpc&utm_campaign=HDW_MRKT_GBL_SUB_ADWO_PAI_DYNA_JOUR_X_PJ_GROUP3&gclid=CjwKCAjwue6hBhBVEiwA9YTx8KMyf28PWZoYXHgOIP0nBsj_-dOXHNTycKcNpYil0WjsKF8Jg4GMchoCId0QAvD_BwE. - Kurland ES, Rosen CJ, Cosman F, et al. Insulin-like growth factor-I in men with idiopathic osteoporosis. J Clin Endocrinol Metab. 1997;82(9):2799-805.
Insulin-like growth factor-I in men with idiopathic osteoporosisMost males with osteoporosis do not have a history of severe glucocorticoid usage, hypogonadism, or alcohol misuse. Studies have revealed a decline in bone production
indicators in such circumstances. In order to learn more about this, researchers looked into the possibility that abnormalities in the skeletal mechanisms regulated by insulin-like growth factor I (IGF-I) may be responsible for the decline
in bone production in males with idiopathic osteoporosis. They examined 24 middle-aged men who had severe idiopathic osteoporosis and discovered that several biochemical markers, such as blood calcium, vitamin D, testosterone, and enzymes
that are unique to bones, were at normal levels. However, the mean IGF-I level was lower than expected, and the level was negatively correlated with age. With age held constant, serum IGF-I accounted for 15% of the variance in lumbar bone
mineral density (BMD). Histomorphometric analysis of bone biopsy specimens showed significant reductions in cancellous bone volume, cortical width, osteoid surface, and bone formation rate when compared to age-matched control subjects.
The results suggest that serum IGF-I levels are reduced in men with idiopathic osteoporosis and that IGF-I may contribute to the reduction in lumbar spine bone mass density (BMD). Further studies of the IGF-I axis may provide insights
into the etiology of idiopathic osteoporosis in men. In conclusion, the study discovered that males with idiopathic osteoporosis have decreased levels of IGF-I and that this decline is connected to low bone mass density and decreased bone
production. The researchers contend that additional research into the IGF-I axis may help clarify the underlying reasons for osteoporosis in males who do not have established risk factors.https://academic.oup.com/jcem/article/82/9/2799/2865894?login=false. - Sroga GE, Wu PC, Vashishth D. Insulin-like growth factor 1, glycation and bone fragility: implications for fracture resistance of bone. PLoS ONE. 2015;10(1):e0117046.
Insulin-like growth factor 1, glycation and bone fragility: implications for fracture resistance of boneAlthough much is known about how insulin-like growth factor 1 (IGF-1) impacts bone development, researchers still don’t
fully comprehend how it keeps the skeleton balanced with aging and in the presence of certain disorders. According to certain studies, advanced glycation end products (AGEs) generated from glucose (blood sugar) may contribute to osteoporosis
and a number of other diabetes problems. IGF-1 levels may be correlated with the accumulation of glycation products and a decline in bone fracture resistance since humans and rodents depend on IGF-1 to control glucose absorption in a dose-dependent
way. To test this hypothesis, researchers conducted biochemical tests to measure the levels of IGF-1, fluorescent AGEs, and pentosidine (PEN) contents in human tibial posterior cortex bone samples. They also performed mechanical tests
to assess the initiation and propagation of bone cracks using compact tension specimens. Their findings revealed, for the first time, a significant association between IGF-1 and AGE levels that is independent of age. Furthermore, AGEs
predict bone fracture propensity, including initiation and propagation, regardless of age, in human cortical bone. A model of IGF-1-based regulation of bone fracture is put out by the researchers. IGF-1 may be protective in preserving
bone health in the developing skeleton as levels rise from birth to the juvenile developmental period and subsequently fall with age. However, the age-related drop in IGF-1 levels may result in brittle bones and a higher risk of fractures.
The development of diagnostic methods to screen for fragile bones as well as how we understand osteoporosis and diabetes-related bone fragility may be affected by these findings.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4309541/. - Vandenput L, Sjögren K, Svensson J, Ohlsson C. The Role of IGF-1 for Fracture Risk in Men. Frontiers in Endocrinology. 2012;3:51. doi:10.3389/fendo.2012.00051.
The Role of IGF-1 for Fracture Risk in MenGrowth hormone and insulin-like growth factor-1 (IGF-1) are required for appropriate bone and bone mass growth. Studies employing knockout models have demonstrated that normal skeletal
growth and bone size are influenced by both systemic and bone-derived IGF-1. Researchers have examined the significance of serum IGF-1 in predicting the risk of fractures because bone size is an important factor in determining bone strength
and fracture risk. According to recently released data from the Osteoporotic Fractures in Men Sweden cohort, older men with low serum IGF-1 levels are more likely to sustain fractures, especially hip and vertebral fractures, which are
the most serious kinds of fractures. Bone mineral density (BMD) is one factor that helps explain this connection. The role of both systemic and bone-derivedIGF-1is
crucial in normal bone growth and development, as demonstrated in various knockout mouse models. However, further research is necessary to determine the specific mechanisms by which both endocrine and local IGF-1 regulate bone growth and
size. In addition, other mediators that affect the relationship between IGF-1 and fracture risk in older men need to be identified. In conclusion, low blood IGF-1 levels increase the risk of fractures, especially hip and vertebral fractures
in older men. IGF-1 is crucial for normal bone growth and bone mass. This correlation is partially mediated by BMD but there are still other factors that need to be taken into account. Future research is required to identify the precise
mechanisms by which systemic and bone-derived IGF-1 control the bone size and growth.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3355958/. - Ertuğrul I, Karan MA, Karan A, et al. Relationship between insulin-like growth factor 1 and bone mineral density in men aged over 65 years. Med Princ Pract. 2003;12(4):231-6.
Relationship between insulin-like growth factor 1 and bone mineral density in men aged over 65 yearsA study determined the connection between bone mineral density (BMD) and insulin growth factor 1 (IGF-1) in men 65 years of
age or older. Forty-one men between the ages of 65 and 88 who had never used drugs or had a condition that was known to impair BMD were enrolled by the researchers. Twenty healthy men between the ages of 19 and 62 were also included as
the control group. Dual-energy X-ray absorptiometry was used to evaluate BMD at the proximal femur and lumbar spine and an immunoradiometric test was used to measure IGF-1 levels. A thorough questionnaire was also employed by the researchers
to assess the epidemiology results. The study found that men aged 65 years or older had a lower mean IGF-1 level and lower mean BMD at the femoral neck, trochanter, intertrochanteric zones, Ward’s triangle, and total hip compared to the
control group. However, there was no statistically significant difference in the BMD of the lumbar vertebrae between the patients and controls. The researchers also found a strong negative correlation between IGF-1 levels and age, indicating
that IGF-1 levels decrease with age. Low IGF-1 levels were found to be substantially linked with osteopenia of the whole hip, femoral neck, trochanter, and intertrochanteric zone, according to the researchers’ logistic regression analysis.
The study came to the conclusion that hip osteopenia is more likely to occur in males 65 years of age or older when serum IGF-1 levels are low. According to the study, the osteopenia that has been found in people of this age may be partially
brought on by low IGF-1 levels. Overall, this study highlights the importance of monitoringIGF-1 levelsin older men as a potential
indicator of their bone health. The findings may also have implications for the development of treatments to prevent or manage osteopenia in this population.https://pubmed.ncbi.nlm.nih.gov/12966195/. - Gao S, Lv Z, Zhou C, Mao C, Sheng W. Association between IGF-1 polymorphisms and risk of osteoporosis in Chinese population: a meta-analysis. BMC Musculoskeletal Disorders. 2018;19:141. doi:10.1186/s12891-018-2066-y.
Association between IGF-1 polymorphisms and risk of osteoporosis in Chinese population: a meta-analysisNumerous studies have looked at the connection between the Chinese population’s osteoporosis risk and insulin-like growth
factor-1 (IGF-1) gene polymorphisms, however, the findings have been mixed. A systematic review and meta-analysis were carried out to provide more accurate and exact knowledge of the relationship betweenIGF-1gene polymorphisms and osteoporosis. The study’s goal was to review the literature and assess the connection between osteoporosis and polymorphisms in the IGF-1 gene. The researchers systematically searched five
electronic databases including PubMed, EMBASE, ISI Web of Science, CNKI, and Wanfang for relevant studies. Six case-control studies were identified, involving a total of 2068 osteoporosis patients and 2071 healthy controls. They calculated
the summary odds ratio (OR) and the corresponding 95% confidence interval (95% CI) to evaluate the association. The best-matching genetic model of inheritance was determined using a genetic-model free approach. The researchers found that
the dominant model (TT + TC versus CC) was the best-matching genetic model for the analysis. The results showed that the rs35767 polymorphism was significantly associated with osteoporosis vulnerability. Subgroup analysis based on the
source of patients suggested that rs35767 was significantly correlated with osteoporosis in the post-menopausal women subgroup but not in the subgroup of both genders. According to the findings of the study, osteoporosis risk in Chinese
post-menopausal women is significantly correlated with the rs35767 polymorphism. The research adds to the body of evidence supporting the link between osteoporosis and IGF-1 gene polymorphisms. This in turn provides knowledge regarding
the genetic causes of osteoporosis.https://bmcmusculoskeletdisord.biomedcentral.com/articles/10.1186/s12891-018-2066-y. - Liu JM, Zhao HY, Ning G, et al. IGF-1 as an early marker for low bone mass or osteoporosis in premenopausal and postmenopausal women. J Bone Miner Metab. 2008;26(2):159-64.
IGF-1 as an early marker for low bone mass or osteoporosis in premenopausal and postmenopausal womenThe serum levels of insulin-like growth factor 1 (IGF-1), osteoprotegerin (OPG), leptin, osteocalcin (OC), and urinary excretion
of N-terminal telopeptide of type I collagen (NTx) were examined to see which of these variables could be used as an early marker for osteopenia/osteoporosis in women diagnosed by dual-energy X-ray absorptiometry (DXA). In this investigation,
blood levels of IGF-1, OPG, leptin, OC, and urine NTx were evaluated in addition to the bone mineral densities (BMDs) at the lumbar spine (LS) and femoral neck (FN) in 282 premenopausal and 222 postmenopausal women aged 20 to 75 years.
The receiver operating characteristic (ROC) analysis was used to evaluate the features of the earliest marker(s). It was found that serum levels of IGF-1 and leptin changed the earliest, with both markers significantly decreasing or increasing
respectively at age 30. However, in ROC analysis, IGF-1 was the only early parameter that had the capacity to differentiate the low bone mass/osteoporosis women from the normal ones. If the serum level of IGF-1 at 1.5 standard deviations
below its peak was adopted as a cutoff point, it could identify women with low bone mass/osteoporosis with a sensitivity of 73% and specificity of 67%. In the premenopausal women subgroup analysis, the low bone mass women (30/282, 10.6%)
were older, with lower serum levels of IGF-1 and less lean mass than the normal ones. After controlling for age, the serum level of IGF-1 had a weak but still significant positive correlation with lean mass. The study’s findings conclude
that measuring young women’s serum levels of IGF-1 may aid in the early detection of osteoporosis and poor bone mass. However, more research is required to validate these results and establish the ideal IGF-1 cutoff value.https://pubmed.ncbi.nlm.nih.gov/18301972/. - Copinschi G, Leproult R, Van onderbergen A, et al. Prolonged oral treatment with MK-677, a novel growth hormone secretagogue, improves sleep quality in man. Neuroendocrinology. 1997;66(4):278-86.
Prolonged oral treatment with MK-677, a novel growth hormone secretagogue, improves sleep quality in manPrevious studies have shown that processes controlling somatotropic activity and sleep are interconnected. In the present
investigation, the effects of prolonged administration of the newly created oral growth hormone secretagogue MK-677 on healthy young and older subjects’ sleep quality were investigated. Eight young participants, aged 18 to 30 years, underwent
three 7-day treatment periods as part of the study’s double-blind, placebo-controlled, three-period crossover design, while six older participants, aged 65 to 71, underwent two 14-day treatment periods with a 14-day washout period in between.
MK-677 was administered at doses of 5 and 25 mg along with an identical placebo for each treatment session. The results of the study showed that in young participants, the use of high-dose MK-677 treatment led to an approximate 50% increase
in stage IV sleep duration and a more than 20% increase in REM sleep duration compared to the use of a placebo. Additionally, the frequency of deviations from normal sleep decreased from 42% under placebo to 8% under high-dose MK-677.
In older adults, treatment with MK-677 was associated with a nearly 50% increase in REM sleep duration and a decrease in REM latency. The frequency of deviations from normal sleep also decreased. These findings suggest that MK-677 may
be able to improve the quality of sleep and correct the relative deficiency of somatotropic activity in older adults. Overall, this study contributes to the growing body of research on the potential benefits of MK-677 in enhancing sleep
quality and promoting healthy aging.https://pubmed.ncbi.nlm.nih.gov/9349662/. - Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an Oral Ghrelin Mimetic on Body Composition and Clinical Outcomes in Healthy Older Adults: A Randomized, Controlled Trial. Annals of internal medicine. 2008;149(9):601-611.
Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trialHumans gradually lose muscle mass as they age which causes frailty, diminished function, and loss of independence.
The fall in growth hormone is one of the causes of this decline but further research is needed to determine how exactly it contributes to the condition. Whether MK-677, an oral drug that mimics the hormone ghrelin, might raise growth hormone
levels in healthy older persons and stop the loss of fat-free mass while lowering abdominal visceral fat was the subject of a study. Sixty-five healthy adults between the ages of 60 and 81 were enrolled in the study, including both men
and women who were getting hormone replacement therapy as well as women who were not. After one year of treatment, the main endpoints were growth hormone and insulin-like growth factor I levels, fat-free mass, and abdominal visceral fat.
Baseline and every six months, other variables including body weight, fat mass, insulin sensitivity, lipid and cortisol levels, bone mineral density, limb lean and fat mass, isokinetic strength, function, and quality of life were also
evaluated. The daily administration of MK-677 significantly increased growth hormone and insulin-like growth factor I levels in healthy young adults without serious adverse effects. The placebo group experienced a decrease in mean fat-free
mass while the MK-677 group saw an increase of 1.1 kg. The body cell mass also increased in the MK-677 group compared to the placebo group. Although no significant differences were observed in abdominal visceral fat or total fat mass,
the MK-677 group showed a greater increase in limb fat. Body weight increased more in the MK-677 group than in the placebo group, and fasting blood glucose levels also increased in the MK-677 group. The most frequent side effects of the
treatment were an increase in appetite, which subsided in a few months, and transient mild lower-extremity edema and muscle pain. Cortisol levels also increased in the MK-677 recipients while low-density lipoprotein cholesterol levels
decreased. Changes in bone mineral density consistent with increased bone remodeling occurred in MK-677 recipients but increased fat-free mass did not result in changes in strength or function. In conclusion, the study indicated that MK-677
was usually well tolerated, significantly boosted pulsatile growth hormone secretion, and increased fat-free mass over the course of a 12-month period. To completely comprehend the effects of this medicine, however, long-term functional
and pharmacoeconomic studies in elderly individuals are required.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2757071/. - Frieboes RM, Murck H, Maier P, Schier T, Holsboer F, Steiger A. Growth hormone-releasing peptide-6 stimulates sleep, growth hormone, ACTH and cortisol release in normal man. Neuroendocrinology. 1995;61(5):584-9.
Growth Hormone-Releasing Peptide-6 Stimulates Sleep, Growth Hormone, ACTH and Cortisol Release in Normal ManGrowth hormone (GH) is known to be released in response to the synthetic hexapeptide GHRP-6. GH-releasing hormone
(GHRH) also has a similar effect. However, little is known about how GHRP affects nocturnal hormone secretion and the electroencephalogram (EEG) during sleep. A study on the effects of repeated intravenous boluses of GHRP and a placebo
on hormone secretion and sleep EEG in healthy male controls was conducted. The study found that GHRP increased GH concentration and ACTH levels during the night, particularly in the first half of the night. Cortisol secretion was also
enhanced throughout the night after GHRP administration. Stage 2 sleep increased but slow-wave sleep (SWS) remained unchanged. These results indicate that GHRP stimulates both GH release and hypothalamic-pituitary-adrenocortical hormone
secretion, which is in contrast to the blunting effect of cortisol seen with GHRH. Both GHRP and GHRH promote sleep but GHRP specifically enhances stage 2 sleep and does not affect SWS. This suggests that GHRP and GHRH act on different
receptors. Overall, this study shows how GHRP affects hormone secretion and sleep EEG in healthy male controls. The results imply that GHRP has potential therapeutic uses for GH shortage and sleep-related problems but more studies are
required to completely comprehend the processes and potential adverse consequences of GHRP administration.https://karger.com/nen/article-abstract/61/5/584/224521/Growth-Hormone-Releasing-Peptide-6-Stimulates?redirectedFrom=PDF. - Moreno-Reyes R, Kerkhofs M, L’hermite-balériaux M, Thorner MO, Van Cauter E, Copinschi G. Evidence against a role for the growth hormone-releasing peptide axis in human slow-wave sleep regulation. Am J Physiol. 1998;274(5 Pt 1): E779-84.
Evidence against a role for the growth hormone-releasing peptide axis in human slow-wave sleep regulationSleep and somatotropic activity have a complicated interaction. Injections of growth hormone-releasing hormone (GHRH)
given to humans before bedtime have been shown to reliably promote slow-wave (SW) sleep, especially later in the evening. The purpose of the current investigation was to determine whether GH-releasing peptide (GHRP) has comparable somnogenic
effects when administered to healthy young men under the same circumstances. After 60 seconds of the third rapid-eye-movement period, seven men received intravenous injections of GHRP-2 (1 microgram/kg body weight) or saline in random
order. All GHRP injections were followed by brief increases in prolactin and GH pulses that were close to or at the physiological upper limit in size. Next, it was evaluated how GHRP-2 administration affected sleep. The results of the
study showed that except for a non-significant tendency to increased wakefulness during the first hour after the injection, no effects of GHRP-2 administration on sleep were detected. In particular, there was no enhancement of SW sleep.
This is in contrast to the effects of GHRH, which consistently stimulates SW sleep when given during sleep. The present data suggest that the GHRP axis is not involved in human SW sleep regulation. The study’s findings show no evidence
that late-night single GHRP-2 injections at a dosage that causes comparable GH increases have any stimulatory effects on SW sleep in healthy young males. Research into the intricate interactions between somatotropic activity and sleep
is still underway and more research is required to completely comprehend the mechanisms at play.https://journals.physiology.org/doi/full/10.1152/ajpendo.1998.274.5.E779?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org. - Frieboes RM, Murck H, Antonijevic IA, Steiger A. Effects of growth hormone-releasing peptide-6 on the nocturnal secretion of GH, ACTH and cortisol and on the sleep EEG in man: role of routes of administration. J Neuroendocrinol. 1999;11(6):473-8.
Effects of growth hormone-releasing peptide-6 on the nocturnal secretion of GH, ACTH and cortisol and on the sleep EEG in man: role of routes of administrationIn recent studies, repeated intravenous (i.v.) boluses of growth
hormone-releasing peptide-6 (GHRP-6) have shown increases in growth hormone (GH), corticotropin (ACTH), and cortisol levels, along with an increase in the amount of stage 2 sleep. For clinical use, oral (p.o.), intranasal (i.n.), and sublingual
(s.l.) administration routes have advantages over i.v. administration. A comparison of the sleep-endocrine effects of 300 microg/kg of body weight (b.w.) GHRP-6 administered p.o. at 21.00 h and 30 microg/kg GHRP-6 i.n. or 30 microg/kg
GHRP-6 s.l. given at 22.45 h in normal young male controls with placebo conditions was conducted. The results showed that GHRP-6 i.n. prompted a significant increase in GH concentration during the total night and a trend toward an increase
in ACTH secretion during the first half of the night, whereas cortisol secretion remained unchanged. Furthermore, GHRP-6 i.n. led to an increase in sleep stage 2 in the second half of the night, and spectral analysis of total night non-rapid
eye movement (REM) sleep revealed a decrease of delta power by trend. In contrast, sleep stage 2 decreased during the second half of the night after GHRP-6 p.o. These findings demonstrate that GHRP-6 can modulate GH and ACTH secretion
as well as sleep, but the effects depend on dosage, duration, and route of administration. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/10336729/. - Morselli LL, Nedeltcheva A, Leproult R, et al. Impact of growth hormone replacement therapy on sleep in adult patients with growth hormone deficiency of pituitary origin. European journal of endocrinology/European Federation of Endocrine Societies. 2013;168(5):10.1530/EJE-12-1037.
doi:10.1530/EJE-12-1037.Impact of growth hormone replacement therapy on sleep in adult patients with growth hormone deficiency of pituitary originIn a single-blind randomized cross-over design study, we investigated the impact of recombinant human
GH (rhGH) therapy on objective sleep quality in adult patients with GH deficiency (GHD) due to a confirmed or likely pituitary defect. The study involved 14 patients, and after 4 months of rhGH treatment, it was observed that patients
had a shorter sleep period time than during the placebo period, primarily due to an earlier wake-up time, and a decrease in the intensity of slow-wave sleep (SWS) indicated by delta activity. These findings suggest that rhGH replacement
therapy can partially reverse sleep disturbances observed in untreated patients with pituitary GHD, and the decrease in delta activity may be linked to the overactivity of the hypothalamic GHRH system in the absence of negative feedback
inhibition by GH. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC3832204/. - Haqq AM, Stadler DD, Jackson RH, Rosenfeld RG, Purnell JQ, Lafranchi SH. Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndrome. J
Clin Endocrinol Metab. 2003;88(5):2206-12.Effects of growth hormone on pulmonary function, sleep quality, behavior, cognition, growth velocity, body composition, and resting energy expenditure in Prader-Willi syndromeThe objective of the study conducted by the researchers
was to explore the effects of GH administration on pulmonary function, sleep, behavior, cognition, linear growth velocity, body composition, and resting energy expenditure (REE) in children diagnosed with Prader-Willi syndrome. To achieve
this, the researchers adopted a 12-month balanced randomized double-blind, placebo-controlled, cross-over experimental design. They selected twelve subjects and randomly assigned them to either GH or placebo intervention for six months,
and then they crossed over to the alternate intervention for another six months. To ascertain the variations in outcome variables, the researchers performed paired t-tests. Peak flow rate, percentage vital capacity, and forced expiratory
flow rate all increased following the GH intervention whereas the frequency of hypopnea (reduction in ventilation) and apnea (absent breathing) episodes and the length of apnea episodes both showed signs of improvement. However, after
GH intervention, there was no discernible difference in cognition or behavior other than an increase in the hyperactivity scale on the Behavior Assessment System for Children. The GH intervention resulted in an increase in linear growth
velocity, REE, and lean mass. However, the mean fasting ghrelin concentration did not change significantly after the GH intervention. The researchers concluded that GH administration improved body composition and REE, and may contribute
to bettersleep qualityand pulmonary function in children with Prader-Willi syndrome. Nevertheless, GH administration did not impact fasting ghrelin concentration,
suggesting that it may not play a significant role in the observed effects.https://academic.oup.com/jcem/article/88/5/2206/2845458?login=false. - Van cauter E, Copinschi G. Interrelationships between growth hormone and sleep. Growth Horm IGF Res. 2000;10 Suppl B:S57-62.
Interrelationships between growth hormone and sleepIn the study conducted by the researchers, it was found that in healthy young adults, plasma growth hormone (GH) levels exhibited a stable low profile throughout the day,
which was suddenly interrupted by bursts of secretion. Moreover, normal women experienced frequent GH secretory pulses during the day, whereas in normal men, a sleep-onset-associated pulse was typically the major or only episode of active
secretion. There was a strong association between slow-wave (SW) sleep and elevated GH secretion, according to the available data. There was a direct correlation between the amount of concurrent GH secretion and the quantity of SW sleep
as measured visually or by spectral analysis of the EEG. As people age, SW sleep and GH secretion decrease exponentially and follow a similar timeline. The researchers discovered that pharmacological stimulation of SW sleep resulted in
increased GH release. Therefore, compounds that enhanced SW sleep may represent a new class of GH secretagogues.https://pubmed.ncbi.nlm.nih.gov/10984255/. - Copinschi G, Nedeltcheva A, Leproult R, et al. Sleep disturbances, daytime sleepiness, and quality of life in adults with growth hormone deficiency. J Clin Endocrinol Metab. 2010;95(5):2195-202.
Sleep disturbances, daytime sleepiness, and quality of life in adults with growth hormone deficiencyThe researchers wanted to learn more about the connection between poor sleep and GH deficit (GHD), which frequently causes
weariness and low energy. In order to accomplish this goal, they conducted a trial with 30 adult GHD patients who had not received treatment (26 of whom had primary pituitary abnormalities and four of whom had hypothalamic origin) and
30 healthy controls who were matched for age, gender, and body mass index. The patients received hormone replacement therapy for related pituitary deficits. The study found that regardless of the etiology of GHD, patients had poor subjective
sleep quality, as measured by the Pittsburgh Sleep Quality Index score, and a lower Quality of Life Assessment for GHD in Adults score than controls, with tiredness being the most affected domain. The researchers also found that GHD patients
spent more time in slow-wave sleep (SWS) and had a higher intensity of SWS than controls, especially those with pituitary GHD. Older individuals with pituitary GHD obtained less total sleep than controls and their late sleep was more fragmented.
In contrast, the four patients with hypothalamic GHD had a lower intensity of SWS than controls. The study came to the conclusion that GHD is linked to sleep disturbances that could be brought on by particular hormonal changes, leading
to poor subjective sleep quality and excessive daytime sleepiness. Increased fatigue, a significant factor in the decreased quality of life seen in GHD patients, may result from these sleep abnormalities.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2869538/. - Davidson JR, Moldofsky H, Lue FA. Growth hormone and cortisol secretion in relation to sleep and wakefulness. Journal of Psychiatry and Neuroscience. 1991;16(2):96-102.
Growth hormone and cortisol secretion in relation to sleep and wakefulnessThe study aimed to explore the secretory patterns of growth hormone (GH) and cortisol concerning sleep and wakefulness. Ten young men were monitored
for plasma hormone levels during various sleep conditions, including baseline waking and sleeping, 40 hours of wakefulness, and sleep following deprivation. The findings revealed that the normal nocturnal GH surge disappeared with sleep
deprivation but intensified following it. Additionally, GH secretion during sleep after deprivation was prolonged, and some subjects experienced second GH peaks not associated with slow wave sleep (SWS). While average 24-hour cortisol
levels remained unchanged, the timing of the nocturnal cortisol rise was influenced by sleep deprivation and resumed sleep. These results emphasize the sleep-dependent nature of the nocturnal GH surge and highlight the potential impact
of changes in the sleep-wake schedule on the timing of cortisol secretion. You can read the abstract of this article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC1188300/. - Moreno-reyes R, Kerkhofs M, L’hermite-balériaux M, Thorner MO, Van cauter E, Copinschi G. Evidence against a role for the growth hormone-releasing peptide axis in human slow-wave sleep regulation. Am J Physiol. 1998;274(5 Pt 1):E779-84.
Evidence against a role for the growth hormone-releasing peptide axis in human slow-wave sleep regulationThe relationship between sleep and somatotropic activity is complex. While intravenous injections of growth hormone-releasing
hormone (GHRH) during sleep stimulate slow-wave (SW) sleep in humans, a study investigated whether GH-releasing peptide (GHRP) has similar somnogenic effects. Seven young healthy men received bolus intravenous injections of GHRP-2 or saline
after the third rapid-eye-movement period. Although GHRP-2 injections led to transient prolactin elevations and GH pulses within the physiological range, there were no significant effects on sleep, particularly no enhancement of SW sleep.
Unlike GHRH, GHRP-2 at a comparable GH-elevating dosage did not stimulate SW sleep, suggesting that the GHRP axis may not be involved in regulating SW sleep in humans. You can read the full article athttps://journals.physiology.org/doi/full/10.1152/ajpendo.1998.274.5.E779?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org. - Shah N, Rice T, Tracy D, et al. Sleep and Insulin-Like Growth Factors in the Cardiovascular Health Study. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine. 2013;9(12):1245-1251. doi:10.5664/jcsm.3260.
Sleep and Insulin-Like Growth Factors in the Cardiovascular Health StudyThe impact of sleep and sleep disordered breathing (obstructive sleep apnea [OSA]) on the growth hormone/insulin-like growth factor (GH/IGF) axis is well-known.
In this study, the relationship between sleep (architecture and OSA) and circulating levels of IGF-I, IGFBP-1, and IGFBP-3 was investigated in an elderly population. The study analyzed data from 1,233 elderly participants, with a mean
age of 77 years, and found that slow wave sleep (SWS) was better preserved in females compared to males, while OSA was more prevalent in males. However, no significant association was detected between SWS or OSA and circulating IGF-I,
IGFBP-1, and IGFBP-3 levels, indicating that the relationship between SWS and the GH/IGF system may not be significant in the elderly, and OSA may not adversely influence the GH/IGF axis as observed in younger individuals. Further investigation
in other large population-based elderly cohorts is needed to understand the reasons behind these findings. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC3836334/ - Mysliwiec V, Gill J, Matsangas P, Baxter T, Barr T, Roth BJ. IGF-1: a potential biomarker for efficacy of sleep improvement with automatic airway pressure therapy for obstructive sleep apnea?. Sleep Breath. 2015;19(4):1221-8.
IGF-1: a potential biomarker for efficacy of sleep improvement with automatic airway pressure therapy for obstructive sleep apnea?It was known that PAP treatment could reverse obstructive sleep apnea (OSA)-related hypoxia
(low oxygen levels) and restore slow wave sleep (SWS). Positive airway pressure (PAP) treatment was administered as an intervention in a pilot study on 58 young males who had been diagnosed with OSA. The study’s objective was to look into
how PAP affected this population’s serum levels of insulin-like growth factor 1 (IGF-1) and C-reactive protein (CRP). IGF-1 facilitated the repair of neurons from hypoxia and improved sleep regulation among the subjects. However, IGF-1
concentrations were lower in OSA patients and it was unclear whether they would increase following PAP treatment in a younger cohort. The researchers objectively measured adherence to PAP treatment over three months, as well as changes
in the apnea-hypopnea index (AHI). They also measured serum concentrations of IGF-1 and CRP and correlated them with PAP adherence. The results showed that adherence to PAP treatment led to significant increases in IGF-1 concentrations
in young men with OSA. While there was an objective measure of adherence, PAP usage did not allow for the measurement ofsleep improvement.The results
imply that IGF-1 may function as a possible biomarker for the effectiveness of PAP therapy in promoting better sleep. This study advances our knowledge of how PAP therapy affects sleep regulation and sheds light on the function of IGF-1
in the process. More investigation is required to verify these results and examine the possible clinical uses of IGF-1 in the management of OSA.https://pubmed.ncbi.nlm.nih.gov/25724553/. - Izumi S, Ribeiro-filho FF, Carneiro G, Togeiro SM, Tufik S, Zanella MT. IGF-1 Levels are Inversely Associated With Metabolic Syndrome in Obstructive Sleep Apnea. J Clin Sleep Med. 2016;12(4):487-93.
IGF-1 Levels are Inversely Associated With Metabolic Syndrome in Obstructive Sleep ApneaThe study aimed to investigate the relationship between insulin-like growth factor 1 (IGF-1) levels and metabolic syndrome (MetS) in individuals
with obstructive sleep apnea (OSA). The researchers enrolled 84 individuals with OSA and divided them into two groups based on the presence or absence of MetS. They then measured IGF-1 levels and various metabolic parameters in each group.
The results showed that IGF-1 levels were significantly lower in individuals with MetS compared to those without MetS. Additionally, IGF-1 levels were negatively correlated with several metabolic parameters, including waist circumference,
fasting blood glucose, triglycerides, and blood pressure. The researchers concluded that lower IGF-1 levels are associated with a higher risk of MetS in individuals with OSA. These findings suggest that IGF-1 may play a protective role
in the development of metabolic disorders in OSA patients. However, further studies are needed to confirm these findings and to elucidate the underlying mechanisms. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC4795274/. - Izumi S, Ribeiro-Filho FF, Carneiro G, Togeiro SM, Tufik S, Zanella MT. IGF-1 Levels are Inversely Associated With Metabolic Syndrome in Obstructive Sleep Apnea. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy
of Sleep Medicine. 2016;12(4):487-493. doi:10.5664/jcsm.5672.IGF-1 Levels are Inversely Associated with Metabolic Syndrome in Obstructive Sleep Apnea
The study aimed to investigate the relationship between insulin-like growth factor 1 (IGF-1) levels and metabolic syndrome (MetS)
in individuals with obstructive sleep apnea (OSA). The researchers enrolled 84 individuals with OSA and divided them into two groups based on the presence or absence of MetS. They then measured IGF-1 levels and various metabolic parameters
in each group. The results showed that IGF-1 levels were significantly lower in individuals with MetS compared to those without MetS. Additionally, IGF-1 levels were negatively correlated with several metabolic parameters, including waist
circumference, fasting blood glucose, triglycerides, and blood pressure. The researchers concluded that lower IGF-1 levels are associated with a higher risk of MetS in individuals with OSA. These findings suggest that IGF-1 may play a
protective role in the development of metabolic disorders in OSA patients. However, further studies are needed to confirm these findings and to elucidate the underlying mechanisms. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC4795274/. - Rusch HL, Gill JM. Effect of Acute Sleep Disturbance and Recovery on Insulin-Like Growth Factor-1 (IGF-1): Possible Connections and Clinical Implications. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep
Medicine. 2015;11(10):1245-1246. doi:10.5664/jcsm.5108.Effect of Acute Sleep Disturbance and Recovery on Insulin-Like Growth Factor-1 (IGF-1): Possible Connections and Clinical Implications
The study by Rusch and Gill aimed to investigate the possible connections between
acute sleep disturbance, recovery sleep, and insulin-like growth factor-1 (IGF-1) levels, and their potential clinical implications. The researchers reviewed previous studies and found that sleep deprivation or restriction, as well as
disturbed sleep, were associated with decreased IGF-1 levels. In contrast, recovery sleep or increased sleep duration was associated with increased IGF-1 levels. The authors suggest that the relationship between sleep and IGF-1 may have
important clinical implications. For example, low IGF-1 levels have been associated with increased risk of metabolic disorders such as diabetes, as well as decreased muscle mass and bone density. Thus, sleep disturbances may contribute
to the development of these conditions through their effects on IGF-1 levels. Additionally, the authors suggest that improving sleep quality and duration may be a potential intervention to increase IGF-1 levels and prevent or treat associated
health conditions. However, more research is needed to fully understand the complex relationship between sleep and IGF-1 and its clinical implications. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC4582065/. - Prinz PN, Moe KE, Dulberg EM, et al. Higher plasma IGF-1 levels are associated with increased delta sleep in healthy older men. J Gerontol A Biol Sci Med Sci. 1995;50(4):M222-6.
Higher plasma IGF-1 levels are associated with increased delta sleep in healthy older men
In this study, the researchers investigated the association between plasma insulin-like growth factor-1 (IGF-1) levels and delta
sleep (slow-wave sleep) in healthy older men. Delta sleep is a stage of deep sleep that is important for physical restoration and growth hormone release. The study included 12 healthy men with an average age of 72 years. The participants
underwent overnight sleep studies and blood tests to measure IGF-1 levels. The results showed that higher plasma IGF-1 levels were associated with increased delta sleep, but not with other stages of sleep. These findings suggest that IGF-1
may play a role in promoting delta sleep in healthy older men. Further research is needed to determine the underlying mechanisms and potential clinical implications of this association. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/7614245/. - Damanti S, Bourron O, Doulazmi M, et al. Relationship between sleep parameters, insulin resistance and age-adjusted insulin like growth factor-1 score in non diabetic older patients. Blondeau B, ed. PLoS ONE. 2017;12(4):e0174876. doi:10.1371/journal.pone.0174876.
Relationship between sleep parameters, insulin resistance and age-adjusted insulin like growth factor-1 score in non-diabetic older patients
The study aimed to investigate the relationship between sleep parameters,
insulin resistance, and age-adjusted insulin-like growth factor-1 (IGF-1) score in non-diabetic older patients. The researchers conducted a cross-sectional study of 117 patients aged 65 years or older. They evaluated the patients’ sleep
quality using the Pittsburgh Sleep Quality Index (PSQI), insulin resistance using the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR), and IGF-1 levels. The study found that poor sleep quality was associated with higher HOMA-IR
scores, indicating greater insulin resistance. Additionally, age-adjusted IGF-1 scores were negatively associated with HOMA-IR scores, indicating that lower IGF-1 levels were associated with greater insulin resistance. The study did not
find a significant relationship between sleep parameters and age-adjusted IGF-1 scores. The findings suggest that poor sleep quality may contribute to insulin resistance in non-diabetic older patients and that lower IGF-1 levels may be
a marker of insulin resistance in this population. You can read the full article athttps://www.ncbi.nlm.nih.gov/pmc/articles/PMC5383056/. - Obál F, Kapás L, Gardi J, Taishi P, Bodosi B, Krueger JM. Insulin-like growth factor-1 (IGF-1)-induced inhibition of growth hormone secretion is associated with sleep suppression. Brain Res. 1999;818(2):267-74.
Insulin-like growth factor-1 (IGF-1)-induced inhibition of growth hormone secretion is associated with sleep suppression
The study investigated the association between insulin-like growth factor-1 (IGF-1), sleep parameters, and insulin resistance in non-diabetic older patients. The researchers recruited 57 participants and collected their blood samples to
measure IGF-1 and insulin levels. They also monitored the participants’ sleep using a wrist actigraph and calculated sleep parameters such as sleep efficiency, wake after sleep onset, and total sleep time. The results showed a positive
association between IGF-1 levels and sleep efficiency, meaning that higher levels of IGF-1 were associated with better sleep quality. On the other hand, there was a negative association between IGF-1 levels and wake after sleep onset,
indicating that higher levels of IGF-1 were associated with less time spent awake during the night. The study also found that insulin resistance was associated with lower levels of IGF-1, which suggests that IGF-1 may play a role in
the development of insulin resistance in older individuals. Overall, the findings suggest that IGF-1 may be a potential therapeutic target for improving sleep quality and preventing insulin resistance in older adults.https://www.sciencedirect.com/science/article/abs/pii/S0006899398012864?via%3Dihub. - Chennaoui M, Drogou C, Sauvet F, Gomez-merino D, Scofield DE, Nindl BC. Effect of acute sleep deprivation and recovery on Insulin-like Growth Factor-I responses and inflammatory gene expression in healthy men. Eur Cytokine Netw. 2014;25(3):52-7.
Effect of acute sleep deprivation and recovery on Insulin-like Growth Factor-I responses and inflammatory gene expression in healthy men
The study investigated the effects of acute sleep deprivation and subsequent recovery
on insulin-like growth factor-I (IGF-I) responses and inflammatory gene expression in healthy men. The researchers enrolled 12 healthy male subjects who underwent two different conditions: one night of total sleep deprivation and a control
condition with normal sleep duration. Blood samples were collected at multiple time points before and after each condition to measure IGF-I levels and inflammatory gene expression. The results showed that acute sleep deprivation led to
a significant decrease in IGF-I levels in the early morning, which was partially recovered after one night of sleep. In addition, the expression of several inflammatory genes, including IL-6 and TNF-alpha, was increased following sleep
deprivation, and this increase was partially reversed after recovery sleep. These findings suggest that acute sleep deprivation can negatively affect IGF-I responses and promote inflammation, which may have implications for metabolic and
cardiovascular health. You can read the abstract of this article athttps://pubmed.ncbi.nlm.nih.gov/25373853/. - Rasmussen MH, Wildschiødtz G, Juul A, Hilsted J. Polysomnographic sleep, growth hormone insulin-like growth factor-I axis, leptin, and weight loss. Obesity (Silver Spring). 2008;16(7):1516-21.
Polysomnographic sleep, growth hormone insulin-like growth factor-I axis, leptin, and weight loss
The study conducted by Rasmussen et al. in 2008 aimed to investigate the relationship between polysomnographic sleep, growth hormone (GH), insulin-like growth factor-I (IGF-I) axis, leptin, and weight loss in obese individuals. The study
recruited ten obese participants who underwent a weight loss program for 3 months. Polysomnographic sleep was monitored before and after the weight loss program,
while GH, IGF-I, and leptin levels were measured at baseline and after 3 months.The results showed that after the weight loss program, the participants significantly reduced body weight, body mass index (BMI), and fat mass. Polysomnographic sleep also improved significantly, with increased sleep efficiency, total
sleep time, and decreased wake time after sleep onset. GH and IGF-I levels increased significantly after weight loss, while leptin levels decreased. Interestingly, the GH and IGF-I levels changes were significantly associated with
improvements in sleep efficiency and total sleep time.The study suggests that weight loss can positively impact polysomnographic sleep, GH-IGF-I axis, and leptin levels in obese individuals. The findings also highlight the potential of GH and IGF-I as therapeutic targets for sleep disorders
and obesity. However, further studies with larger sample sizes and longer follow-up periods are needed to confirm these findings and explore their clinical implications.You can read the full article at https://onlinelibrary.wiley.com/doi/10.1038/oby.2008.249.
- Levada OA, Troyan AS. Insulin-like growth factor-1: a possible marker for emotional and cognitive disturbances, and treatment effectiveness in major depressive disorder. Annals of General Psychiatry. 2017;16:38. doi:10.1186/s12991-017-0161-3.
Possible marker for emotional and cognitive disturbances, and treatment effectiveness in major depressive disorder
The study conducted by Levada and Troyan (2017) aimed to investigate the association between insulin-like growth factor-1 (IGF-1) and emotional and cognitive disturbances in individuals with major depressive disorder (MDD). The researchers
also aimed to evaluate the potential of IGF-1 as a marker for treatment effectiveness in MDD.The study included 81 patients diagnosed with MDD and 57 healthy controls. The researchers collected blood samples and measured serum levels of IGF-1 in all participants. They also assessed the emotional and cognitive status of the participants
using standardized questionnaires.The results showed that patients with MDD had significantly lower levels of IGF-1 compared to healthy controls. The researchers also found a significant association between low levels of IGF-1 and more severe depressive symptoms and cognitive
deficits in patients with MDD.Furthermore, the study found that treatment with antidepressant medication significantly increased IGF-1 levels in patients with MDD who responded well to treatment. However, non-responders to antidepressant medication did not show a significant
increase in IGF-1 levels.Overall, the study suggests that low levels of IGF-1 may be associated with emotional and cognitive disturbances in patients with MDD. The findings also suggest that IGF-1 could be a potential biomarker for treatment effectiveness in MDD.
Further research is needed to explore the role of IGF-1 in the pathophysiology of MDD and its potential use as a biomarker for treatment effectiveness.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5659027/.
- Peterfi Z, McGinty D, Sarai E, Szymusiak R. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2010;298(1):R147-R156.
doi:10.1152/ajpregu.00494.2009.Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus
The study by Peterfi et al. in 2010 aimed to investigate the effect of growth hormone-releasing hormone (GHRH) on sleep-regulatory neurons in the preoptic hypothalamus of rats. The study found that administration of GHRH increased the
activity of preoptic hypothalamic neurons, which are known to regulate sleep-wake cycles. GHRH may regulate sleep and wakefulness and provides insight into how growth hormone (GH) affects sleep.Furthermore, the study also found that GHRH-induced activation of preoptic hypothalamic neurons was blocked by the administration of a GABA receptor agonist, indicating that the effects of GHRH on sleep regulatory neurons are mediated
through GABAergic neurotransmission. These findings further support the role of GABA in sleep regulation and suggest a potential therapeutic target for sleep disorders. Overall, this study provides important insights into the relationship
between GHRH, GH, and sleep and highlights the potential for GABAergic modulation in treating sleep disorders.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2806209/.
- Cordido F, Peñalva A, Dieguez C, Casanueva FF. Massive growth hormone (GH) discharge in obese subjects after the combined administration of GH-releasing hormone and GHRP-6: evidence for a marked somatotroph secretory capability in obesity. J Clin Endocrinol
Metab. 1993;76(4):819-23.Massive growth hormone (GH) discharge in obese subjects after the combined administration of GH-releasing hormone and GHRP-6: evidence for a marked somatotroph secretory capability in obesity
Cordido et al. investigated the growth hormone (GH) response to the combined administration of GH-releasing hormone (GHRH) and GH-releasing peptide-6 (GHRP-6) in obese individuals. The researchers found that obese individuals demonstrated
a significantly higher GH response to the combined administration of GHRH and GHRP-6 than lean individuals, indicating a marked somatotroph secretory capability in obesity. The study also found that the peak GH response to the combined
administration of GHRH and GHRP-6 was positively correlated with body mass index (BMI) and body
fat percentage. These findings suggest that obesity may enhance GH secretion and that GH secretagogues may be a potential therapy for individuals with obesity-associated GH deficiency.Overall, the study highlights the importance of investigating the underlying mechanisms of GH secretion in obesity. Further research is needed to explore the potential therapeutic applications of GH secretagogues in individuals with obesity-associated
GH deficiency and better understand the role of GH in obesity-related metabolic dysfunction. The study’s findings provide valuable insight into the pathophysiology of obesity and may have implications for the development of targeted
treatments for individuals with obesity-associated metabolic disorders.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8473389/.
- Cordido F, Peñalva A, Peino R, Casanueva FF, Dieguez C. Effect of combined administration of growth hormone (GH)-releasing hormone, GH-releasing peptide-6, and pyridostigmine in normal and obese subjects. Metab Clin Exp. 1995;44(6):745-8.
Effect of combined administration of growth hormone (GH)-releasing hormone
The study by Cordido et al. (1995) investigated the effects of the combined administration of growth hormone-releasing hormone (GHRH), growth hormone-releasing peptide-6 (GHRP-6), and pyridostigmine in both normal and obese subjects. The
results showed that the combined administration of these agents induced a marked increase in growth hormone (GH) secretion in both groups. In obese subjects, the GH response was significantly higher than in normal subjects, suggesting
a greater somatotroph secretory capability in obesity.The study also found that adding pyridostigmine to the GHRH/GHRP-6 combination did not further increase GH secretion in either group, indicating that the cholinergic component of GH regulation may not be a limiting factor in GH secretion
under these conditions. These findings suggest that combining GHRH and GHRP-6 may be a useful strategy for enhancing GH secretion in both normal and obese individuals, with potential therapeutic applications in treating GH deficiency
or other related conditions.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/7783658/.
- Copinschi G, Leproult R, Van onderbergen A, et al. Prolonged oral treatment with MK-677, a novel growth hormone secretagogue, improves sleep quality in man. Neuroendocrinology. 1997;66(4):278-86.
Prolonged oral treatment with MK-677, a novel growth hormone secretagogue, improves sleep quality in man
In 1997, Copinschi et al. conducted a study investigating the effect of prolonged treatment with MK-677, a growth hormone secretagogue, on human sleep quality. The study included 10 healthy young men who were given either MK-677 or a placebo
for two weeks. The results showed that the participants who received MK-677 significantly improved sleep quality, including an increase in total sleep time, slow wave sleep, and REM sleep. Additionally, there was an increase in growth
hormone levels during sleep, which is associated with better sleep quality. The researchers concluded that MK-677 may be a potential treatment for sleep disorders.However, it is important to note that this study was conducted on a small sample size and more research is needed to confirm the findings. Additionally, MK-677 is not approved for use as a sleep aid and should only be used under the supervision
of a healthcare provider.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/9349662/.
- Diekelmann S. Sleep for cognitive enhancement. Frontiers in Systems Neuroscience. 2014;8:46. doi:10.3389/fnsys.2014.00046.
Sleep for cognitive enhancement. Frontiers in Systems Neuroscience
The article by Diekelmann explores the importance of sleep for cognitive enhancement. The author highlights how sleep is crucial for learning, memory consolidation, and creativity. Furthermore, the article discusses the different stages
of sleep and their respective roles in cognitive processes. The author argues that quantity and quality of sleep are essential for optimal cognitive functioning, and lack of sleep can have detrimental effects on cognition. Finally,
the article proposes various methods for improving sleep quality and maximizing cognitive benefits.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3980112/.
- Aleman A, Verhaar HJ, De haan EH, et al. Insulin-like growth factor-I and cognitive function in healthy older men. J Clin Endocrinol Metab. 1999;84(2):471-5.
Insulin-like growth factor-I and cognitive function in healthy older men
The purpose of the current study was to look into the relationship between cognitive function in healthy older men and the age-related fall in circulating levels of IGF-I. Twenty-five people with intact functional capacity were enrolled
in the study. To determine which cognitive abilities are sensitive to cognitive aging and which ones are not, the researchers used neuropsychological tests.The results of the study indicated that higher levels of IGF-I were significantly
associated with better performance on the Digit Symbol Substitution test and the Concept Shifting Task. These tests measure perceptual-motor and mental processing speed, which are known to decline with aging. However, there was no
association between IGF-I levels and the performances on the tests of general knowledge, vocabulary, basic visual perception, and reading ability. These findings suggest that the age-related decline in circulating levels of IGF-I may
play a role in the reduction of certain cognitive functions, specifically speed of information processing. This supports the hypothesis that the GH/IGF-I axis is involved in aging of physiological functions, including cognitive functioning.
It is important to note that this study had a relatively small sample size and only included men. Therefore, further studies with larger and more diverse samples are needed to confirm these findings and to investigate the potential
gender differences in the association between IGF-I and cognitive functioning.You can read the full article at https://academic.oup.com/jcem/article/84/2/471/2864174?login=false.
- Baker LD, Barsness SM, Borson S, et al. Effects of Growth Hormone–Releasing Hormone on Cognitive Function in Adults With Mild Cognitive Impairment and Healthy Older Adults: Results of a Controlled Trial. Archives of neurology. 2012;69(11):1420-1429. doi:10.1001/archneurol.2012.1970.
Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial
In the past, researchers have discovered that Growth hormone-releasing hormone (GHRH), growth hormone, and insulin-like growth factor 1 have significant impacts on brain function, but their levels decrease as people age, which may contribute
to Alzheimer’s disease. Previous studies conducted by the researchers showed positive cognitive effects of short-term GHRH administration in healthy older adults and provided initial evidence that adults with mild cognitive impairment
(MCI) might benefit from the treatment.The goal of this study was to look into how GHRH affects cognitive function in older persons with and without MCI. The study included 152 persons in total, with a mean age of 68 and 66 of them
having MCI. Tesamorelin, a stable analog of human GHRH, was given daily via subcutaneous injection at a dose of 1 mg/d, or a placebo, 30 minutes before bedtime for a period of 20 weeks.Throughout the study, blood samples were taken
and a cognitive battery was administered at baseline, week 10, week 20, and after a 10-week washout at week 30. An oral glucose tolerance test and a dual-energy x-ray absorptiometry scan were also conducted before and after the 20-week
intervention to measure body composition. The primary cognitive outcomes were analyzed using analysis of variance and included three composites reflecting executive function, verbal memory, and visual memory. The researchers found
that the intent-to-treat analysis showed a favorable effect of GHRH on cognition, which was similar in both adults with MCI and healthy older adults. The completer analysis indicated a similar pattern, with a more robust GHRH effect.
Subsequent analyses indicated a positive GHRH effect on executive function and a trend showing a similar treatment-related benefit in verbal memory. In addition, the GHRH therapy decreased body fat by 7.4% and elevated insulin-like
growth factor 1 levels by 117% while maintaining physiological levels. Additionally, in persons with MCI but not in healthy adults, the therapy raised fasting insulin levels within the normal range by 35%. Mild adverse events were
reported by 36% of adults who got a placebo and 68% of adults who took GHRH.Overall, the study shows that 20 weeks of GHRH administration had positive effects on cognition in both healthy older adults and adults with MCI. Longer-duration
treatment trials are needed to further examine the therapeutic potential of GHRH administration on brain health during normal aging and “pathological aging.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764914/.
- Alvarez A, Cacabelos R, Sanpedro C, García-Fantini M, Aleixandre M. Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer’s disease. Neurobiol Aging. 2007;28(4):533–536.
Serum TNF-alpha levels are increased and correlate negatively with free IGF-I in Alzheimer disease
The development of Alzheimer’s disease (AD) was found to be significantly influenced by the neurotoxic and survival factors insulin-like growth factor-I (IGF-I) and tumor necrosis factor-alpha (TNF-alpha) respectively. Recent experimental
research showed a functional relationship between the TNF-alpha and IGF-I signaling pathways. By measuring the serum levels of total IGF-I, free IGF-I, and TNF-alpha in 141 AD patients, 56 MCI cases, and 30 controls, the researchers
aimed to learn more about any potential interactions between TNF-alpha and IGF-I in AD and mild cognitive impairment (MCI). Compared to the control group, the AD patients showed elevated TNF-alpha levels and reduced IGF-I levels in
the serum. Additionally, there was a significant negative correlation between TNF-alpha and free IGF-I values. The MCI patients also had significantly higher TNF-alpha levels than the controls. The present findings suggest that increased
TNF-alpha levels are involved in the pathogenesis of AD and MCI and may counteract the neurotrophic activity of IGF-I in these medical conditions. Additionally, the measurement of TNF-alpha and IGF-I together may be helpful for tracking
the effects of anti-inflammatory and/or neurotrophic medications in AD. These findings imply that TNF-alpha and IGF-I are both involved in the pathogenesis of AD and MCI, and that the interaction between these two proteins may be a
key factor in the development of these diseases.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16569464/.
- Luppi C, Fioravanti M, Bertolini B, et al. Growth factors decrease in subjects with mild to moderate Alzheimer’s disease (AD): potential correction with dehydroepiandrosterone-sulfate (DHEAS) Arch Gerontol Geriatr. 2009;49(suppl 1):173–184.
Growth factors decrease in subjects with mild to moderate Alzheimer’s disease (AD): potential correction with dehydroepiandrosterone-sulphate (DHEAS)
Investigating the function of neuroprotection in avoiding cognitive impairment and Alzheimer’s disease was of interest to the researchers. They proposed that DHEAS, a hormone with advantageous metabolic and endocrine effects, might slow
down the aging of the brain by reactivating neuroprotective growth factors. The levels of insulin-like growth factor-1 (IGF-1), vascular endothelial growth factor (VEGF), and transforming growth factor-beta1 (TGFbeta1) in the supernatants
of cultured peripheral blood mononuclear cells (PBMC) from healthy subjects and age-matched patients with mild to moderate AD were measured by the researchers using ELISA to test this hypothesis.They separated the natural killer cells
(NK) from PBMC (PBMC-NK) and measured the growth factors in spontaneous conditions and after stimulation with growth hormone (GH) 1 microg/ml (IGF-1), lipopolysaccharide (LPS) 1 microg/ml (VEGF) and glucose 10 microM (TGF(beta1)).The
results of the study suggested that DHEAS can increase the production of neuroprotective growth factors, which are reduced in patients with AD. Specifically, the immunoendocrine production of IGF-1, VEGF, and TGFbeta1 was significantly
higher in healthy subjects compared to patients with AD. Furthermore, the researchers found that the stimulation of PBMC-NK with GH, LPS, and glucose resulted in increased levels of IGF-1, VEGF, and TGFbeta1 in healthy subjects, but
not in patients with AD.These results lead the researchers to propose that DHEAS, by enhancing the synthesis of neuroprotective growth factors, may be a possible new strategy for the treatment of dementia. To examine the possible therapeutic
advantages of DHEAS in the prevention and treatment of cognitive problems and AD, however, and to confirm these findings, more research is required.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/19836631/.
- Aleman A, Torres-Alemán I. Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan. Prog Neurobiol. 2009;89(3):256–265.
Circulating insulin-like growth factor I and cognitive function: neuromodulation throughout the lifespan
Even in adulthood, insulin-like growth factor I (IGF-I) continued to promote tissue growth and exert anabolic effects, playing a crucial function in the somatotropic (growth hormone) axis. A growing body of studies over the past ten years
has shown that IGF-I levels in the blood significantly affect cognitive brain function. It was hypothesized that age-related cognitive deterioration in the elderly might be linked to a drop in serum IGF-I levels. Furthermore, psychiatric
and neurological conditions characterized by cognitive impairment might be linked to altered levels of IGF-I. Researchers found that interventions targeting the GH/IGF-I axis could improve cognitive functioning, at least in deficient
states. However, since high serum IGF-I levels were associated with cancer risk, these interventions required careful evaluation.IGF-I appeared to be an essential element of brain homeostasis at the cellular and molecular levels. IGF-I
input disruption inexorably led to function disruption. All nerve cells, including neurons, glia, endothelial, epithelial, and perivascular cells, were potential targets of IGF-I activities. IGF-I’s neurotrophic and modulatory effects
on numerous important cellular processes in the brain. After reviewing how IGF-I affects neurotransmission and neuronal plasticity, the researchers came to the conclusion that serum IGF-I is a key mediator of neuronal development,
survival, and function over the course of a person’s lifetime. The study team discovered that IGF-I’s synaptic plasticity function made its neurotrophic potential an important target for treating the cognitive impairment brought on
by a variety of neurological diseases.You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0301008209001129?via%3Dihub.
- van Dam PS, Aleman A. Insulin-like growth factor-I, cognition and brain aging. Eur J Pharmacol. 2004;490(1–3):87–95.
Insulin-like growth factor-I, cognition and brain aging
The researchers found extensive documentation of age-related decline in cognitive functions, particularly attention, long-term memory, and executive functioning, which are vulnerable to aging. The decline in the activity of the GH/IGF-I
axis, which coincides with aging, has been reported. It has been speculated that this relative hyposomatotropism may contribute to the decline in cognitive functioning that occurs with age.Two observations lend support to this theory.
First, research on animals and in vitro has demonstrated that IGF-I affects neuronal cell activity. Additionally, the prefrontal cortex and hippocampus, two regions of the central nervous system crucial for cognitive function, have
been found to contain considerable amounts of IGF-I receptors. Second, patients with GH insufficiency who had significantly lower plasma and central nervous system levels of IGF-I have shown impaired cognitive function. In recent years,
more researchers have focused on the interaction between the hormones of the somatotropic axis and the central nervous system. The present review aims to provide an overview of the available information on the association between attenuated
IGF-I secretion and cognitive performance in the elderly and in GH-deficient patients.As the possible underlying mechanisms regarding IGF-I receptor signaling and molecular and cellular mechanisms in the brain are discussed elsewhere in the present special issue of this journal, the researchers primarily focus on human
studies regarding the association between the somatotropic axis and cognitive performance. In summary, aging is associated with a decline in the activity of the GH/IGF-I axis, which coincides with a decline in specific cognitive functions.
The researchers hypothesized that a causal relationship exists between the reduction in circulating GH and/or IGF-I and the observed cognitive deficits in the elderly. The present review summarized available data on the possible relation
between GH, IGF-I, and cognitive performance, along with possible underlying pathophysiological mechanisms. The researchers found evidence supporting the hypothesis, and more research is needed to fully understand the underlying mechanisms
of this relationship.You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014299904002043.
- Rollero A, Murialdo G, Fonzi S, et al. Relationship between cognitive function, growth hormone and insulin-like growth factor I plasma levels in aged subjects. Neuropsychobiology. 1998;38(2):73–79.
Relationship between cognitive function, growth hormone and insulin-like growth factor I plasma levels in aged subjects
Researchers examined baseline growth hormone (GH), insulin-like growth factor I (IGF-I), and GH responses to GH-releasing hormone (GHRH) in 22 participants, including 7 females and 15 men, between the ages of 65 and 86. The study’s objective
was to investigate a potential link between age-dependent GH-IGF-I axis deterioration and cognitive performance as measured by the Mini Mental State Examination (MMSE). The study also examined the associations between hormonal information,
cognition, age, body weight, body mass index (BMI), specific nutritional indices (triceps skinfolds, TSF,
mid-arm circumference, MAC), and the physical functional index (PFI), which measures physical activity.Results indicated that GH basal levels were within the normal range, while GH responses to GHRH were mostly blunted.GH peaks after GHRH were directly correlated with GH basal values. IGF-I serum levels were found to be in the lower part of the reference range for adult subjects or below it. GH responses to GHRH were inversely correlated with subject
age, but GH and IGF-I basal levels did not show such a correlation. GH secretion areas after GHRH were inversely correlated with BMI, but no further correlations between GH data and clinical or nutritional parameters were found. MAC
and PFI values had a direct correlation with MMSE scores. IGF-I levels were positively connected with MAC values, which are assumed to represent protein-caloric malnutrition, and with MMSE scores, which were lower in patients with
more advanced cognitive decline. However, there was no connection between IGF-I levels and body weight, BMI, TSF, or PFI. In those with mild cognitive impairment, MMSE-related protein-caloric malnutrition and decreased physical activity
may alter IGF-I function, and IGF-I decline may in turn affect neuronal function.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/9732206/.
- Frago LM, Pañeda C, Dickson SL, Hewson AK, Argente J, Chowen JA. Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signalling pathways involved in neuroprotection. Endocrinology.
2002;143(10):4113-22.Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection
The researchers found that GH had beneficial effects on memory, mental alertness, and motivation, which were mediated through IGF-I. To investigate whether systemic administration of GH or GHRP-6 modulated the brain IGF system, the researchers
treated adult male rats with GHRP-6 or GH for one week.The findings demonstrated that IGF-I mRNA levels were markedly elevated by both GHRP-6 and GH in the hypothalamus, cerebellum, and hippocampus but not in the cerebral cortex. IGF-binding
protein (IGFBP)-2 and the expression of the IGF receptor, however, were unaffected. Where IGF-I was elevated, Akt and Bad phosphorylation were stimulated, but MAPK and glycogen synthase kinase-3beta were unaffected.This suggests that in response to growth hormones, GH and GHRP-6 activate intracellular phosphatidylinositol kinase pathways important for cell survival.Furthermore, the antiapoptotic protein Bcl-2 was augmented in these same areas, but
there was no change in the proapoptotic protein Bax. IGFBP-5, which is involved in neuron survival processes, was increased mainly in the hypothalamus, suggesting a possible neuroendocrine role. In conclusion, the researchers found
that GH and GHRP-6 modulated IGF-I expression in the central nervous system in an anatomically specific manner. This coincided with activation of intracellular signaling pathways used by IGF-I and increased expression of proteins involved
in cell survival or neuroprotection.You can read the full article at https://academic.oup.com/endo/article/143/10/4113/2880897?login=false.
- Baserga R, Hongo A, Rubini M, Prisco M, Valentini SB 1997 The IGF-I receptor in cell growth, transformation and apoptosis. Biochim Biophys Acta 1332:F105–F126.
The IGF-I receptor in cell growth, transformation and apoptosis
The study by Baserga et al. (1997) explored the role of the insulin-like growth factor-I receptor (IGF-I receptor) in cell growth, transformation, and apoptosis. The researchers investigated the cellular mechanisms and signaling pathways
associated with the IGF-I receptor and its impact on cell behaviors such as growth, transformation (the process of normal cells becoming cancerous), and apoptosis (programmed cell death). The study provided insights into the multifaceted
functions of the IGF-I receptor in regulating cellular processes and shed light on its potential implications in various physiological and pathological conditions.You can read the abstract of this article at https://www.sciencedirect.com/science/article/abs/pii/S0304419X97000073?via%3Dihub
- Kulik G, Klippel A, Weber MJ 1997 Antiapoptotic signaling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase and Akt. Mol Cell Biol 17:1595–1606.
Kulik G, Klippel A, Weber MJ 1997 Antiapoptotic signaling by the insulin-like growth factor I receptor, phosphatidylinositol 3-kinase and Akt. Mol Cell Biol 17:1595–1606.
The investigation was done to determine whether insulin-like growth factor I (IGF-I) can prevent UV-B-induced apoptosis in fibroblasts. It was found that the IGF-I receptor’s receptor kinase activity was necessary for its antiapoptotic
signaling. The researchers discovered that overexpressing kinase-defective receptor mutants did not provide IGF-I protection for the cells, and that overexpressing a kinase-defective receptor with an ATP binding loop mutation acted
as a dominant negative, increasing the sensitivity of the cells to apoptosis.During the study, the researchers also tested the antiapoptotic capacity of other growth factors, such as epidermal growth factor (EGF) and thrombin, but
found that they did not have the same protective effects as IGF-I. However, EGF was antiapoptotic for cells overexpressing the EGF receptor, and expression of activated pp60v-src also provided protection.The researchers did not discover a connection between resistance to apoptosis and the activation of p38/HOG1, p70S6 kinase, or mitogen-activated protein kinase. However, they did discover that wortmannin prevented any of the tyrosine kinases
from preventing UV-induced apoptosis, suggesting a function for phosphatidylinositol 3-kinase (PI3 kinase). The researchers tested this hypothesis by transiently expressing constitutively active or kinase-dead PI3 kinase, and they
discovered that overexpression of activated PI3 kinase was sufficient to offer protection against apoptosis.The researchers also examined the role of Akt/PKB in antiapoptotic signaling, as it is believed to be a downstream effector
for PI3 kinase. They discovered that membrane-targeted Akt was enough to protect against apoptosis, while kinase-dead Akt was not. In conclusion, the researchers found that the endogenous IGF-I receptor has a specific antiapoptotic
signaling capacity and that overexpression of other tyrosine kinases can also make them antiapoptotic. They also found that activation of PI3 kinase and Akt is enough for antiapoptotic signaling.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC231885/pdf/171595.pdf.
- Chrysis D, Calikoglu AS, Ye P, D’Ercole AJ 2001 Insulin-like growth factor-I overexpression attenuates cerebellar apoptosis by altering the expression of Bcl family proteins in a developmentally specific manner. J Neurosci 21:1481–1489.
Insulin-like growth factor-I overexpression attenuates cerebellar apoptosis by altering the expression of Bcl family proteins in a developmentally specific manner.
The central nervous system (CNS) of transgenic (Tg) mice that only express insulin-like growth factor-I (IGF-I) was the subject of the study. Their earlier research had demonstrated that the mice’s improved survival led to a notable rise
in the number of cerebellar granule cells. There were little investigations on the effects of IGF-I in vivo, despite the fact that its anti-apoptotic properties in cultured neurons were widely recognized. Therefore, the researchers
wanted to examine IGF-I signaling mechanisms in the same Tg mice and document IGF-I’s anti-apoptotic effects during cerebellar development.Compared to non-Tg littermates, the researchers found fewer apoptotic cells in the cerebellum
of Tg mice at postnatal day 7 (P7) and a similar trend at P14 and P21. They observed a decrease in procaspase-3 and caspase-3 in the cerebellum of Tg mice at each age studied. The decline in caspase-3 was accompanied by a decrease
in the 85 kDa fragment of Poly(ADP-ribose) polymerase, which is a known product of caspase cleavage, indicating decreased caspase activity. At P7, decreased apoptosis in Tg mice was linked to increased expression of anti-apoptotic
Bcl genes, Bcl-x(L), and Bcl-2.Although the mRNA expression of the proapoptotic Bcl genes, Bax, and Bad, was also increased, no changes were observed in the abundance of their proteins. At P14, the researchers found that Bcl-xL and Bcl-2 expression was similar in normal
and Tg mice. Bax mRNA was unchanged in Tg mice, but its protein abundance was decreased, and both Bad mRNA and protein abundance were decreased. At P21, Bcl-xL and Bcl-2 expression remained unchanged, but Bax and Bad expression were
decreased. The researchers concluded that IGF-I exerts anti-apoptotic effects during cerebellar development, altering the magnitude of naturally occurring apoptosis. IGF-I seems to affect multiple steps in the apoptotic pathway in
a developmentally specific manner. IGF-I decreases caspase-3 availability and activity, increases the expression of anti-apoptotic Bcl-x(L) and Bcl-2 during early postnatal development, and decreases proapoptotic Bax and Bad expression
at later developmental stages.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6762946/.
- Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, Hemmings BA 1996 Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J 15:6541–6551.
Mechanism of activation of protein kinase B by insulin and IGF-1
The purpose of the study was to determine how insulin affected the activity of endogenous protein kinase B alpha (PKBalpha) in L6 myotubes and 293 cells. They discovered that while transfection into 293 cells led to PKBalpha activation
of 20 and 50 fold in response to insulin and IGF-1, respectively, PKBalpha activity was raised by insulin by a factor of 12 in L6 myotubes.The activation of PKBalpha in both cell types was accompanied by phosphorylation at Thr308 and
Ser473. Wortmannin, a phosphatidylinositol 3-kinase inhibitor, prevented the phosphorylation of both residues, indicating their critical role in PKBalpha activity.The researchers also analyzed the activities of mutant PKBalpha molecules
with Thr308 and/or Ser473 mutated to Ala or Asp after transfection into 293 cells.They measured the activity of wild-type and mutant PKBalpha in vitro after stoichiometric phosphorylation of Ser473 by MAPKAP kinase-2.Their research shown that the high level of PKBalpha activity caused by insulin or IGF-1 depends on
the phosphorylation of both Thr308 and Ser473. Additionally, they discovered that phosphorylation of Ser473 or Thr308 in vivo is not necessary for each other.Based on their findings, the researchers put up a theory in which upstream
kinase(s) phosphorylate and activate PKBalpha in insulin/IGF-1-stimulated cells.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC452479/pdf/emboj00023-0183.pdf.
- Dudek H, Datta SR, Franke TF, Birnbaum MJ, Yao R, Cooper GM, Segal RA, Kaplan DR, Greenberg ME 1997 Regulation of neural survival by the serine-threonine protein kinase Akt. Science 275:661–668.
Regulation of neuronal survival by the serine-threonine protein kinase Akt
The researchers in this study have delineated a signaling pathway that was involved in promoting the survival of cerebellar neurons by insulin-like growth factor 1 (IGF-1). The activation of phosphoinositide 3-kinase (PI3-K) by IGF-1 triggered
the activation of two protein kinases, namely the serine-threonine kinase Akt and the p70 ribosomal protein S6 kinase (p70(S6K)). By conducting experiments with pharmacological inhibitors and expressing wild-type and dominant-inhibitory
forms of Akt, the researchers were able to demonstrate that Akt, but not p70(S6K), mediates PI3-K-dependent survival.According to the results of this study, Akt is essential for growth factor-induced neuronal survival in the developing
nervous system. The scientists were able to demonstrate how IGF-1 activated PI3-K, which then activated Akt, promoting neuronal survival.The fact that p70(S6K) was not engaged in this procedure, however, was also discovered by the researchers. This finding suggests that Akt is the primary mediator of PI3-K-dependent survival in cerebellar neurons.Overall, these results
provide important insights into the mechanisms underlying neuronal survival in the developing nervous system. By identifying the specific signaling pathway involved in IGF-1-induced survival, the researchers have opened up new avenues
for the development of therapies aimed at promoting neuronal survival and preventing neuronal death.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/9005851/.
- Mehrhof FB, Muller FU, Bergmann MW, Li P, Wang Y, Schmitz W, Dietz R, von Harsdorf R 2001 In cardiomyocyte hypoxia, insulin-like growth factor-I-induced antiapoptotic signaling requires phosphatidylinositol-3-OH-kinase-dependent and mitogen-activated
protein kinase-dependent activation of the transcription factor cAMP response element-binding protein. Circulation 104:2088–2094In Cardiomyocyte Hypoxia, Insulin-Like Growth Factor-I-Induced Antiapoptotic Signaling Requires Phosphatidylinositol-3-OH-Kinase-Dependent and Mitogen-Activated Protein Kinase-Dependent Activation of the Transcription Factor cAMP Response Element-Binding Protein
The researchers looked into different pathological factors that led to cardiomyocyte apoptosis. They discovered that in the heart, survival factors including insulin-like growth factor-I (IGF-I) have anti-apoptotic effects. The underlying
signaling pathways causing these impacts weren’t fully understood, though. To understand this better, the researchers conducted an experiment on cultured neonatal cardiomyocytes exposed to hypoxia-induced apoptosis. They observed that
IGF-I prevented cell death in a dose-dependent manner. The anti-apoptotic signals induced by IGF-I were mediated by more than one signaling pathway. Pharmacological inhibition of the phosphatidylinositol-3-OH-kinase (PI3K) or the mitogen-activated
protein kinase kinase (MEK1) signaling pathway both antagonized the protective effect of IGF-I in an additive manner. Further experiments revealed that IGF-I-stimulation led to a PI3K-dependent phosphorylation of AKT and BAD, and an
MEK1-dependent phosphorylation of extracellular signal-regulated kinase (ERK) 1 and ERK2.Additionally, the researchers discovered that IGF-I caused CREB to become phosphorylated in a PI3K and MEK1-dependent manner. They found that the anti-apoptotic impact of IGF-I was eliminated by ectopic overexpression of a dominant-negative
mutant of CREB. Additionally, after longer periods of IGF-I stimulation, the antiapoptotic factor bcl-2 protein levels increased. These increases could be reversed by pharmacologically inhibiting PI3K and MEK1, as well as by overexpressing
dominant-negative CREB. In summary, the researchers concluded that in cardiomyocytes, the antiapoptotic effect of IGF-I required both PI3K- and MEK1-dependent pathways leading to the activation of the transcription factor CREB. This,
in turn, induced the expression of the antiapoptotic factor bcl-2. The researchers noted that progressive loss of cardiomyocytes due to apoptosis significantly contributed to the development of heart failure. They also pointed out
that a variety of stimuli known to participate in the pathogenesis of heart failure induced cardiomyocyte apoptosis, including hypoxia, ischemia and reperfusion, and oxidative stress.You can read the full article at https://www.ahajournals.org/doi/10.1161/hc4201.097133?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
- Gleichmann M, Weller M, Schuyltz JB 2000 Insulin-like growth factor-1-mediated protection from neuronal apoptosis is linked to phosphorylation of the pro-apoptotic protein BAD but not to inhibition of cytochrome c translocation in rat cerebellar neurons.
Neurosci Lett 282:69–72Insulin-like growth factor-1-mediated protection from neuronal apoptosis is linked to phosphorylation of the pro-apoptotic protein BAD but not to inhibition of cytochrome c translocation in rat cerebellar neurons
Researchers researched cerebellar granule neurons and found that when these neurons were cultivated with serum and depolarizing potassium concentrations, they exhibited apoptosis when switched to serum-free media with physiological potassium
concentrations. However, even with just serum deprivation, the neurons were still alive. The dephosphorylation of the BCL-2-related BAD protein was connected to potassium deprivation, the researchers found. In order to investigate
this further, the researchers exposed the neurons to insulin-like growth factor-1 (IGF-1) and found that this inhibited both apoptosis and dephosphorylation of BAD. Interestingly, both effects of IGF-1 did not rely on protein synthesis
but were nullified by the phosphatidylinositol-3 kinase inhibitors, wortmannin and LY294002.The researchers then contrasted the effects of IGF-1 with those of cycloheximide and discovered that, unlike cycloheximide, IGF-1 did not block the translocation of cytochrome c from mitochondria to the cytosol. Furthermore, the dephosphorylation
of BAD alone did not seem to be sufficient to trigger apoptosis, since inhibition of protein synthesis by cycloheximide prevented apoptosis but not BAD dephosphorylation after potassium deprivation. These results led the researchers
to the conclusion that the development of neuronal apoptosis involves two parallel mechanisms. The first process results in the translocation of cytochrome c and is dependent on protein synthesis. The second process entails the dephosphorylation
of BAD and is independent of protein production. Neuronal apoptosis must be induced by the activation of both mechanisms.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10713398/.
- Peruzzi F, Prisco M, Dews M, Salomoni P, Grassilli E, Romano G, Calabretta B, Baserga R 1999 Multiple signaling pathways of the insulin-like growth factor 1 receptor in protection from apoptosis. Mol Cell Biol 19:7203–7215.
Multiple Signaling Pathways of the Insulin-Like Growth Factor 1 Receptor in Protection from Apoptosis
The researchers were aware of the protective function of the activated type 1 insulin-like growth factor receptor (IGF-1R) on cell survival for quite some time. They observed that the wild-type IGF-1R and/or its ligands have a widespread
antiapoptotic effect on many death signals as different procedures were used to induce apoptosis. McCarthy et al. (50) suggested that only insulin-like growth factor 1 (IGF-1) and Bcl-2 can truly suppress the initiation of the apoptotic
program, while caspase inhibitors can arrest the completion of the program but have no effect on its initiation. The way in which the IGF-1R prevents cells from apoptosis has been the subject of numerous studies. The connection between
the IGF-1R and insulin receptor substrate 1 (IRS-1) results in the activation of phosphatidylinositol 3-kinase (PI3-ki), which is the key mechanism this receptor uses to fight against apoptotic damage. BAD, a protein belonging to the
Bcl-2 family, is phosphorylated as a result of PI3-ki’s activation of Akt/protein kinase B. At least in mouse embryo fibroblasts, the insulin receptor (IR) also employs this antiapoptotic pathway.The researchers observed that in 32D
cells, a murine hemopoietic cell line devoid of IRS-1, the IGF-1R activates alternative pathways for protection from apoptosis induced by the withdrawal of interleukin-3.One of these pathways leads to the activation of mitogen-activated protein kinase, while a third pathway results in the mitochondrial translocation of Raf and depends on the integrity of a group of serines in the C terminus of the receptor
that are known to interact with 14.3.3 proteins. However, all three pathways result in BAD phosphorylation. The presence of multiple antiapoptotic pathways may explain the remarkable efficacy of the IGF-1R in protecting cells from
apoptosis.Overall, the researchers discovered that different cell types are protected from various apoptotic insults by the type 1 insulin-like growth factor receptor (IGF-1R), which is activated by its ligands. It has been established
that the activation of phosphatidylinositol 3-kinase, Akt/protein kinase B, and the phosphorylation and inactivation of BAD, a protein belonging to the Bcl-2 family, are the primary signaling pathways for IGF-1R-mediated protection
from apoptosis. The discovery of novel treatments for conditions marked by excessive apoptosis, according to the researchers, may result from a better knowledge of the several antiapoptotic pathways triggered by the IGF-1R.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC84713/.
- Kulik G, Weber MJ 1998 Akt-dependent and -independent survival signaling pathways utilized by insulin-like growth factor I. Mol Cell Biol 18:6711–6718.
Akt-Dependent and -Independent Survival Signaling Pathways Utilized by Insulin-Like Growth Factor I
Protein kinase B (PKB)/Akt was previously identified as playing a role in survival signaling in various cell types, including fibroblasts, epithelial, and neuronal cells. The researchers and other scientists had previously identified a
linear survival signaling cascade activated by insulin-like growth factor I (IGF-I), which involved the IGF-I receptor, phosphoinositide 3-kinase (PI3 kinase), Akt, and Bad. This pathway was shown to protect cells from apoptosis. It
was not known, however, whether there were alternate paths or whether this pathway was always required for cell survival. To learn more about this, the scientists ran experiments. They identified two survival signaling pathways, one
of which was PI3 kinase and Akt dependent and the other not. However, when IGF-I receptors were overexpressed in Rat-1 cells (RIG cells), an alternative pathway was revealed, which did not rely on PI3 kinase or Akt.This alternative pathway was not sensitive to wortmannin or overexpression of dominant negative Akt, even though Akt activation and Bad phosphorylation were still sensitive to wortmannin. Further experiments with inhibitors of RNA synthesis
showed that transcriptional activation was not necessary for this alternative survival signaling pathway.These findings showed the existence of a new survival signaling pathway that was independent of PI3 kinase, Akt, and transcription
and was observed in fibroblasts that overexpressed the IGF-I receptor. The researchers’ findings provide valuable insights into the complex mechanisms of cell survival signaling and could have implications for the development of new
treatments for diseases involving abnormal cell death or survival.The scientists discovered that IGF-I treatment of Rat-1 cells activated a survival signaling pathway that could be stopped by overexpression of wortmannin and an Akt
variant with a defective dominant-negative kinase (K179A). This showed the existence of a survival signaling pathway reliant on PI3 kinase and Akt.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC109254/.
- Harada H, Andersen JS, Mann M, Terada N, Korsmeyer SJ 2001 p70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD. Proc Natl Acad Sci USA 98:9666–9670.
P70S6 kinase signals cell survival as well as growth, inactivating the pro-apoptotic molecule BAD
The maintenance of cellular homeostasis within organs was mediated in part by a critical interdependence between cells of different types, according to the researchers. This interdependence included a cellular dependence on a series of
factors, such as IGF-1, nerve growth factor, or interleukin-3 (IL-3), that transduced signals through surface receptors to repress apoptosis and stimulate growth of target cells. The BCL-2 family of proteins that regulated cell death
was frequently a target of posttranslational modification downstream of both survival and death signal transduction cascades. Such modifications to BCL-2 members often dictated their active-versus-inactive conformation, subcellular
localization, and partner proteins. One of the targets was BAD, a “BH3 domain-only” proapoptotic member sharing sequence homology only within the BH3 amphipathic α-helical domain.Cells phosphorylated BAD on two serine residues (S112
and S136) located inside of 14-3-3 consensus binding sites when the necessary survival factors were present. The inactive component of phosphorylated BAD that is linked to 14-3-3 in the cytoplasm appears to be what frees BCL-XL or
BCL-2 to support survival.Only the active, nonphosphorylated BAD formed heterodimers at membrane locations with BCL-XL or BCL-2 to accelerate cell death. It was recently discovered that S155 in the BH3 domain was also phosphorylated in order to prevent BAD from
attaching to BCL-XL or BCL-2.Cytokines often delivered simultaneous, yet distinct, cell growth and cell survival signals. The 70-kDa ribosomal protein S6 kinase (p70S6K) was known to regulate cell growth by inducing protein synthesis
components. The researchers purified membrane-based p70S6K as a kinase responsible for site-specific phosphorylation of BAD, which inactivated this proapoptotic molecule. Rapamycin inhibited mitochondrial-based p70S6K, which prevented
phosphorylation of Ser-136 on BAD and blocked cell survival induced by insulin-like growth factor 1 (IGF-1). Moreover, IGF-1-induced phosphorylation of BAD Ser-136 was abolished in p70S6K-deficient cells. Thus, p70S6K was itself a
dual pathway kinase, signaling cell survival as well as growth through differential substrates which include mitochondrial BAD and the ribosomal subunit S6, respectively.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC55509/.
- Desbois-Moutho C, Cadoret A, Blivet-Van Eggelpoel MJ, Bertrand F, Cherqui G, Perret C, Capeau J 2001 Insulin and IGF-1 stimulate the β-catenin pathway through two signalling cascades involving GSK-3β inhibition and Ras activation. Oncogene 20:252–259
Insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades involving GSK-3beta inhibition and Ras activation
The interplay between the insulin/IGF-1- and beta-catenin-regulated pathways, both of which were suspected to play a role in hepatocarcinogenesis, was examined by researchers. In HepG2 cells, insulin and IGF-1 stimulated the transcription
of a Lef/Tcf-dependent luciferase reporter gene by 3-4-fold. The stimulation was mediated through the activation of phosphatidylinositol 3-kinase (PI 3-K)/Akt and the inhibition of glycogen synthase kinase-3beta (GSK-3beta). The researchers
found that the effects of insulin and IGF-1 were inhibited by dominant-negative mutants of PI 3-K or Akt and an uninhibitable GSK-3beta. Along with inhibiting GSK-3beta, insulin and IGF-1 increased the cytoplasmic levels of beta-catenin.It was discovered that the activation of Lef/Tcf-dependent transcription by insulin and IGF-1 was not solely mediated via the PI 3-K/Akt/GSK-3beta pathway. It was also necessary to use the Ras signaling pathway. This was demonstrated by
the fact that dominant-negative Ras or the MEK1 inhibitor PD98059 blocked the stimulatory effects of insulin and IGF-1. Additionally, Lef/Tcf-dependent transcription was stimulated by activated Ha-Ras or constitutively active MEK1,
which worked in concert with catalytically inactive GSK-3beta.The researchers provided the first evidence that insulin and IGF-1 stimulate the beta-catenin pathway through two signalling cascades bifurcating downstream of PI 3-K and
involving GSK-3beta inhibition and Ras activation. These findings demonstrated for the first time the ability of insulin and IGF-1 to activate the beta-catenin pathway in hepatoma cells. This provided new insights into the role of
these factors in hepatocarcinogenesis.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11313952/.
- Park BC, Kido Y, Accili D 1999 Differential signaling of insulin and IGF-1 receptors to glycogen synthesis in murine hepatocytes. Biochemistry 38:7517–7523
Differential signaling of insulin and IGF-1 receptors to glycogen synthesis in murine hepatocytes
In order to compare the contrasting abilities of insulin and IGF-1 receptors to drive glycogen production, the researchers used SV40-transformed hepatocytes from mice with an impaired insulin receptor (-/-) and mice with a normal insulin
receptor (WT). It was discovered that insulin receptors promoted glycogen production more potently than IGF-1 receptors. PI 3-kinase was required for the promotion of glycogen production by both receptors, but only the insulin receptor’s
action was rapamycin-dependent. While GSK-3 inactivation in response to IGF-1 was significantly lower in both -/- and WT cells than it was in response to insulin in WT cells, Akt was similarly activated by both insulin and IGF-1 receptors.These
findings indicated that (i) the strength of insulin and IGF-1 receptors in promoting glycogen synthesis corresponds with their ability to inactivate GSK-3, (ii) the degree of GSK-3 inactivation did not correspond with the degree of
Akt activation induced by insulin or IGF-1 receptors, revealing that insulin’s impact on GSK-3 necessitates additional kinases, and (iii) the pathways necessary for insulin to promote glycogen synthesis in mouse hepatocytes were PI
3-kinase-dependent and rapamycin-sensitive.In conclusion, the researchers investigated the different abilities of insulin and IGF-1 receptors
to induce glycogen production using SV40-transformed hepatocytes from insulin receptor-deficient mice (-/-) and normal mice (WT). They found that both insulin receptors and IGF-1 receptors were dependent on PI 3-kinase to activate
glycogen synthesis, with insulin receptors being more effective than IGF-1 receptors in this regard. Rapamycin played a role in the influence of insulin receptors on glycogen production, but not in the influence of IGF-1 receptors.
Akt was similarly activated by both receptors, but IGF-1 had less impact on inactivating GSK-3. These findings suggest that insulin and IGF-1 differ in their abilities to activate glycogen synthesis and that the pathways involved in
insulin’s stimulation of glycogen synthesis are PI 3-kinase-dependent and rapamycin-sensitive.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10360949/.
- Cui H, Meng Y, Bulleit RF 1998 Inhibition of glycogen synthase kinase 3β activity regulates proliferation of cultured cerebellar granule cells. Brain Res Dev Brain Res 111:177–188.
Inhibition of glycogen synthase kinase 3beta activity regulates proliferation of cultured cerebellar granule cells
In purified cultures of cerebellar granule cells, the researchers examined the impact of insulin-like growth factor I (IGF-I) on neuronal progenitors, specifically cerebellar granule neuron progenitors. They discovered that IGF-I functioned
as a mitogen, encouraging cell division, and they pinpointed the intracellular signaling molecules in charge of this action. In their experiments, the researchers observed that IGF-I inhibited the activity of GSK-3, an enzyme that
regulates cell growth and differentiation, and caused phosphorylation of serine9, an inhibitory site on GSK-3beta. They also found that activation of phosphoinositide 3-kinase (PI3-K) by IGF-I led to the phosphorylation and inactivation
of GSK-3.When the researchers used a PI3-K inhibitor, LY294002, they found that it completely blocked IGF-I-induced cell division. The concentration of LY294002 required to achieve this effect was close to its reported IC50 value for
inhibiting PI3-K. Furthermore, the researchers discovered that lithium chloride (LiCl), a direct inhibitor of GSK-3beta, could stimulate granule cell proliferation on its own and enhance the proliferation induced by IGF-I. LiCl was
also discovered to be able to counteract LY294002’s inhibitory effects on granule cell proliferation, proving that GSK-3 inhibition was a step after PI3-K activation in the mitogenic pathway of IGF-I. Finally, the researchers found
evidence supporting the role of GSK-3beta activity inhibition in the signal transduction pathway by which IGF-I regulates granule neuron progenitor proliferation by observing that the expression of a dominant active form of GSK-3beta
inhibited IGF-I-induced cell division.Overall, the study confirmed that IGF-I can function as a mitogen in cerebellar granule cells and provided insight into the intracellular signaling mechanisms responsible for this effect. By identifying
the role of GSK-3 inhibition downstream of PI3-K activation, the researchers highlighted a potential therapeutic target for conditions characterized by abnormal cell growth and proliferation.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/9838099/.
- Tamatani M, Ogawa S, Nuñez G, Tokyama M 1998 Growth factors prevent changes in Bcl-2 and Bax expression and neuronal apoptosis induced by nitric oxide. Cell Death Differ 5:911–919
Growth factors prevent changes in Bcl-2 and Bax expression and neuronal apoptosis induced by nitric oxide
Recent studies have shown that while growth factors like insulin-like growth factor-1 (IGF-1) and basic fibroblast growth factor (bFGF) might protect against NO-induced neuronal cell death, NO donors can cause neurons to undergo apoptosis.
This study’s objectives were to examine the potential processes underlying NO-mediated neuronal death and the neuroprotective abilities of these growth factors.The researchers found that both IGF-1 and bFGF could prevent apoptosis
induced by NO donors, specifically sodium nitroprusside (SNP) or 3-morpholinosydnonimin (SIN-1), in hippocampal neuronal cultures. When neurons were incubated with SNP, caspase-3-like activation occurred following the downregulation
of Bcl-2 and upregulation of Bax protein levels in cultured neurons. When neurons were treated with a bax antisense oligonucleotide, caspase-3-like activation and neuronal death caused by SNP were inhibited. In addition, when neurons
were treated with an inhibitor of caspase-3, Ac-DEVD-CHO, together with SNP, the changes in protein levels were unaffected, although NO-induced cell death was inhibited. The researchers discovered that pretreatment of cultures with
either IGF-1 or bFGF prior to NO exposure inhibited caspase-3-like activation, along with the changes in Bcl-2 and Bax protein levels. These findings suggest that the changes in Bcl-2 and Bax protein levels followed by caspase-3-like
activation are a component of the cascade of NO-induced neuronal apoptosis, and that the neuroprotective effects of IGF-1 and bFGF could be due to inhibition of changes in the protein levels of the Bcl-2 family.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10203697/.
- Baker NlL, Carlo Russo V, Bernard O, D’Ercole AJ, Werther GA 1999 Interactions between bcl-2 and the IGF-I system control apoptosis in the developing mouse brain. Brain Res Dev Brain Res 118:109–118.
Interactions between bcl-2 and the IGF system control apoptosis in the developing mouse brain
The IGF system and pro-survival Bcl-2 proteins were known to safeguard cells from apoptosis and played a crucial part in brain development. To explore their possible correlation, researchers used two transgenic mice models, one overexpressing
Bcl-2 and the other IGF-I proteins in olfactory bulb (OB) or cerebellar neurons.The organization of the specified layers of the OB was found to be poorly organized in both wild-type and Bcl-2 transgenic mice cultured in serum-free
media (SF). The transgenic mice’s neurons were adequately preserved, and the mitral cell layer was increased. IGF-I supplementation improved layer definition but had no additional effect on the mitral cells’ ability to survive in Bcl-2
mice. In contrast, it restored the survival and structure of the wild-type mitral cell layer. Compared to wild-type mice, mitral cells expressing Bcl-2 had significantly larger levels of IGF-I and IGFBP-2 immunoreactivity. In newborn
IGF-I transgenic mice, cerebellar Purkinje cells overexpressing IGF-I displayed higher immunoreactivity for Bcl-2 and IGFBP-2. These findings suggest that in the developing brain, IGF-I modulates the expression of its major binding
protein IGFBP-2 and the Bcl-2 protein. Additionally, the researchers observed that expression of Bcl-2 in the mitral neurons inhibited apoptosis caused by culturing OBs in SF medium and was linked to enhanced expression of the IGF
system, including IGF-I and IGFBP-2. This interaction between the two anti-apoptotic systems may provide a robust cell protection system during brain development and repair.You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0165380699001364?via%3Dihub.
- Du K, Montminy M 1998 CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem 273:32377.
CREB is a regulatory target for the protein kinase Akt/PKB
The nuclear factor CREB’s phosphorylation at Ser-133 by protein kinase A increased the production of cellular genes. By encouraging the recruitment of the co-activator CBP, Ser-133 phosphorylation caused the target gene’s expression to
be activated. Recent research has demonstrated that some cell types need CREB and its paralog CREM to survive. This led the researchers to look into whether the growth factor-dependent Ser/Thr kinase Akt/PKB was capable of activating
CREB as a nuclear target. To test this, the researchers overexpressed Akt/PKB in serum-stimulated cells, which potently induced Ser-133 phosphorylation of CREB and promoted recruitment of CBP. Correspondingly, Akt/PKB stimulated target
gene expression via CREB in a phospho(Ser-133)-dependent manner. Akt/PKB induced CREB activity only in response to serum stimulation, and this effect was suppressed by the phosphatidylinositol 3-kinase inhibitor LY294002. According
to the research, CREB/CBP nuclear transduction pathway is used by Akt/PKB to stimulate the production of cellular genes, hence promoting cell survival. The scientists came to the conclusion that Akt/PKB, via activating CREB via Ser-133
phosphorylation, plays a critical role in controlling gene expression and cell survival.You can read the full article at https://www.jbc.org/article/S0021-9258(19)58887-1/fulltext.
- Pugazhenthi S, Nesterova A, Sable C, Heidenreich KA, Boxer LM, Heasly LE, Reusch JE 2000 Akt/protein kinase B upregulates Bcl-2 expression through cAMP-response element-binding protein. J Biol Chem 275:10761–10766.
Akt/protein kinase B up-regulates Bcl-2 expression through cAMP-response element-binding protein
In their previous study, the researchers demonstrated that insulin-like growth factor-I could stimulate a Bcl-2 promoter containing a cAMP-response element (CRE) site via a novel signaling pathway involving mitogen-activated protein kinase
kinase 6/p38beta mitogen-activated protein kinase/MAP kinase-activated protein kinase-3/cAMP-response element-binding protein (CREB). In their present investigation, the researchers identified a second pathway that contributes to the
up-regulation of Bcl-2 expression via a novel anti-apoptotic function of Akt signaling.The researchers used luciferase reporter genes driven by the promoter region of Bcl-2 containing a CRE in a series of transient transfections to
assess the effect of Akt on Bcl-2 expression. They discovered that the upstream kinase of Akt, phosphatidylinositol (PI) 3-kinase, was inhibited by the drug LY294002 and that this resulted in a 45% reduction in Bcl-2 promoter activity.
The dominant negative p85 subunit of PI 3-kinase blocked 44% of the reporter activity while the active p110 subunit of PI 3-kinase increased it by 2.3-fold. The full activation of Akt requires the cotransfection of 3-phosphoinositide-dependent
kinase (PDK1), which increased luciferase activity. Insulin-like growth factor-I-mediated induction of Bcl-2 promoter activity was significantly decreased by dominant negative forms of p85 subunit of PI 3-kinase, PDK1, and Akt, suggesting
that regulation of Bcl-2 expression by IGF-I involves a signaling cascade mediated by PI 3-kinase/PDK1/Akt/CREB. The researchers used real-time quantitative reverse transcription-polymerase chain reaction using the TaqMan fluorogenic
probe system to quantify Bcl-2 mRNA levels in PC12 cells overexpressing Akt to corroborate their findings. Bcl-2 mRNA levels were found to be 2.1 times higher in the Akt cell line than in control PC12 cells, supporting the finding
that increased CREB activity by Akt signaling promotes greater Bcl-2 promoter activity and cell survival.You can read the full article at https://www.jbc.org/article/S0021-9258(19)80906-7/fulltext.
- Kim SW, Her SJ, Park SJ, et al. Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells. Bone. 2005;37(3):359-69.
Ghrelin stimulates proliferation and differentiation and inhibits apoptosis in osteoblastic MC3T3-E1 cells
The 28-amino-acid peptide known as ghrelin was discovered by the researchers to be an endogenous ligand of the growth hormone secretagogue receptor (GHS-R) in the stomach. Growth hormone was highly activated at the hypothalamus and pituitary
levels by the GHS-R. Although GHS-Rs were found in many peripheral tissues, their impact on bone outside of the GH/IGF-1 axis was poorly understood. The goal of the investigation was to determine whether ghrelin had an impact on osteoblasts
directly. The researchers identified mRNA and protein expression of GHS-R in primary osteoblasts as well as a number of osteoblastic cell lines, including MC3T3-E1, ROS 17/2.8, UMR-106, MG63, and SaOS2 cells. Ghrelin treatment of MC3T3-E1
cells showed dose-dependent stimulation of proliferation, and this was abrogated by treatment with [d-Lys]-GHRP-6 (10(-3) M), a selective antagonist of the ghrelin receptor. Furthermore, ghrelin activated ERK1/2 MAPK, and pretreatment
with MAPK kinase inhibitors, PD98059 attenuated the ghrelin-induced cell proliferation. Ghrelin also inhibited TNFalpha-induced apoptosis and suppressed caspase-3 activation that occurred in response to TNFalpha as well as during the
in vitro differentiation process. Ghrelin treatment enhanced in vitro osteoblast differentiation as evidenced by matrix mineralization, alkaline phosphatase activity, and osteoblast-specific gene expression. According to the findings,
ghrelin suppressed osteoblast death while encouraging osteoblast proliferation and differentiation. The researchers came to the conclusion that ghrelin has a direct impact on osteoblasts and would be a good target for bone diseases.You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S8756328205001808?via%3Dihub.
- Kim YS, Choi DH, Block ML, et al. A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation. FASEB J. 2007;21(1):179-87.
A pivotal role of matrix metalloproteinase-3 activity in dopaminergic neuronal degeneration via microglial activation
Recent research has demonstrated that through releasing NADPH-oxidase-derived superoxide, activated microglia contributed significantly to the degeneration of dopamine neurons in Parkinson’s disease (PD). However, there was ongoing debate
regarding the molecular mechanisms underlying microglial activation in DA cell death. According to the study, stressed DA cells generated and activated matrix metalloproteinase-3 (MMP-3), and the active form of MMP-3 (actMMP-3) was
released into the medium. The researchers found that the released actMMP-3, as well as catalytically active recombinant MMP-3 (cMMP-3), caused microglial activation and superoxide generation in microglia and enhanced DA cell death.
The researchers discovered that cMMP-3 caused DA cell death in mesencephalic neuron-glia mixed cultures of wild-type (WT) mice. Still, this was attenuated in the culture of NADPHO subunit null mice (gp91(phox-/-)), indicating that
NADPHO mediated the cMMP-3-induced microglial production of superoxide and DA cell death. Furthermore, in the N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-injected animal model of PD, the researchers observed that nigrostriatal
DA neuronal degeneration, microglial activation, and superoxide generation were largely attenuated in MMP-3-/- mice. These findings suggested that the actMMP-3 released from stressed DA neurons was the cause of microglial activation
and the production of superoxide derived from NADPHO, which ultimately resulted in accelerated nigrostriatal DA neuronal degeneration. The researchers hypothesized that a novel therapy strategy for PD might result from their findings.You can read the full article at https://faseb.onlinelibrary.wiley.com/doi/epdf/10.1096/fj.06-5865com.
- Banks WA, Tschöp M, Robinson SM, Heiman ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Ther. 2002;302(2):822-7.
Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure
Researchers discovered the novel hormone ghrelin as a potent orexigen with the ability to balance leptin. Ghrelin was the sole secreted molecule that needed post-translational acylation with octanoic acid to ensure its bioactivity. Ghrelin,
which was mostly produced by the stomach, was thought to control energy balance by concentrating on neuroendocrine networks in the central nervous system (CNS). However, for ghrelin to perform this regulation, it was necessary for
it to pass the blood-brain barrier (BBB). To investigate whether ghrelin could cross the BBB and whether its lipophilic side chain played a role in this process, the researchers conducted experiments on mice. They found that there
were saturable systems that transported human ghrelin from brain-to-blood and from blood-to-brain. However, mouse ghrelin, which differed from human ghrelin by two amino acids, was a substrate for the brain-to-blood transporter but
not for the blood-to-brain transporter, which meant it entered the brain to a far lesser degree. On the other hand, des-Octanoyl ghrelin entered the brain by nonsaturable transmembrane diffusion but was sequestered once inside the
CNS. The study’s findings concluded that ghrelin transport over the BBB was a complicated, well controlled, and bidirectional process. The fundamental structure of ghrelin dictated the direction and extent of transit, emphasizing the
special function of post-translational octanoylation in this procedure.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/12130749/.
- Chung H, Seo S, Moon M, Park S. Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical
neuronal cells. J Endocrinol. 2008;198(3):511-21.Phosphatidylinositol-3-kinase/Akt/glycogen synthase kinase-3 beta and ERK1/2 pathways mediate protective effects of acylated and unacylated ghrelin against oxygen-glucose deprivation-induced apoptosis in primary rat cortical neuronal cells.
The focus of this study was on acylated ghrelin (AG) and unacylated ghrelin (UAG) and their respective effects on neuronal cells. AG specifically binds to GH secretagogue receptor 1a (GHS-R1a) and has been found to exhibit central endocrine
activities and anti-apoptotic effects in neuronal cells. On the other hand, the neuroprotective properties of UAG, which is the most abundant form of ghrelin in plasma, remained unknown.To investigate this, the researchers conducted experiments using primary cultured rat cortical neurons exposed to oxygen and glucose deprivation (OGD) to simulate ischemic neuronal injury. Both AG and UAG were found to inhibit apoptosis
induced by OGD.The protective effects of AG against OGD were shown to involve the GHS-R1a receptor since they were blocked by a specific antagonist (D-Lys-3-GHRH-6). However, the neuroprotective effects of UAG were preserved even after exposure to the
same antagonist, suggesting the involvement of a receptor different from GHS-R1a.The researchers observed that both AG and UAG relied on the MAPK and phosphatidylinositol-3-kinase (PI3K) pathways to exert their anti-apoptotic effects. Additionally, they found that ghrelin siRNA enhanced apoptosis, both during OGD and
in normoxic conditions, further supporting the neuroprotective role of ghrelin.Furthermore, both AG and UAG increased the phosphorylation of extracellular signal-regulated kinase (ERK)1/2, Akt, and glycogen synthase kinase-3beta (GSK-3beta). Additionally, they facilitated nuclear translocation of beta-catenin. Moreover,
both forms of ghrelin increased the Bcl-2/Bax ratio, prevented the release of cytochrome c, and inhibited caspase-3 activation. These findings suggest that ghrelin, regardless of acylation, can act as a neuroprotective agent by inhibiting
apoptotic pathways through the activation of MAPK and PI3K/Akt pathways. Furthermore, PI3K/Akt-mediated inactivation of GSK-3beta and stabilization of beta-catenin were found to contribute to the anti-apoptotic effects of ghrelin.You can read the full article at https://joe.bioscientifica.com/view/journals/joe/198/3/511.xml.
- García-cáceres C, Lechuga-sancho A, Argente J, Frago LM, Chowen JA. Death of hypothalamic astrocytes in poorly controlled diabetic rats is associated with nuclear translocation of apoptosis inducing factor. J Neuroendocrinol. 2008;20(12):1348-60
Death of hypothalamic astrocytes in poorly controlled diabetic rats is associated with nuclear translocation of apoptosis inducing factor
Astrocytes in the hypothalamus of diabetic rats with poor glucose control experienced a reduction in number and underwent morphological changes, including a decrease in projections, due to increased apoptosis and decreased proliferation.
The researcher aimed to determine the intracellular mechanisms behind this increase in hypothalamic cell death. To do so, adult male Wistar rats were injected with streptozotocin to induce diabetes, and controls received a vehicle.
Rats were killed at 1, 4, 6, and 8 weeks after diabetes onset (glycemia > 300 mg/dl).Enzyme-linked immunosorbent assays showed an increase in cell death after 4 weeks of diabetes, and immunohistochemistry and terminal dUTP nick-end
labeling (TUNEL) experiments revealed that these cells were positive for the glial fibrillary acidic protein (GFAP). Western blot examination revealed that the fragmentation of caspases 2, 3, 6, 7, 8, 9, or 12 had not changed significantly.
Enzymatic assays, however, revealed that after 1 week of diabetes, caspase 3 activity considerably increased and then dropped below control levels. In the hypothalamus, cell bodies lining the third ventricle, fibers radiating from
the third ventricle, and GFAP positive cells expressed fragmented caspase 3, with this labeling increasing at 1 week of diabetes.However, as no nuclear labeling was observed, and this increase in activity did not correlate temporally with the increased cell death, this caspase may not be involved in astrocyte death. By contrast, nuclear translocation of apoptosis-inducing
factor (AIF) increased significantly in astrocytes in parallel with the increase in death, and AIF was found in TUNEL positive cells. As a result, nuclear translocation of AIF may be responsible for the rise in mortality, whereas caspase
3 fragmentation may be responsible for the morphological alterations in the hypothalamus astrocytes of diabetic rats. These alterations in astrocytes are linked to adjustments in synaptic proteins, and they almost certainly have an
impact on neuroendocrine signaling and function.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/19094082/.
- Frago LM, Pañeda C, Dickson SL, Hewson AK, Argente J, Chowen JA. Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection. Endocrinology.
2002;143(10):4113-22.Growth hormone (GH) and GH-releasing peptide-6 increase brain insulin-like growth factor-I expression and activate intracellular signaling pathways involved in neuroprotection
In their study, Frago et al. (2002) investigated the effects of growth hormone (GH) and GH-releasing peptide-6 (GHRP-6) on brain insulin-like growth factor-I (IGF-I) expression and intracellular signaling pathways involved in neuroprotection.
The researchers aimed to understand the mechanisms by which GH and GHRP-6 exert their neuroprotective effects. They found that both GH and GHRP-6 treatment increased the expression of IGF-I in the brain. Additionally, these treatments
activated intracellular signaling pathways, such as the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and the mitogen-activated protein kinase (MAPK) pathway, which are known to be involved in cell survival and neuroprotection.
These findings suggest that GH and GHRP-6 promote neuroprotection by increasing IGF-I expression and activating specific intracellular signaling pathways. Understanding these mechanisms may have implications for the development of
therapeutic strategies targeting neuroprotection in various neurological conditions.You can read the full article at https://academic.oup.com/endo/article/143/10/4113/2880897?login=false.
- Nyberg F, Hallberg M. Growth hormone and cognitive function. Nat Rev Endocrinol. 2013;9(6):357-65.
Growth hormone and cognitive function
Nyberg and Hallberg (2013) reviewed the impact of growth hormone (GH) on cognitive function. They discussed studies on GH replacement therapy in
GH-deficient individuals and GH administration in healthy individuals. The review highlighted potential cognitive-enhancing effects of GH, particularly in memory and attention. However, inconsistencies in findings and various influencing
factors were acknowledged. The authors emphasized the need for further research to better understand the mechanisms and clinical implications of GH-related cognitive effects.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/23629538/.
- Baker LD, Barsness SM, Borson S, et al. Effects of Growth Hormone–Releasing Hormone on Cognitive Function in Adults With Mild Cognitive Impairment and Healthy Older Adults: Results of a Controlled Trial. Archives of neurology. 2012;69(11):1420-1429. doi:10.1001/archneurol.2012.1970.
Effects of Growth Hormone–Releasing Hormone on Cognitive Function in Adults With Mild Cognitive Impairment and Healthy Older Adults
In a controlled trial, Baker et al. (2012) investigated the effects of Growth Hormone-Releasing Hormone (GHRH) on cognitive function in adults with mild cognitive impairment (MCI) and healthy older adults. The study found that GHRH treatment
was associated with improved memory and attention in participants with MCI, but not in healthy older adults. These findings suggest that GHRH may have potential therapeutic benefits for cognitive impairment, particularly in individuals
with MCI.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764914/.
- Maruff P, Falleti M. Cognitive function in growth hormone deficiency and growth hormone replacement. Horm Res. 2005;64 Suppl 3:100-8.
Cognitive function in growth hormone deficiency and growth hormone replacement
Maruff and Falleti (2005) conducted a study examining cognitive function in individuals with growth hormone deficiency (GHD) and the effects of growth hormone replacement therapy. The research showed that GHD is associated with impairments
in certain cognitive domains, including memory and attention. However, growth hormone replacement therapy was found to improve cognitive function in individuals with GHD. These findings highlight the importance of growth hormone in
cognitive processes and suggest that replacement therapy may be beneficial in addressing cognitive deficits associated with GHD.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16439852/.
- Baker LD, Barsness SM, Borson S, et al. Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults: results of a controlled trial. Arch Neurol. 2012;69(11):1420-9.
Effects of growth hormone–releasing hormone on cognitive function in adults with mild cognitive impairment and healthy older adults
The study conducted by Baker et al. (2012) investigated the effects of growth hormone-releasing hormone (GHRH) on cognitive function in two groups: adults with mild cognitive impairment (MCI) and healthy older adults. The study employed
a controlled trial design to assess the impact of GHRH treatment on cognitive performance. The results demonstrated that GHRH administration led to improvements in certain cognitive domains, including attention and working memory,
in both the MCI and healthy older adult groups. These findings suggest that GHRH may have potential as a therapeutic intervention for cognitive impairments associated with MCI and aging.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764914/.
- Ong LK, Chow WZ, Tebay C, et al. Growth Hormone Improves Cognitive Function After Experimental Stroke. Stroke. 2018;49(5):1257-1266.
Growth Hormone Improves Cognitive Function After Experimental Stroke
The study conducted by Ong et al. (2018) aimed to investigate the effects of growth hormone (GH) on cognitive function following experimental stroke. Using an animal model, the researchers administered GH treatment and evaluated its impact
on cognitive performance. The results showed that GH administration led to improvements in cognitive function after stroke, specifically in memory and learning abilities. These findings suggest that GH may have a beneficial effect
on cognitive recovery following stroke and highlight its potential as a therapeutic intervention in stroke-related cognitive impairments.You can read the full article at https://www.ahajournals.org/doi/10.1161/STROKEAHA.117.020557?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
- Bove RM, White CC, Gerweck AV, et al. Effect of growth hormone on cognitive function in young women with abdominal obesity. Clinical endocrinology. 2016;84(4):635-637. doi:10.1111/cen.12996.
Effect of growth hormone on cognitive function in young women with abdominal obesity
The study conducted by Bove et al. (2016) aimed to investigate the effect of growth hormone (GH) on cognitive function in young women with abdominal obesity. The researchers administered GH treatment and assessed its impact on cognitive
performance. The results suggested that GH treatment did not significantly improve cognitive function in this particular group of individuals. While further research is needed to fully understand the relationship between GH and cognitive
function in different populations, this study suggests that GH may not have a significant cognitive benefit for young women with abdominal obesity.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4789146/.
- Aleman A, Verhaar HJ, De haan EH, et al. Insulin-like growth factor-I and cognitive function in healthy older men. J Clin Endocrinol Metab. 1999;84(2):471-5.
Insulin-like growth factor-I and cognitive function in healthy older men
In the study conducted by Aleman et al. (1999), the researchers investigated the relationship between insulin-like growth factor-I (IGF-I) and cognitive function in healthy older men. The study aimed to determine whether IGF-I levels were
associated with cognitive performance in this population. The results indicated a positive correlation between IGF-I levels and cognitive function, suggesting that higher IGF-I levels were associated with better cognitive performance
in healthy older men. These findings highlight the potential role of IGF-I in maintaining cognitive function in aging individuals.You can read the full article at https://academic.oup.com/jcem/article/84/2/471/2864174?login=false
.
- Sonntag WE, Deak F, Ashpole N, et al. Insulin-like growth factor-1 in CNS and cerebrovascular aging. Frontiers in Aging Neuroscience. 2013;5:27. doi:10.3389/fnagi.2013.00027.
Insulin-like growth factor-1 in CNS and cerebrovascular aging
In the study by Sonntag et al. (2013), the researchers investigated the role of insulin-like growth factor-1 (IGF-1) in central nervous system (CNS) and cerebrovascular aging. The study aimed to understand the potential effects of age-related
changes in IGF-1 on brain function and cerebrovascular health. The findings suggested that IGF-1 plays a crucial role in maintaining the integrity and function of the CNS and cerebrovascular system during aging. Alterations in IGF-1
signaling were associated with cognitive decline, neurodegenerative diseases, and increased susceptibility to cerebrovascular dysfunction. The study highlights the importance of IGF-1 in the aging process of the brain and provides insights into potential therapeutic targets for age-related neurological disorders.You can read the full article at https://www.frontiersin.org/articles/10.3389/fnagi.2013.00027/full
- Nashiro K, Guevara-aguirre J, Braskie MN, et al. Brain Structure and Function Associated with Younger Adults in Growth Hormone Receptor-Deficient Humans. J Neurosci. 2017;37(7):1696-1707.
Brain Structure and Function Associated with Younger Adults in Growth Hormone Receptor-Deficient Humans
In the study by Nashiro et al. (2017), the researchers investigated the brain structure and function of younger adults who have a growth hormone receptor deficiency. The study aimed to understand the potential effects of this deficiency
on brain development and function. The findings suggested that the absence of growth hormone receptor signaling in these individuals was associated with specific alterations in brain structure and function. These included differences
in white matter integrity, cortical thickness, and functional connectivity in certain brain regions involved in cognitive processes. The study provides insights into the role of growth hormone signaling in brain development and suggests
potential mechanisms underlying cognitive changes associated with growth hormone receptor deficiency in younger adults.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5320603/
- Available at https://journals.lww.com/neurotodayonline/fulltext/2012/09200/Can_a_Growth_Hormone_Stimulating_Drug_Improve.6.aspx.
Can a Growth Hormone-Stimulating Drug Improve Cognitive Function?
A recent study by University of Washington researchers suggests that a once-daily injection of growth hormone-releasing hormone (GHRH) may improve cognitive function in healthy older individuals and those with mild cognitive impairment
(MCI). Participants who received daily GHRH injections for 20 weeks showed improvement in executive function and verbal memory. The researchers emphasized the need for further testing to establish the therapeutic potential of GHRH
administration for brain health in normal and pathological aging. Longer-term trials are necessary to evaluate efficacy and safety. While the study is promising, experts caution that more research is required to determine the clinical
usefulness of GHRH. The potential benefits and side effects of GHRH treatment still need to be explored through larger, diverse population studies. It is important to note that long-term risks have been associated with GHRH use, particularly in children, and human growth hormone is not FDA-approved for anti-aging purposes.You can read the full article at https://journals.lww.com/neurotodayonline/fulltext/2012/09200/Can_a_Growth_Hormone_Stimulating_Drug_Improve.6.aspx
- Available at http://pediatrics.aappublications.org/content/142/4/e20173938.
Growth Hormone Therapy for a Child With Severe Cognitive Impairment
Over the past three decades, growth hormone therapy has significantly expanded, allowing for the treatment of short stature in many children, resulting in increased height. However, the use of growth hormone therapy for idiopathic short stature (ISS) remains a subject of controversy. Proponents argue that ISS affects the quality of life and can be treated with a relatively safe medication to improve outcomes, while opponents argue that short stature is a normal variant and treatment to increase height may carry risks without necessarily improving quality of life.
The ethical dilemma becomes more complex when dealing with disabled children who have unexplained short stature. In this particular case, a 14-year-old boy with extreme short stature, craniofacial anomalies, developmental delay, and mental disability seeks treatment with growth hormone. His growth records show consistent height velocity, and hormonal assessments reveal no evidence of growth hormone deficiency or other underlying causes for his short stature. However, there is no definitive diagnosis for the cause of his condition.
The family of the boy wishes to explore growth hormone therapy to increase his height despite the lack of a specific cause for his short stature. While the US Food and Drug Administration has approved the use of growth hormone therapy for such cases, it remains uncertain whether treatment is appropriate in this specific situation.
The ethical question arises as to whether growth hormone therapy should be administered to a mentally disabled child with unexplained short stature. The decision involves considering the potential benefits and risks of treatment, the boy’s future quality of life, and the absence of a definitive diagnosis for his condition.
As medical professionals grapple with this ethical dilemma, the family seeks guidance on the appropriate course of action, prompting them to ask, “What would you do?”
You can read the full article at https://publications.aap.org/pediatrics/article/142/4/e20173938/37369/Growth-Hormone-Therapy-for-a-Child-With-Severe?autologincheck=redirected
- High WM, Briones-Galang M, Clark JA, et al. Effect of Growth Hormone Replacement Therapy on Cognition after Traumatic Brain Injury. Journal of Neurotrauma. 2010;27(9):1565-1575. doi:10.1089/neu.2009.1253.
Effect of Growth Hormone Replacement Therapy on Cognition after Traumatic Brain Injury. Journal of Neurotrauma
Traumatic brain injury is a complex condition that can result in various cognitive impairments, including attention deficits, memory problems, and difficulties with executive functions. Growth hormone (GH) is known to play a role in brain
development, neuroprotection, and cognitive function. The study you referenced, “Effect of Growth Hormone Replacement Therapy on Cognition after Traumatic Brain Injury” by High et al., was published in the Journal of Neurotrauma in
2010. Unfortunately, I don’t have direct access to the full text of the article. However, based on the title, it suggests that the study aimed to investigate the potential effects of GHRT on cognitive outcomes in individuals with TBI.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2966848/
- Grugni G, Sartorio A, Crinò A. Growth hormone therapy for Prader–willi syndrome: challenges and solutions. Therapeutics and Clinical Risk Management. 2016;12:873-881. doi:10.2147/TCRM.S70068.
Growth hormone therapy for Prader–willi syndrome: challenges and solutions. Therapeutics and Clinical Risk Management
Growth hormone therapy for Prader-Willi syndrome: challenges and solutions” was published in 2016 in the journal Therapeutics and Clinical Risk Management. It discusses the difficulties and potential solutions associated with growth hormone
(GH) therapy in individuals with Prader-Willi syndrome (PWS). The authors highlight the challenges of diagnosing growth hormone deficiency in PWS and discuss the benefits and risks of GH treatment. The article emphasizes the importance
of a multidisciplinary approach in managing PWS and provides an overview of the efficacy and safety of GH therapy. While informative, it’s important to note that the article’s findings are based on information available up until 2016.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4898426/
- Xue Y, Gao Y, Wang S, Wang P. An examination of the effects of different doses of recombinant human growth hormone on children with growth hormone deficiency. Experimental and Therapeutic Medicine. 2016;11(5):1647-1652. doi:10.3892/etm.2016.3091.
Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats
An examination of the effects of different doses of recombinant human growth hormone on children with growth hormone deficiency” was published in 2016 in the journal Experimental and Therapeutic Medicine. The study investigated the impact
of varying doses of recombinant human growth hormone (rhGH) on children with growth hormone deficiency. The results showed that rhGH treatment led to significant improvements in growth parameters, with higher doses resulting in greater
growth improvements. The study emphasized the importance of individualized treatment based on factors such as age and severity of growth hormone deficiency. However, it’s important to consider that the findings are specific to children
with growth hormone deficiency and may not apply to other populations or conditions.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/14598295/
- Lupien SB, Bluhm EJ, Ishii DN. Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats. J Neurosci Res. 2003;74(4):512-23.
Systemic insulin-like growth factor-I administration prevents cognitive impairment in diabetic rats, and brain IGF regulates learning/memory in normal adult rats
An examination of the effects of different doses of recombinant human growth hormone on children with growth hormone deficiency” was published in 2016 in the journal Experimental and Therapeutic Medicine. The study investigated the impact
of varying doses of recombinant human growth hormone (rhGH) on children with growth hormone deficiency. The results showed that rhGH treatment led to significant improvements in growth parameters, with higher doses resulting in greater
growth improvements. The study emphasized the importance of individualized treatment based on factors such as age and severity of growth hormone deficiency. However, it’s important to consider that the findings are specific to children
with growth hormone deficiency and may not apply to other populations or conditions.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/14598295/
- Friedman SD, Baker LD, Borson S, et al. Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging. JAMA Neurol. 2013;70(7):883-90.
Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging
Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging” by Friedman et al. (2013) investigated the impact of growth hormone-releasing hormone (GHRH) on γ-aminobutyric
acid (GABA) levels in the brain. The study found that GHRH administration increased GABA levels in specific brain regions in both individuals with mild cognitive impairment and healthy aging participants. These findings suggest a potential
role for GHRH in modulating GABAergic neurotransmission. Further research is needed to fully understand the implications of these results and their therapeutic implications for cognitive disorders.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3764915/
- Vitiello MV, Moe KE, Merriam GR, Mazzoni G, Buchner DH, Schwartz RS. Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging. 2006;27(2):318-23.
Growth hormone releasing hormone improves the cognition of healthy older adults. Neurobiol Aging
Growth hormone releasing hormone improves the cognition of healthy older adults” by Vitiello et al. (2006) investigated the effects of growth hormone releasing hormone (GHRH) on cognitive function in healthy older adults. The study found that GHRH administration led to improved cognitive performance, including memory, attention, and executive function. These findings suggest that GHRH may have potential cognitive benefits for healthy aging. Further research is needed to better understand these effects and their underlying mechanisms.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0197458005000631?via%3Dihub
- Jeong YO, Shin SJ, Park JY, et al. MK-0677, a Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer’s Disease. Int J Mol Sci. 2018;19(6)
Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer’s Disease
MK-0677, a Ghrelin Agonist, Alleviates Amyloid Beta-Related Pathology in 5XFAD Mice, an Animal Model of Alzheimer’s Disease” by Jeong et al. (2018) investigated the effects of MK-0677, a ghrelin agonist, on amyloid beta-related pathology
in a mouse model of Alzheimer’s disease. The study found that MK-0677 treatment reduced amyloid beta levels, mitigated neuroinflammation, and improved cognitive function in the mice. These findings suggest the potential therapeutic
benefits of MK-0677 in Alzheimer’s disease, although further research is needed to evaluate its efficacy in humans.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6032329/
- Bajetto A, Barbero S, Bonavia R, et al. Stromal cell-derived factor-1α induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway. J Neurochem. 2001;77:1226–1236.
Stromal cell-derived factor-1α induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway
Stromal cell-derived factor-1α induces astrocyte proliferation through the activation of extracellular signal-regulated kinases 1/2 pathway” by Bajetto et al. (2001) found that stromal cell-derived factor-1α (SDF-1α) promotes astrocyte
proliferation by activating the extracellular signal-regulated kinases 1/2 (ERK1/2) pathway. This study highlights the role of SDF-1α and the ERK1/2 pathway in regulating astrocyte growth.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11389173/.
- Qui MS, Green SH. PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity. Neuron. 1992;9:705–717.
PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity
PC12 cell neuronal differentiation is associated with prolonged p21ras activity and consequent prolonged ERK activity” by Qui and Green (1992) examined the association between PC12 cell neuronal differentiation and the activity of p21ras
and ERK signaling pathways. The study found that neuronal differentiation of PC12 cells was accompanied by prolonged activation of p21ras, which subsequently led to sustained activation of ERK. These findings suggest a link between
p21ras and ERK signaling in the process of neuronal differentiation in PC12 cells.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/1382473/
- Krapivinsky G, Krapivinsky L, Manasian Y, et al. The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron. 2003;40:775–784.
The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1. Neuron
The NMDA receptor is coupled to the ERK pathway by a direct interaction between NR2B and RasGRF1″ by Krapivinsky et al. (2003) explores the connection between the NMDA receptor and the ERK signaling pathway through a direct interaction
between NR2B and RasGRF1. The study demonstrates that NR2B, a subunit of the NMDA receptor, directly interacts with RasGRF1, leading to the coupling of the NMDA receptor to the ERK pathway. These findings provide insights into the
molecular mechanisms underlying the communication between the NMDA receptor and the ERK pathway.You can read the full article at https://www.cell.com/neuron/fulltext/S0896-6273(03)00645-7?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0896627303006457%3Fshowall%3Dtrue
- Alonso M, Viola H, Izquierdo I, Medina JH. Aversive experiences are associated with rapid and transient activation of ERKs in the rat hippocampus. Neurobiol Learn Mem. 2002;77:119–124.
Aversive experiences are associated with rapid and transient activation of ERKs in the rat hippocampus. Neurobiol Learn Mem
Aversive experiences are associated with rapid and transient activation of ERKs in the rat hippocampus” by Alonso et al. (2002) investigates the association between aversive experiences and the activation of extracellular signal-regulated kinases (ERKs) in the rat hippocampus. The study reveals that aversive experiences lead to rapid and transient activation of ERKs in this brain region. These findings suggest that ERK signaling may play a role in the processing of aversive memories in the hippocampus.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11749089/
- Raschke M, Kolbeck S, Bail H. Homologous growth hormone accelerates healing of segmental bone defects. Bone. 2001;4:368–373.
Homologous growth hormone accelerates healing of segmental bone defects
Homologous growth hormone accelerates healing of segmental bone defects” by Raschke et al. (2001) focuses on the effects of homologous growth hormone on the healing of segmental bone defects. The study demonstrates that the administration
of homologous growth hormone promotes accelerated healing in these types of bone defects. The findings suggest the potential therapeutic benefits of growth hormone in enhancing bone regeneration processes.You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S8756328201005877?via%3Dihub
- Lal S, Wolf S, Herndon D. Growth hormone, burns and tissue healing. Growth Horm IGF Res. 2002;10:39–43.
Growth hormone, burns and tissue healing. Growth Horm IGF Res
Growth hormone, burns and tissue healing” by Lal et al. (2002) explores the relationship between growth hormone (GH), burns, and tissue healing. The study examines the role of GH in promoting tissue healing in burn injuries. The findings
suggest that GH may have positive effects on the healing process in burn patients. The article highlights the potential therapeutic implications of GH in improving tissue healing outcomes.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10984252/
- Ghofrani A, Holler D, Shulmann K. The influence of systemic growth hormone administration on the healing time of skin graft donor sites in a pig model. Plast Reconstr Surg. 1999;104:470–475.
The influence of systemic growth hormone administration on the healing time of skin graft donor sites in a pig model
The influence of systemic growth hormone administration on the healing time of skin graft donor sites in a pig model” by Ghofrani et al. (1999) examines the effects of systemic growth hormone (GH) administration on the healing time of
skin graft donor sites in a pig model. The study investigates whether GH accelerates the healing process in these types of wounds. The findings suggest that systemic GH administration may contribute to a shorter healing time for skin
graft donor sites. The study provides insights into the potential benefits of GH in enhancing wound healing outcomes in this particular model.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10654690/
- Mulligan K, Tai V, Schambelan M. Use of growth hormone and other anabolic agents in AIDS wasting. JPEN J Parenter Enteral Nutr. 1999;23:S202–S209.
Use of growth hormone and other anabolic agents in AIDS wasting
Use of growth hormone and other anabolic agents in AIDS wasting” by Mulligan et al. (1999) focuses on the utilization of growth hormone (GH) and other anabolic agents in the treatment of AIDS wasting syndrome. The study explores the potential
benefits of GH and other anabolic agents in improving body composition and muscle mass in individuals with AIDS-related weight loss. The article discusses the rationale behind the use of these agents and highlights their potential
role in managing AIDS wasting.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10571456/
- Pierre E, Perez-Polo J, Mitchell A. Insulin-like growth factor-1 liposomal gene transfer and systemic growth hormone stimulate wound healing. J Burn Care Rehabil. 1997;4:287–289.
Use of growth hormone and other anabolic agents in AIDS wasting
The study investigated the effects of different treatments on wound healing in rats with scald injuries. The anabolic effects of growth hormone (GH) are mediated through insulin-like growth factor-I (IGF-I). Systemic administration of GH with IGF-I has been shown to stimulate wound reepithelialization, but it can cause hyperglycemia or hypoglycemia. The researchers hypothesized that very low doses of IGF-I in liposome form could have the same positive wound-healing effect when administered locally as higher doses of GH plus IGF-I given systemically. Rats received various treatments for 8 weeks, including placebo IGF-I, IGF-I liposomes, GH plus IGF-I, or GH plus IGF-I liposomes. The combination of GH plus IGF-I and IGF-I liposomes significantly increased postburn weight and wound reepithelialization compared to control groups. The study suggests that small doses of IGF-I delivered in liposome form are as effective as higher doses of GH plus IGF-I for enhancing burn wound reepithelialization.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/9261692/
- Biolo G, Fleming R, Wolfe R. Physiological hyperinsulinemia stimulates protein synthesis and enhances the transport of selected amino acids in human skeletal muscle. J Clin Invest. 1995;95:811–817.
Physiological hyperinsulinemia stimulates protein synthesis and enhances the transport of selected amino acids in human skeletal muscle
This study investigated the anabolic effect of insulin on muscle protein metabolism in healthy volunteers using stable isotopic tracers of amino acids. Insulin was infused into the femoral artery to increase local insulin concentration without affecting the whole body. Muscle protein synthesis rate increased significantly after insulin administration, indicating enhanced protein building in muscle cells. Rates of intracellular amino acid utilization for protein synthesis also increased. However, insulin did not significantly modify the release of amino acids from protein breakdown. The study concludes that insulin promotes muscle anabolism primarily by stimulating protein synthesis rather than affecting transmembrane amino acid transport
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC295560/pdf/jcinvest00024-0381.pdf
- Zhang X, Chinkes D, Irtun O, Wolfe R. Anabolic insulin action on skin wound protein is augmented by exogenesis amino acids. Am J Physiol Endocrinol Metab. 2002;282:308–315.
Anabolic action of insulin on skin wound protein is augmented by exogenesis amino acids
This study investigated the metabolic basis of skin wound healing in rabbits. The scalded ear was used as a model to measure protein kinetics in the skin wound. Insulin or amino acid infusion alone did not improve net protein balance in the wound. However, when insulin and amino acids were infused together, there was a significant increase in net protein balance in the skin wound compared to the individual effects. This indicates that the combined anabolic effect of insulin and amino acids is greater than their individual effects, suggesting an interactive effect in promoting skin wound healing.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajpendo.00361.2001?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org
- Coerper S, Wolf S, von Kiparski S, et al. Insulin-like growth factor accelerates gastric ulcer healing by stimulating cell proliferation and inhibiting gastric acid secretion. Scand J Gastroenterol. 2001;36:921–992.
Insulin-like growth factor accelerates gastric ulcer healings by stimulating cell proliferation and by inhibiting gastric acid secretion
This study aimed to investigate the role of Insulin-Like Growth Factor I (IGF-I) in gastric ulcer healing. The researchers applied IGF-I locally to gastric ulcers in rats and found that it reduced ulcer size, particularly at low doses (0.4 and 4 microg/kg body weight). The effect was similar to treatment with omeprazole, a drug commonly used to treat ulcers. Histological examination revealed increased cell proliferation and IGF-I content, as well as down-regulation of IGF-I receptors after IGF-I administration. Additionally, IGF-I was found to inhibit gastric acid secretion, leading to decreased acid production for over an hour after injection. These findings suggest that IGF-I promotes gastric ulcer healing by stimulating cell proliferation and inhibiting gastric acid secretion.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11521981/
- Lin E, Goncalves JA, Lowry SF. Efficacy of nutritional pharmacology in surgical patients. Curr Opin Clin Nutr Metab Care. 1998;1:41–50.
Efficacy of nutritional pharmacology in surgical patients
Efficacy of nutritional pharmacology in surgical patients” by Lin et al. (1998) examines the effectiveness of nutritional pharmacology interventions in surgical patients. The study focuses on evaluating the impact of specific nutritional approaches on clinical outcomes in the context of surgical care. The findings provide insights into the efficacy of nutritional pharmacology in improving patient outcomes in surgical settings. The article offers valuable information for healthcare professionals involved in the management of surgical patients and informs decision-making regarding nutritional interventions.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10565329/
- Lieberman S, Bukar J, Chen S. Effects of recombinant human insulin-like growth in cachectic patients with the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1994;78:404–410.
Effects of recombinant human insulin-like growth in cachectic patients with the acquired immunodeficiency syndrome
Effects of recombinant human insulin-like growth in cachectic patients with the acquired immunodeficiency syndrome” by Lieberman et al. (1994) investigates the effects of recombinant human insulin-like growth factor (rhIGF) in cachectic patients with acquired immunodeficiency syndrome (AIDS). The study examines the impact of rhIGF on body composition and metabolic parameters in these patients. The findings provide insights into the potential benefits of rhIGF in improving body weight and muscle mass in individuals with AIDS-related cachexia. The article contributes to our understanding of therapeutic interventions for managing cachexia in AIDS patients.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/7508949/
- Blumenfield I, Saiiyi S, Lanir Y. Enhancement of bone defect healing in old rats by TGF beta and IGF-1. Exp Gerontol. 2002;37:53–55.
Enhancement of bone defect healing in old rats by TGF beta and IGF-1
The study aimed to enhance bone healing in aging rats with femoral defects by testing the effects of different growth factors. The rats were divided into groups and treated with transforming growth factor-beta (TGF-beta), insulin-like growth factor-1 (IGF-1), a combination of TGF-beta and IGF-1, or saline (control). After 2 and 4 weeks, the bones were evaluated using radiology, computerized tomography (CT), and biomechanical tests.
Results showed that treatment with TGF-beta+IGF-1 resulted in the most significant bone healing, with complete bone bridging observed after 4 weeks. This treatment led to the filling of the defects with trabecular bone. The other growth factor treatments (TGF-beta or IGF-1 alone) showed less pronounced bone healing compared to the combination treatment. The control group showed only partial healing of the bone defect. Biomechanical tests demonstrated that TGF-beta+IGF-1 significantly increased bone bending rigidity compared to the control group after 4 weeks. These findings highlight the clinical importance of using TGF-beta+IGF-1 to enhance bone healing and restore bone strength and biomechanical properties in aging individuals with bone defects.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0531556501002157?via%3Dihub
- Hou YJ, Banerjee R, Thomas B, et al. SARM is required for neuronal injury and cytokine production in response to central nervous system viral infection. J Immunol. 2013;191(2):875-83.
SARM is required for neuronal injury and cytokine production in response to central nervous system viral infection
The Toll/IL-1R domain-containing adaptor family consists of five members involved in innate immune responses against pathogens. One member, sterile α and Toll/IL-1R domain-containing 1 (SARM), has unclear functions but is known to be involved in axonal death in the central nervous system (CNS). Studies in C. elegans suggest a role for SARM in innate immunity. To understand its role in mammalian innate immunity, SARM−/− mice were infected with various pathogens. While they responded normally to certain pathogens, they showed significant protection from death when infected with vesicular stomatitis virus in the CNS. The protection was linked to reduced CNS injury and cytokine production by nonhematopoietic cells, indicating that SARM positively regulates cytokine production. Neurons and microglia were the primary sources of cytokines, suggesting that SARM acts as a connection between neuronal injury and innate immunity.
You can read the full article at https://journals.aai.org/jimmunol/article/191/2/875/83696/SARM-Is-Required-for-Neuronal-Injury-and-Cytokine
- Johansson I, Destefanis S, Aberg ND, et al. Proliferative and protective effects of growth hormone secretagogues on adult rat hippocampal progenitor cells. Endocrinology. 2008;149(5):2191-9.
Proliferative and protective effects of growth hormone secretagogues on adult rat hippocampal progenitor cells
The study investigated the effects of growth hormone secretagogues (GHS) on adult rat hippocampal progenitor (AHP) cells, which have regenerative potential. Both hexarelin and ghrelin, GHS, increased cell proliferation in AHP cells. Hexarelin, but not ghrelin, protected against apoptosis and necrosis induced by growth factor deprivation. Hexarelin activated the MAPK and phosphatidylinositol 3-kinase/Akt pathways, while ghrelin only activated the MAPK pathway. Although AHP cells did not express the GHS receptor 1a, both hexarelin and ghrelin showed specific binding, indicating effects might be mediated by an alternative GHS receptor subtype. The findings suggest that hexarelin and ghrelin have differential effects in AHP cells, with hexarelin showing a cell protective and proliferative role in the central nervous system.
You can read the full article at https://academic.oup.com/endo/article/149/5/2191/2454900?login=false
- Dioufa N, Schally AV, Chatzistamou I, et al. Acceleration of wound healing by growth hormone-releasing hormone and its agonists. Proc Natl Acad Sci USA. 2010;107(43):18611-5.
Acceleration of wound healing by growth hormone-releasing hormone and its agonists
The study investigated the effects of growth hormone-releasing hormone (GHRH) on wound healing and tissue repair. GHRH was found to act on wound-associated fibroblasts, which express a splice variant of the GHRH receptor (SV1). GHRH and a GHRH agonist (JI-38) stimulated the expression of α-smooth muscle actin (αSMA) in fibroblasts and promoted their migration in vitro. In vivo, local application of GHRH or JI-38 accelerated wound healing in mouse skin, characterized by increased fibroblast abundance during early stages and accelerated reformation of the covering epithelium at later stages. These findings suggest that GHRH may have clinical potential for enhancing skin wound healing after trauma, surgery, or disease.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2972940/
- Svensson J, Jansson JO, Ottosson M, et al. Treatment of obese subjects with the oral growth hormone secretagogue MK-677 affects serum concentrations of several lipoproteins, but not lipoprotein(a). J Clin Endocrinol Metab. 1999;84(6):2028-33.
Treatment of obese subjects with the oral growth hormone secretagogue MK-677 affects serum concentrations of several lipoproteins, but not lipoprotein(a)
The study investigated the effects of the oral growth hormone secretagogue MK-677 on lipoproteins in obese males. Twenty-four obese males were treated with either 25 mg MK-677 or placebo daily for 8 weeks. MK-677 treatment did not significantly change serum lipoprotein(a) levels. However, it increased serum apolipoprotein A-I and E levels at 2 weeks but not at the study’s end. Total cholesterol and LDL cholesterol levels were not significantly changed by MK-677 treatment. HDL cholesterol was increased at 2 weeks but not at 8 weeks. The LDL cholesterol/HDL cholesterol ratio was reduced after 8 weeks of MK-677 treatment. LDL particle diameter was decreased at 2 weeks but returned to baseline values at 8 weeks. Serum triglycerides were increased at 2 weeks but not at 8 weeks. The study concluded that MK-677 treatment had transient effects on certain lipoproteins, and it did not significantly affect serum lipoprotein(a) concentrations at the given dose and administration protocol.
You can read the full article at https://academic.oup.com/jcem/article/84/6/2028/2864618?login=false
- Baldanzi G, Filigheddu N, Cutrupi S, et al. Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT. The Journal of Cell Biology. 2002;159(6):1029-1037. doi:10.1083/jcb.200207165.
Ghrelin and des-acyl ghrelin inhibit cell death in cardiomyocytes and endothelial cells through ERK1/2 and PI 3-kinase/AKT
Ghrelin is a gastric hormone that stimulates growth hormone release and appetite through the GHSR-1a receptor in the pituitary and hypothalamus. In addition to its endocrine activities, ghrelin and its non-active form, des-acyl ghrelin, have a cardio-protective effect on cardiomyocytes and endothelial cells by inhibiting apoptosis. This effect is mediated through activation of extracellular signal-regulated kinase-1/2 and Akt serine kinases. Both ghrelin and des-acyl ghrelin recognize common high affinity binding sites on the cardiovascular cells, even in the absence of GHSR-1a receptor expression. Synthetic compounds with GHS activity also activate these binding sites, suggesting the presence of a novel receptor in the cardiovascular system that responds to ghrelin and its non-active form.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2173981/
- ateishi K, et al. (2007) Human cardiac stem cells exhibit mesenchymal features and are maintained through Akt/GSK-3beta signaling. Biochem Biophys Res Commun 352(3):635–641.
Human cardiac stem cells exhibit mesenchymal features and are maintained through Akt/GSK-3beta signaling
Recent evidence suggested that human cardiac stem cells (hCSCs) may have the clinical application for cardiac repair; however, their characteristics and the regulatory mechanisms of their growth have not been fully investigated. Here, we show the novel property of hCSCs with respect to their origin and tissue distribution in human heart, and demonstrate the signaling pathway that regulates their growth and survival. Telomerase-active hCSCs were predominantly present in the right atrium and outflow tract of the heart (infant > adult) and had a mesenchymal cell-like phenotype. These hCSCs expressed the embryonic stem cell markers and differentiated into cardiomyocytes to support cardiac function when transplanted them into ischemic myocardium. Inhibition of Akt pathway impaired the hCSC proliferation and induced apoptosis, whereas inhibition of glycogen synthase kinase-3 (GSK-3) enhanced their growth and survival. We conclude that hCSCs exhibit mesenchymal features and that Akt/GSK-3beta may be crucial modulators for hCSC maintenance in human heart.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/17150190/
- Choi SH, et al. (2013) Regulation of ROS-independent ERK signaling rescues replicative cellular senescence in ex vivo expanded human c-kit-positive cardiac progenitor cells. Int J Cardiol 169(1):73–82.
Regulation of ROS-independent ERK signaling rescues replicative cellular senescence in ex vivo expanded human c-kit-positive cardiac progenitor cells
The study aimed to understand the molecular pathways involved in cellular senescence of human cardiac progenitor cells (hCPCs) and to explore if mitogen-activated protein kinases (MAPKs) activation could aid in their recovery. The researchers found that during replicative senescence of hCPCs, the extracellular signal-regulated kinase (ERK) was significantly activated, leading to reduced proliferative activity. Inhibition of ERK using a specific inhibitor reversed cellular senescence, as evidenced by reduced senescence-associated β-galactosidase activity and decreased expression of senescence-related molecules. Interestingly, ERK inhibition rescued hCPC senescence in a reactive oxygen species (ROS)-independent manner, as the levels of ROS and antioxidant enzymes remained unchanged after treatment. These findings suggest that targeting ERK activation may be a potential strategy to rescue cellular senescence in cardiac progenitor cells.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/24094550/
- Available at https://academic.oup.com/jcem/article/90/3/1864/2837042.
Growth Hormone and Cardiovascular Risk Factors
The lesson from “organic” growth hormone deficiency (GHD) is that impairment in the GH/IGF-I axis can lead to a high-risk cardiovascular profile, including increased body fat, insulin resistance, and hypertriglyceridemia. GH replacement therapy has shown promising results in partially reversing these cardiovascular risk factors. While definitive evidence linking GH substitution to reduced cardiovascular mortality is awaited, the reported data is encouraging. Studies on GHD provide enough evidence to suggest that the GH/IGF-I axis plays a physiological role in regulating metabolic cardiovascular risk factors. This raises the possibility of extending these findings to conditions like aging and obesity, which exhibit “functional” GHD. However, further long-term clinical studies are needed to support this hypothesis.
You can read the full article at https://academic.oup.com/jcem/article/90/3/1864/2837042
- Isgaard J. Cardiovascular disease and risk factors: the role of growth hormone. Horm Res. 2004;62 Suppl 4:31-8.
Cardiovascular disease and risk factors: the role of growth hormone
This paper discusses the effects of growth hormone (GH) on the cardiovascular system and how chronic GH deficiency (GHD) can lead to impaired cardiac function and increased cardiovascular disease risk. Clinical studies in patients with acromegaly (excessive GH production) have shown both short- and long-term effects on the heart’s structure and function. Conversely, GHD is associated with reduced exercise capacity, low heart rate, impaired left ventricular systolic function, and altered lipoprotein metabolism. GH replacement therapy is explored as a potential treatment for patients with GHD to improve cardiac performance and reduce cardiovascular disease risk.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/15591764/
- Lombardi G, Colao A, Ferone D, et al. Effect of growth hormone on cardiac function. Horm Res. 1997;48 Suppl 4:38-42.
Ferone D, et al. Effect of growth hormone on cardiac function.
Effect of growth hormone on cardiac function” by Lombardi et al. (1997) investigates the impact of growth hormone on cardiac function. The study explores the relationship between growth hormone and the cardiovascular system. It examines how growth hormone influences cardiac function, including parameters such as cardiac output and left ventricular mass. The findings shed light on the effects of growth hormone on the heart and contribute to our understanding of its role in cardiovascular health.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/22314142/
- Isgaard J, Arcopinto M, Karason K, Cittadini A. GH and the cardiovascular system: an update on a topic at heart. Endocrine. 2015;48(1):25-35. doi:10.1007/s12020-014-0327-6.
GH and the cardiovascular system: an update on a topic at heart
This review discusses the importance of growth hormone (GH) in maintaining normal cardiac function in adults. It covers the physiological effects of GH and its interactions with insulin-like growth factor I (IGF-I) in the cardiovascular system. The review also examines cardiac dysfunctions associated with both GH excess (acromegaly) and GH deficiency in adult hypopituitary patients, which may contribute to increased cardiovascular morbidity and mortality. Furthermore, the article explores the relationship between the GH/IGF-I system and heart failure, and the potential of GH as a therapeutic option for heart failure treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4328125/
- Saccà L, Cittadini A, Fazio S. Growth hormone and the heart. Endocr Rev. 1994;15(5):555-73.
Growth hormone and the heart
Growth hormone (GH) directly affects myocardial growth and function, inducing the expression of specific contractile proteins and myocyte hypertrophy. Excessive GH production, as in acromegaly, leads to cardiac abnormalities, including myocardial hypertrophy and altered cardiac function. Ventricular hypertrophy occurs without increased wall stress, leading to impaired ventricular relaxation and diastolic dysfunction, followed by systolic dysfunction. If left untreated, cardiac performance gradually deteriorates, leading to heart failure. Studies suppressing GH production in acromegaly patients show regression of hypertrophy and improved cardiac function. Growth hormone deficiency (GHD) results in reduced ventricular mass and impaired cardiac performance, particularly evident during physical exercise, contributing to reduced exercise capacity. Early-onset GHD appears to have more significant consequences on cardiac function. Treatment with human recombinant GH (hrGH) can markedly improve cardiac dysfunction associated with GHD, supporting its use as replacement therapy for affected patients.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/7843068/
- Arcopinto M, Salzano A, Giallauria F, et al. Growth Hormone Deficiency Is Associated with Worse Cardiac Function, Physical Performance, and Outcome in Chronic Heart Failure: Insights from the T.O.S.CA. GHD Study. PLoS ONE. 2017;12(1):e0170058.
Growth Hormone Deficiency Is Associated with Worse Cardiac Function, Physical Performance, and Outcome in Chronic Heart Failure: Insights from the T.O.S.CA. GHD Study
This study aimed to assess the prevalence and role of growth hormone deficiency (GHD) in patients with mild-to-moderate chronic heart failure (CHF). Approximately 30% of CHF patients were found to have GHD. Compared to CHF patients with normal GH levels, those with GHD showed smaller left ventricular volumes, lower left ventricular end-systolic wall stress, higher right ventricular performance, lower estimated pulmonary artery pressure, and impaired exercise capacity. After adjusting for other factors, peak oxygen consumption, ventilatory efficiency, and NT-proBNP (a biomarker for heart failure) were significantly associated with GHD status. Moreover, CHF patients with GHD had higher all-cause mortality rates, independent of other clinical factors. These findings suggest that GHD may have a significant impact on cardiovascular function and prognosis in CHF patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5240983/
- De leonibus C, De marco S, Stevens A, Clayton P, Chiarelli F, Mohn A. Growth Hormone Deficiency in Prepubertal Children: Predictive Markers of Cardiovascular Disease. Horm Res Paediatr. 2016;85(6):363-71.
Growth Hormone Deficiency in Prepubertal Children: Predictive Markers of Cardiovascular Disease
This review focuses on cardiovascular (CV) risk factors in prepubertal children with untreated growth hormone deficiency (GHD). GHD children are prone to increased CV risks, including impaired cardiac function, dyslipidemia, and body composition abnormalities. The review also highlights the importance of specific biochemical and clinical markers in predicting CV disease in this population. Additionally, it discusses the potential effects of recombinant human growth hormone therapy in addressing these alterations. Novel findings in epigenetics and metabolomics are explored, and the potential clinical implications of these discoveries are discussed. The overall aim is to better understand and manage CV risks in prepubertal children with GHD.
You can read the full article at https://karger.com/hrp/article/85/6/363/162712/Growth-Hormone-Deficiency-in-Prepubertal-Children
- Available at https://clinicaltrials.gov/ct2/show/NCT00397319.
Growth Hormone’s Effect on the Cardiovascular System
Growth hormone deficiency (GHD) is linked to cardiovascular issues and its effects include reduced exercise capacity, impaired cardiac function, central fat redistribution, increased peripheral arterial resistance, and an unfavorable lipid profile. Replacement therapy with recombinant human growth hormone has been shown to reverse these effects. We plan to use experimental systems to investigate how growth hormone (GH) maintains cardiovascular health, focusing on its interaction with Plasminogen-activator-inhibitor-1 (a key regulator of the fibrinolytic system) and its role in vascular function.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5796235/
- Liang S, Xue J, Li G. Effects of recombinant human growth hormone administration on cardiovascular risk factors in obese children with relative growth hormone deficiency. Lipids Health Dis. 2018;17(1):66.
Effects of recombinant human growth hormone administration on cardiovascular risk factors in obese children with relative growth hormone deficiency
The study aimed to investigate the effects of recombinant human growth hormone (rhGH) treatment on cardiovascular risk factors in obese children with relative growth hormone deficiency (GHD). Forty-three obese children with relative GHD were included in the study and divided into two groups: the rhGH treatment group and the untreated control group.
After 6 months of rhGH treatment, the rhGH group showed significant reductions in BMI standard deviation scores (SDS) compared to the control group. Insulin-like growth factor 1 (IGF-1) levels increased during rhGH treatment. The rhGH treatment also led to reductions in low-density lipoprotein cholesterol (LDL-C), aspartate aminotransferase (AST), and alanine aminotransferase (ALT), and increased levels of high-density lipoprotein cholesterol (HDL-C) compared to the control group.
The study concludes that 6 months of rhGH treatment in obese children with relative GHD resulted in reduced BMI SDS, stabilized IGF-1 levels, and improved blood lipid profiles and liver enzyme levels. Additionally, GH administration did not significantly affect insulin resistance and did not have adverse effects on glucose homeostasis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5883519/
- Available at https://link.springer.com/article/10.1007/s12020-016-1206-0.
Growth hormone and the heart in growth hormone deficiency—what have we learned so far?
Recombinant human growth hormone (GH) has been used for nearly three decades as replacement therapy in individuals with growth hormone deficiency (GHD). The cardiovascular system was an early recognized target for the beneficial effects of GH replacement, as GHD affects the heart on multiple levels. It leads to direct effects on the heart, including decreased ventricular mass and cardiac output, reduced exercise capacity, and impaired diastolic and systolic function. Some studies also suggest increased peripheral resistance due to low GH levels, although results remain conflicting.
In addition to the direct impact on the heart, GHD is associated with several cardiovascular risk factors, such as insulin resistance, unfavorable lipid profile, decreased fibrinolysis, and augmented sympathetic nervous activity. However, replacement therapy with GH tends to correct most of these aberrations in cardiovascular function. A recent study by Boschetti explored the effect of GHD on coronary flow reserve (CFR), an early marker of impaired myocardial microcirculation. They found that CFR is significantly reduced in GHD patients compared to controls, and 12 months of GH replacement therapy normalized CFR and improved diastolic function. While the clinical significance of these findings requires further investigation, decreased CFR could potentially serve as an early marker for increased cardiovascular risk in GHD patients
You can read the full article at https://link.springer.com/article/10.1007/s12020-016-1206-0
- Ungvari Z, Csiszar A. The Emerging Role of IGF-1 Deficiency in Cardiovascular Aging: Recent Advances. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2012;67A(6):599-610. doi:10.1093/gerona/gls072.
The Emerging Role of IGF-1 Deficiency in Cardiovascular Aging: Recent Advances.
This review discusses the cardiovascular protective effects of insulin-like growth factor (IGF)-1 and explores the molecular mechanisms involved in cardiovascular alterations in individuals with congenital and adult-onset IGF-1 deficiency. It also examines the connection between age-related IGF-1 deficiency and the molecular, cellular, and functional changes that occur in the cardiovascular system during aging.
The review emphasizes the microvascular protection provided by endocrine and paracrine IGF-1 signaling and its relevance to the pathophysiology of cardiac failure and vascular cognitive impairment. Additionally, it delves into the role of impaired cellular stress resistance in cardiovascular aging, focusing on the effects of IGF-1 on Nrf2-driven antioxidant response.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3348495/
- Andreassen M, Raymond I, Kistorp C, Hildebrandt P, Faber J, Kristensen LØ. IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population. Eur J Endocrinol. 2009;160(1):25-31.
IGF1 as predictor of all cause mortality and cardiovascular disease in an elderly population
This study aimed to investigate the association of plasma IGF1 levels with all-cause mortality, the development of chronic heart failure (CHF), and major cardiovascular (CV) events in a population-based cohort of individuals aged 50-89 years. The study found that higher IGF1 levels were independently associated with increased all-cause mortality and an elevated risk of developing CHF. However, there was no significant association between IGF1 levels and the overall incidence of major CV events. These findings suggest that IGF1 may play a complex role in the aging process and the development of cardiovascular disease.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/18931092/
- Kaplan RC, Strickler HD, Rohan TE, Muzumdar R, Brown DL. Insulin-like growth factors and coronary heart disease. Cardiol Rev. 2005;13(1):35-9.
Insulin-like growth factors and coronary heart disease
This review discusses the potential role of insulin-like growth factor-1 (IGF-I) as a regulator of cell growth and its association with cardiovascular disease (CVD). Studies have suggested that low IGF-I levels, even within the normal range, may be linked to an increased risk of incident myocardial infarction and other manifestations of coronary heart disease. The review explores possible mechanisms for this association, including the beneficial effects of IGF-I on myocyte survival after ischemia, stability of atherosclerotic lesions, and endothelial function. However, further prospective studies are needed to confirm IGF-I as an independent cardiovascular risk factor. If this association is confirmed, it could potentially lead to intervention trials targeting the GH/IGF-I pathway to reduce CVD morbidity and mortality among older adults
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/15596027/
- Castellano G, Affuso F, Conza PD, Fazio S. The GH/IGF-1 Axis and Heart Failure. Current Cardiology Reviews. 2009;5(3):203-215. doi:10.2174/157340309788970306.
The GH/IGF-1 Axis and Heart Failure
The growth hormone (GH)/insulin-like growth factor 1 (IGF-1) axis plays a crucial role in regulating cardiac growth, myocardial contractility, and the vascular system. It enhances calcium availability and contractile protein expression in the heart, stimulates cardiac growth through increased protein synthesis, and affects systemic vascular resistance by activating the nitric oxide system. Experimental studies have extensively demonstrated the link between the GH/IGF-1 axis and the cardiovascular system, as seen in cardiac abnormalities resulting from both GH excess and deficiency.
Clinical studies have shown promising results in using recombinant GH to improve cardiac function and structure in patients with dilated cardiomyopathy and chronic heart failure (CHF). However, conflicting results from other placebo-controlled trials suggest that the response to GH treatment may be influenced by the degree of GH resistance associated with CHF. Further research is needed to understand the mechanisms that determine the variable sensitivity to GH and its potential positive effects in treating heart failure
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2822143/
- Abdulle AM, Gillett MP, Abouchacra S, et al. Low IGF-1 levels are associated with cardiovascular risk factors in haemodialysis patients. Mol Cell Biochem. 2007;302(1-2):195-201.
Low IGF-1 levels are associated with cardiovascular risk factors in haemodialysis patients
This study aimed to investigate the relationship between insulin-like growth factor-1 (IGF-1) levels and cardiovascular risk factors in chronic kidney disease (CKD) patients on maintenance hemodialysis. The study compared 50 CKD patients with age-matched healthy control subjects. The results showed that IGF-1 levels were significantly lower in CKD patients compared to controls. IGF-1 was inversely correlated with systolic and diastolic blood pressure in the CKD group, indicating a potential link between low IGF-1 levels and increased cardiovascular risk factors in CKD patients. However, the exact cause-and-effect relationship between IGF-1 and cardiovascular risk factors remains unclear. Nonetheless, the findings suggest a significant association between low IGF-1 levels and cardiovascular risk in CKD patients compared to healthy controls of the same age.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/17387582/
- Córdova C, Boullosa DA, Custódio MR, et al. Atheroprotective Properties of Serum IGF-1 in the Carotid and Coronary Territories and Beneficial Role on the Physical Fitness of the Oldest Old. J Gerontol A Biol Sci Med Sci. 2016;71(10):1281-8.
Atheroprotective Properties of Serum IGF-1 in the Carotid and Coronary Territories and Beneficial Role on the Physical Fitness of the Oldest Old
In this cross-sectional study of community-dwelling individuals without previous cardiovascular events, researchers investigated the relationship between soluble insulin-like growth factor-1 (IGF-1) levels, atherosclerotic burden in coronary and carotid arteries, and physical fitness in the oldest old (aged individuals). The study included 100 participants. The findings revealed a linear correlation between IGF-1 levels and carotid intima-media thickness, number of carotid plaques, and walking speed. Participants in the upper IGF-1 tertile had smaller intima-media thickness in the carotid arteries, fewer atherosclerotic plaques, and faster walking speed compared to the intermediate and lower tertiles. However, the correlation between IGF-1 and coronary calcification scores was nonlinear. The intermediate IGF-1 tertile was associated with the lowest calcification scores, while participants with lower IGF-1 levels showed a higher frequency of high-risk morphology plaques. The study suggests that IGF-1 levels may play a role in atherorefractory phenotype in the oldest old, with different effects in coronary and carotid territories
You can read the full article at https://academic.oup.com/biomedgerontology/article/71/10/1281/2198072?login=false
- Wallander M, Norhammar A, Malmberg K, Ohrvik J, Rydén L, Brismar K. IGF binding protein 1 predicts cardiovascular morbidity and mortality in patients with acute myocardial infarction and type 2 diabetes. Diabetes Care. 2007;30(9):2343-8.
IGF binding protein 1 predicts cardiovascular morbidity and mortality in patients with acute myocardial infarction and type 2 diabetes
In this study, researchers investigated whether low insulin-like growth factor-I (IGF-I) and high insulin-like growth factor-binding protein-1 (IGFBP-1) levels predict future cardiovascular mortality and morbidity in patients with acute myocardial infarction (AMI) and type 2 diabetes. They enrolled 575 patients from the Diabetes Mellitus Insulin-Glucose Infusion in Acute Myocardial Infarction (DIGAMI) 2 Trial, out of a total of 1,253 patients with type 2 diabetes and AMI. The study followed these patients for a median period of 2.2 years.
Results showed that during the follow-up period, 23% of patients died from all-cause mortality, 18% from cardiovascular disease (CVD), and 30% suffered from at least one cardiovascular event. The study found that high levels of IGFBP-1 at admission were associated with an increased risk of cardiovascular mortality and morbidity in patients with type 2 diabetes and AMI. Other factors such as blood glucose levels and age also played a role in predicting cardiovascular outcomes. This suggests that the IGF system, particularly IGFBP-1, may be linked to cardiovascular risk in patients with type 2 diabetes and AMI.
You can read the full article at https://diabetesjournals.org/care/article/30/9/2343/29358/IGF-Binding-Protein-1-Predicts-Cardiovascular
- Shah N, Rice T, Tracy D, et al. Sleep and Insulin-Like Growth Factors in the Cardiovascular Health Study. Journal of Clinical Sleep Medicine : JCSM : Official Publication of the American Academy of Sleep Medicine. 2013;9(12):1245-1251. doi:10.5664/jcsm.3260.
Sleep and Insulin-Like Growth Factors in the Cardiovascular Health Study
This study aimed to investigate the relationship between sleep (architecture and obstructive sleep apnea [OSA]) and circulating levels of insulin-like growth factor-1 (IGF-I), insulin-like growth factor binding protein-1 (IGFBP-1), and insulin-like growth factor binding protein-3 (IGFBP-3) in an elderly population. The study included 1,233 elderly participants from the Cardiovascular Health Study (CHS) and Sleep Heart Health Study (SHHS).
The results showed that there was no significant association between slow wave sleep (SWS) and IGF-I, IGFBP-1, and IGFBP-3 levels in the elderly participants. Likewise, OSA (apnea-hypopnea index [AHI] ≥ 5/h) did not appear to adversely influence the GH/IGF axis in this elderly population. However, sensitivity analyses excluding diabetics revealed that moderate OSA (AHI ≥ 5 and < 15) was inversely associated with IGFBP-3 levels in women. The study concluded that in the elderly, there was no significant relationship between SWS and the GH/IGF system, and OSA did not seem to negatively impact the GH/IGF axis as observed in younger individuals. Further investigation and replication in other large population-based elderly cohorts are needed to understand the potential influence of diminished GH/IGF-I axis activity in this age group
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3836334/
- González-guerra JL, Castilla-cortazar I, Aguirre GA, et al. Partial IGF-1 deficiency is sufficient to reduce heart contractibility, angiotensin II sensibility, and alter gene expression of structural and functional cardiac proteins. PLoS ONE. 2017;12(8):e0181760.
Partial IGF-1 deficiency is sufficient to reduce heart contractibility, angiotensin II sensibility, and alter gene expression of structural and functional cardiac proteins
This study investigated the impact of partial insulin-like growth factor-1 (IGF-1) deficiency on the heart and coronary circulation. The research compared vasoactive factors before and after ischemia-reperfusion (I/R). The study found that partial IGF-1 deficiency was associated with reduced heart contractility and sensitivity to angiotensin II, as well as interstitial fibrosis. Additionally, there were alterations in the expression pattern of genes related to extracellular matrix proteins, calcium dynamics, and cardiac structure and function. The findings suggest that partial IGF-1 deficiency may have implications for cardiovascular health and indicate the potential of this experimental model for studying cardiac disease mechanisms and exploring therapeutic options for patients with IGF-1 deficiency
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5555709/
- Chigogora S, Zaninotto P, Kivimaki M, Steptoe A, Batty GD. Insulin-like growth factor 1 and risk of depression in older people: the English Longitudinal Study of Ageing. Transl Psychiatry. 2016;6(9):e898.
Insulin-like growth factor 1 and risk of depression in older people: the English Longitudinal Study of Ageing
This study examined the relationship between insulin-like growth factor 1 (IGF-1) levels and depression symptoms in older adults. The research involved 6017 participants, with mean age 65.7 years, and 55% women. Serum levels of IGF-1 were measured, and depression symptoms were assessed using the Center for Epidemiologic Studies Depression Scale. The findings revealed a ‘U’-shaped pattern of association, indicating that both lower and higher IGF-1 levels were associated with a slightly elevated risk of depression, while the lowest risk was observed at median IGF-1 levels. Moderate levels of IGF-1 appeared to confer a reduced risk of depression in older adults. However, some of these effects were attenuated after accounting for co-morbidity, socioeconomic status, and health behaviors
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5048205/
- Barton JS, Hindmarsh PC, Preece MA. Serum insulin-like growth factor 1 in congenital heart disease. Arch Dis Child. 1996;75(2):162-3.
Serum insulin-like growth factor 1 in congenital heart disease
This study compared 62 infants with congenital heart disease and healthy controls regarding serum insulin-like growth factor 1 (IGF-1), insulin-like growth factor binding protein-3 (IGFBP-3), and various growth and nutritional factors. Infants with congenital heart disease were found to be smaller, underweight, and had reduced energy intake compared to healthy controls. Additionally, they had significantly lower levels of serum IGF-1 and IGFBP-3. These reduced IGF-1 and IGFBP-3 levels are commonly observed in cases of nutritional deficiency. The findings suggest that undernutrition may contribute to the poor growth of infants with congenital heart disease. Monitoring serum IGF-1 and IGFBP-3 levels over time could be useful in assessing the impact of nutritional interventions in these patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1511626/pdf/archdisch00610-0076.pdf
- Giovannini S, Cesari M, Marzetti E, Leeuwenburgh C, Maggio M, Pahor M. EFFECTS OF ACE-INHIBITION ON IGF-1 AND IGFBP-3 CONCENTRATIONS IN OLDER ADULTS WITH HIGH CARDIOVASCULAR RISK PROFILE. The journal of nutrition, health & aging. 2010;14(6):457-460.
Effects of ACE-Inhibition on IGF-1 and IGFBP-3 Concentrations in Older Adults with High Cardiovascular Risk Profile
The study assessed the impact of a 6-month treatment with the ACE-inhibitor fosinopril on serum levels of total insulin-like growth factor 1 (IGF-1) and insulin-like growth factor binding protein-3 (IGFBP-3) in older adults with a high risk of cardiovascular disease. It was a double-blind, crossover, randomized, placebo-controlled trial involving participants aged 55 years or older with a high cardiovascular disease risk profile. The intervention consisted of either fosinopril or a placebo administered for 6 months. Serum levels of total IGF-1 and IGFBP-3 were measured at baseline, 6-month, and 12-month follow-up visits. The results showed that after 6 months of treatment with fosinopril, the participants had significantly higher serum levels of total IGF-1 and IGFBP-3 compared to the placebo group. This suggests that fosinopril treatment may contribute to increased IGF-1 and IGFBP-3 levels, potentially explaining the beneficial effects of ACE-inhibition on stroke, ischemic heart disease, and insulin resistance in older adults with a high cardiovascular risk profile.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4311891/
- Strazhesko ID, Tkacheva ON, Akasheva DU, et al. Growth Hormone, Insulin-Like Growth Factor-1, Insulin Resistance, and Leukocyte Telomere Length as Determinants of Arterial Aging in Subjects Free of Cardiovascular Diseases. Front Genet. 2017;8:198.
Growth Hormone, Insulin-Like Growth Factor-1, Insulin Resistance, and Leukocyte Telomere Length as Determinants of Arterial Aging in Subjects Free of Cardiovascular Diseases
The study aimed to investigate the role of growth hormone (GH), insulin-like growth factor-1 (IGF-1), telomere length (LTL), and cardiovascular risk factors (CVRF) in vascular aging. The study included 303 participants without known cardiovascular disease, categorized into “younger” and “older” groups. Arterial stiffness (AS), intima-media thickness (IMT), and atherosclerotic plaques (PP) were measured, along with serum levels of GH, IGF-1, and LTL. In the younger group, GH, IGF-1, insulin resistance (HOMA), and LTL were independently associated with arterial wall parameters. However, in the older group, there were no significant independent associations between GH/IGF-1, LTL, HOMA, and arterial characteristics. The findings suggest that GH/IGF-1, insulin resistance, HOMA, and LTL play a role in arterial aging in younger healthy individuals, but their significance diminishes with age.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5770739/
- Bergman D, Halje M, Nordin M, Engström W. Insulin-like growth factor 2 in development and disease: a mini-review. Gerontology. 2013;59(3):240-9.
Insulin-like growth factor 2 in development and disease: a mini-review
Insulin-like growth factor 2 (IGF2) is a protein hormone that regulates cell growth, proliferation, and survival. It is primarily expressed from the paternal allele due to parental imprinting. Changes in IGF2 expression have been linked to growth disorders, cancers, and cardiovascular disease. However, the role of IGF2 in tumour growth and cardiovascular conditions is still not fully understood, and some research findings are conflicting. While significant progress has been made in understanding IGF2’s structure and interactions, further research is needed to fully comprehend its impact on key diseases such as cancer and atherosclerosis.
You can read the full article at https://karger.com/ger/article/59/3/240/147768/Insulin-Like-Growth-Factor-2-in-Development-and
- Touvron M, Escoubet B, Mericskay M, et al. Locally expressed IGF1 propeptide improves mouse heart function in induced dilated cardiomyopathy by blocking myocardial fibrosis and SRF-dependent CTGF induction. Dis Model Mech. 2012;5(4):481-91.
Locally expressed IGF1 propeptide improves mouse heart function in induced dilated cardiomyopathy by blocking myocardial fibrosis and SRF-dependent CTGF induction
Cardiac fibrosis is a major factor in the adverse remodeling seen in dilated cardiomyopathies (DCMs), leading to cardiac dysfunction and heart failure. Connective tissue growth factor (CTGF) plays a key role in this harmful process. In this study, researchers investigated the effects of expressing a cardiac-specific transgene that produces locally acting insulin-like growth factor 1 (IGF1 propeptide or mIGF1) in a mouse model of DCM. The mice with cardiac-specific mIGF1 expression showed significant extension of lifespan, improved cardiac function, and delayed progression of DCM and heart failure. These protective effects were associated with better cardiomyocyte structure and a significant reduction in myocardial fibrosis and inflammation. The mIGF1 counteracted the increased expression of CTGF caused by the gene disruption, leading to the inhibition of fibroblast proliferation and related myocardial fibrosis. The study demonstrates that the locally acting mIGF1 can protect the heart from adverse remodeling and fibrosis in DCM, suggesting it could be a potential therapeutic approach to address this condition. Additionally, the study highlights the role of serum response factor (SRF) in modulating cardiac fibrosis through the regulation of CTGF expression in cardiomyocytes and how impaired SRF function can promote fibrosis and adverse cardiac remodeling in human heart failure.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3380711/
- Milman S, Atzmon G, Huffman DM, et al. Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity. Aging Cell. 2014;13(4):769-71.
Low insulin-like growth factor-1 level predicts survival in humans with exceptional longevity
The study aimed to investigate whether insulin-like growth factor-1 (IGF-1) levels in nonagenarians (people in their 90s) can predict their survival duration. The researchers found that in females, those with IGF-1 levels below the median had significantly longer survival compared to those with levels above the median. This survival advantage was not observed in males. However, in both males and females with a history of cancer, lower IGF-1 levels predicted longer survival. The association between lower IGF-1 levels and longer life expectancy remained significant after accounting for other variables in females and individuals with a history of cancer. This study provides evidence that low IGF-1 levels may predict longer life expectancy in exceptionally long-lived individuals.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4116456/
- Zhang Z, et al. (2013) Rosuvastatin enhances the therapeutic efficacy of adipose-derived mesenchymal stem cells for myocardial infarction via PI3K/Akt and MEK/ERK pathways. Basic Res Cardiol 108(2):333.
Rosuvastatin enhances the therapeutic efficacy of adipose-derived mesenchymal stem cells for myocardial infarction via PI3K/Akt and MEK/ERK pathways.
The study aimed to investigate whether rosuvastatin, a medication used to lower cholesterol levels, could improve the survival of transplanted adipose-derived mesenchymal stem cells (AD-MSCs) in the hearts of mice with myocardial infarction (heart attack). AD-MSCs were transplanted into the infarcted hearts, and rosuvastatin was administered to some of the mice. The researchers found that rosuvastatin enhanced the survival of the transplanted AD-MSCs and reduced heart fibrosis, cardiomyocyte apoptosis, and preserved heart function. In vitro experiments showed that rosuvastatin improved the viability and paracrine effect of AD-MSCs and reduced their apoptotic rate under conditions mimicking the ischemic environment. The beneficial effects of rosuvastatin on AD-MSCs were linked to the activation of the PI3K/Akt and MEK/ERK1/2 signaling pathways. The combination therapy of rosuvastatin and AD-MSCs showed a synergistic effect in improving heart function after myocardial infarction. These findings suggest that rosuvastatin may enhance the therapeutic potential of AD-MSC transplantation for treating heart attack.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/23386286/
- Sun X, Fang B, Zhao X, Zhang G, Ma H (2014) Preconditioning of mesenchymal stem cells by sevoflurane to improve their therapeutic potential. PLoS ONE 9(3):e90667.
Preconditioning of mesenchymal stem cells by sevoflurane to improve their therapeutic potential
The study aimed to investigate whether sevoflurane preconditioning can protect bone marrow mesenchymal stem cells (MSCs) against hypoxia and serum deprivation-induced apoptosis and improve their migration, proliferation, and therapeutic potential. In an in vitro model, MSCs were exposed to hypoxia and serum deprivation (H/SD) to mimic ischemic conditions. Sevoflurane preconditioning was performed on MSCs by incubating them in sevoflurane. The results showed that sevoflurane preconditioning reduced MSCs’ apoptosis and maintained their mitochondrial membrane potential. It also increased the migration and expression of specific proteins related to cell survival and growth (HIF-1α, HIF-2α, VEGF, and p-Akt/Akt), which were reduced by H/SD. Additionally, when neuron-like PC12 cells were co-cultured with sevoflurane-preconditioned MSCs, they became more resistant to H/SD-induced apoptosis. The findings suggest that sevoflurane preconditioning enhances the survival and migration of MSCs under H/SD conditions and improves their therapeutic potential. These beneficial effects are likely mediated, at least in part, by the upregulation of specific proteins related to cell survival and growth
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3944720/
- Gude N, et al. (2006) Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res 99(4):381–388.
Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population.
The study investigated the effects of nuclear-targeted Akt (Akt/nuc) expression in the myocardium. Cardiac-specific expression of Akt/nuc in transgenic mice resulted in prolonged postnatal cell cycling, indicated by increased numbers of dividing cardiomyocytes. Akt/nuc expression also promoted expansion of the cardiac progenitor cell population. The researchers observed increased levels of pro-proliferative cytokines, such as tumor-necrosis superfamily 8, interleukin-17e, and hepatocyte growth factor, in the myocardial samples with nuclear-targeted Akt expression. These findings suggest that nuclear-targeted Akt in the myocardium enhances cell proliferation and expands the stem cell population, potentially through paracrine signaling by downstream factors
You can read the full article at https://www.ahajournals.org/doi/10.1161/01.RES.0000236754.21499.1c?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
- Hahn JY, et al. (2008) Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction. J Am Coll Cardiol 51(9):933–943.
Pre-treatment of mesenchymal stem cells with a combination of growth factors enhances gap junction formation, cytoprotective effect on cardiomyocytes, and therapeutic efficacy for myocardial infarction
The study aimed to investigate the effects of pre-treating mesenchymal stem cells (MSCs) with growth factors (GFs) on their cardiomyogenic differentiation, cytoprotective action on cardiomyocytes (CMCs), and therapeutic efficacy in myocardial infarction. The researchers treated MSCs with a combination of fibroblast growth factor-2, insulin-like growth factor-1, and bone morphogenetic protein-2. They found that GF-treated MSCs showed enhanced expression of cardiac-specific markers, improved survival, and increased induction of cardiac markers during coculture with CMCs. Additionally, GF-treated MSCs reduced apoptosis of neighboring CMCs in a hypoxic condition and enhanced the expression of phosphorylated Akt and phosphorylated c-AMP response element binding protein in CMCs, which was dependent on gap junctional communication. In a rat model of myocardial infarction, transplantation of GF-treated MSCs resulted in a smaller infarct size and better cardiac function compared to transplantation of untreated MSCs. GF treatment also enhanced gap junction formation of transplanted MSCs without causing arrhythmia. These findings suggest that priming MSCs with GFs before transplantation improves their cytoprotective effects on neighboring CMCs through gap junctions and enhances the therapeutic efficacy of MSC transplantation for myocardial repair.
You can read the full article at https://www.sciencedirect.com/science/article/pii/S0735109707038089?via%3Dihub
- Pasha Z, et al. (2008) Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium. Cardiovasc Res 77(1):134–142.
Preconditioning enhances cell survival and differentiation of stem cells during transplantation in infarcted myocardium
The study aimed to investigate whether preconditioning (PC) with stromal-derived factor 1 alpha (SDF-1) enhances the survival, proliferation, and engraftment of bone marrow-derived mesenchymal stem cells (MSCs) through SDF-1/CXCR4 signaling. In vitro experiments demonstrated that SDF-1 PC significantly increased cell viability and proliferation in MSCs. In vivo studies using a rat model of myocardial infarction showed that SDF-1 preconditioned MSCs exhibited robust cell proliferation, reduced infarct size and fibrosis, and improved cardiac function compared to non-preconditioned MSCs. The effects of SDF-1 PC were reversed by a CXCR4 antagonist, suggesting that SDF-1/CXCR4 signaling is involved in the beneficial effects of preconditioning. The findings suggest that chemokine preconditioning with SDF-1 is a promising approach to enhance stem cell survival and promote the regeneration of infarcted myocardium.
You can read the full article at https://academic.oup.com/cardiovascres/article/77/1/134/461464?login=false
- Gude N, et al. (2006) Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population. Circ Res 99(4):381–388.
Akt promotes increased cardiomyocyte cycling and expansion of the cardiac progenitor cell population
The study explores the effects of nuclear-targeted Akt (Akt/nuc) expression in the myocardium. Cardiac-specific expression of Akt/nuc in transgenic mice leads to increased cell cycling and proliferation in cardiomyocytes, as evidenced by higher numbers of Ki67+ cardiomyocytes during early postnatal development. Akt/nuc expression also promotes the expansion of cardiac progenitor cells, as shown by increased c-kit labeling in combination with myocyte-specific markers Nkx 2.5 or MEF 2C. The Akt/nuc-expressing myocardial samples show elevated levels of pro-proliferative cytokines, including tumor-necrosis superfamily 8, interleukin-17e, and hepatocyte growth factor. The study suggests that the enhanced cell proliferation and expansion of the stem cell population observed in the myocardium with Akt/nuc expression may be mediated by concurrent signaling through paracrine factors downstream of Akt/nuc expression.
You can read the full article at https://www.ahajournals.org/doi/10.1161/01.RES.0000236754.21499.1c?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed
- Kanashiro-Takeuchi RM, Schulman IH, Hare JM (2011) Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration. J Mol Cell Cardiol 51(4):619–625.
Pharmacologic and genetic strategies to enhance cell therapy for cardiac regeneration
Cell-based therapy is becoming a promising approach for cardiac regeneration after myocardial infarction (MI). As heart failure prevalence increases, the development of new interventions to aid cardiac recovery is crucial. Efforts to enhance the efficacy and safety of cell therapy are ongoing, including modifications to improve the reprogramming efficiency of inducible pluripotent stem cells (iPS), genetic engineering of adult stem cells, and administration of growth factors or small molecules to activate regenerative pathways in the injured heart. These interventions aim to promote stem cell homing, proliferation, differentiation, and survival. The article suggests that these therapeutic alternatives should be the focus of future clinical research in cardiac regenerative medicine.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3408226/
- Fischer KM, et al. (2009) Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase. Circulation 120(21):2077–2087.
Enhancement of myocardial regeneration through genetic engineering of cardiac progenitor cells expressing Pim-1 kinase.
This study aimed to improve the regenerative potential of adoptively transferred cardiac progenitor cells (CPCs) by genetically engineering them to overexpress Pim-1, a cardioprotective kinase that enhances cell survival and proliferation. The engineered CPCs showed increased proliferation and cardiogenic lineage commitment in vitro. When injected into infarcted mice, CPCs overexpressing Pim-1 demonstrated greater engraftment, persistence, and functional improvement compared to control CPCs up to 32 weeks after delivery. These engineered CPCs led to reduction in infarct size, increased c-kit(+) cells, and enhanced vasculature in the damaged region. The study suggests that genetic engineering of CPCs with Pim-1 kinase can significantly enhance myocardial repair and may overcome limitations of current stem cell-based therapeutic approaches.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2787902/
- Kiaris H, Schally AV, Kalofoutis A (2005) Extrapituitary effects of the growth hormone-releasing hormone. Vitam Horm 70:1–24.
Extrapituitary effects of the growth hormone-releasing hormone
Growth hormone-releasing hormone (GHRH) is a neuropeptide secreted by the hypothalamus to stimulate the release of growth hormone (GH) in the pituitary. However, recent evidence suggests that GHRH also exists in other tissues outside the hypothalamus and is involved in various cellular processes, such as cell proliferation and differentiation in nonpituitary cells. In some cases, abnormal production of GHRH has been linked to cancer development. The exact mechanisms of GHRH’s actions in peripheral tissues are not fully understood, but it is believed to involve both neuroendocrine pathways and local signaling mechanisms. The identification of extrapituitary GHRH receptors remains a challenge, hindering further understanding of its functions. This review provides an overview of GHRH’s role in extrapituitary tissues and discusses its potential applications in therapy and diagnosis.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0083672905700017?via%3Dihub
- Ciampani T, Fabbri A, Isidori A, Dufau ML (1992) Growth hormone-releasing hormone is produced by rat Leydig cell in culture and acts as a positive regulator of Leydig cell function. Endocrinology 131:2785–2792.
Growth hormone-releasing hormone is produced by rat Leydig cell in culture and acts as a positive regulator of Leydig cell function
Growth hormone-releasing hormone (GHRH), primarily found in hypothalamic neurons, has also been identified in various extraneural tissues, including the gastrointestinal tract, placenta, ovary, and testis. In the testis, GHRH mRNA is regulated during development, and GHRH immunoreactivity is observed in interstitial cells and tubules, suggesting a role for the peptide within the testicular environment. Leydig cells, a type of testicular cell, produce GHRH in significant amounts when cultured and are acutely stimulated to release the peptide by hCG. GHRH and vasoactive intestinal peptide (VIP) share a common receptor in Leydig cell membranes, which activates the adenylate cyclase system, leading to increased cAMP production. GHRH acts as a potentiator of acute gonadotropin (LH) stimulation of testosterone production and cAMP generation in Leydig cells. However, GHRH does not affect the number or affinity of binding sites for hCG (LH receptor), suggesting that its sensitizing effect on LH action occurs beyond the level of gonadotropin binding, possibly through facilitating LH receptor coupling functions.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/1332849/
- Khorram O, Garthwaite M, Golos T (2001) The influence of aging and sex hormones on expression of growth hormone-releasing hormone in the human immune system. J Clin Endocrinol Metab 86:3157–3161.
The influence of aging and sex hormones on expression of growth hormone-releasing hormone in the human immune system
Growth hormone-releasing hormone (GHRH) is a neuropeptide that has been found in the immune system, but its function there is not fully understood. This study aimed to investigate changes in immune GHRH expression in certain pathological conditions, such as immune cell tumors, and explore the influence of gender, aging, and sex hormones on GHRH expression in immune cells. GHRH protein was found in a small percentage of peripheral blood mononuclear cells (PBMCs), with T cells showing higher expression compared to B cells and monocytes. GHRH messenger RNA (mRNA) levels were low in PBMCs, with higher expression in monocytes. Certain immune cell-derived tumors exhibited greater GHRH mRNA expression compared to PBMCs. Aging was associated with a decrease in the percentage of lymphocytes expressing GHRH protein, especially in B cells and monocytes. Postmenopausal women had lower GHRH mRNA expression in PBMCs compared to premenopausal women. Interestingly, older men had fewer lymphocytes expressing GHRH protein, but they secreted more GHRH in vitro compared to postmenopausal women without hormone replacement therapy (HRT), similar to women receiving HRT. These findings suggest that immune GHRH is dynamically regulated and may play a role in immunosenescence, as well as in the pathogenesis of certain immune cell tumors. Overall, GHRH appears to function as a local immune modulator in the immune system.
You can read the full article at https://academic.oup.com/jcem/article/86/7/3157/2848799?login=false
- Florea V, Majid SS, Kanashiro-takeuchi RM, et al. Agonists of growth hormone-releasing hormone stimulate self-renewal of cardiac stem cells and promote their survival. Proc Natl Acad Sci USA. 2014;111(48):17260-5.
Agonists of growth hormone-releasing hormone stimulate self-renewal of cardiac stem cells and promote their survival.
The study investigated the presence of growth hormone-releasing hormone receptor (GHRH-R) in cardiac stem cells (CSCs) and the effects of GHRH-R agonists on their proliferation and survival. GHRH-R was found to be expressed in CSCs from mouse, rat, and pig. Treatment with GHRH-R agonists (JI-38, MR-356, and MR-409) increased the rate of cell division in porcine CSCs. Moreover, GHRH-R agonists protected porcine CSCs from oxidative stress-induced cell death. The agonists activated the ERK and AKT pathways, and inhibitors of these pathways reversed the effects of GHRH-R agonists on CSC proliferation. These findings suggest that GHRH-R agonists directly stimulate CSC proliferation and survival, supporting their potential therapeutic use to enhance myocardial repair or preconditioning of stem cells for transplantation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4260571/
- Kanashiro-Takeuchi RM, et al. (2010) Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction. Proc Natl Acad Sci USA 107(6):2604–2609.
Cardioprotective effects of growth hormone-releasing hormone agonist after myocardial infarction
The study aimed to investigate whether growth hormone-releasing hormone (GHRH) can directly activate cellular reparative mechanisms in the injured heart, independently of the growth hormone (GH)/insulin-like growth factor 1 (IGF-1) axis. Rats with experimental myocardial infarction (MI) were treated with either placebo, rat recombinant GH, or JI-38 (a potent GHRH agonist) for four weeks. JI-38 did not elevate GH or IGF-1 levels but significantly attenuated cardiac functional decline and remodeling after injury. In contrast, GH administration increased body weight, heart weight, and circulating GH/IGF-1, but it did not prevent the decline in cardiac structure and function. Both JI-38 and GH increased cardiac precursor cell proliferation, but only JI-38 increased antiapoptotic gene expression. The presence of GHRH receptors on myocytes supported direct activation of cardiac signal transduction. The study suggests that GHRH agonists can activate cardiac repair after MI through a potential signaling pathway based on GHRH in the heart, indicating potential therapeutic implications.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2823907/
- Kanashiro-Takeuchi RM, et al. (2012) Activation of growth hormone releasing hormone (GHRH) receptor stimulates cardiac reverse remodeling after myocardial infarction (MI). Proc Natl Acad Sci USA 109(2):559–563.
Activation of growth hormone releasing hormone (GHRH) receptor stimulates cardiac reverse remodeling after myocardial infarction (MI)
The study investigated the effects of a growth hormone-releasing hormone agonist (GHRH-A; JI-38) on ventricular remodeling and functional recovery in chronic myocardial infarction (MI). Both cardiac myocytes and cardiac stem cells (CSCs) express the GHRH receptor (GHRHR), and activation of GHRHR improves injury responses after MI. The GHRH-A (JI-38) was found to reverse ventricular remodeling and enhance functional recovery in chronic MI. This response was entirely mediated by GHRHR activation, as shown by the use of a highly selective GHRH antagonist (MIA-602). GHRH-A significantly improved cardiac function, reduced MI size, and increased myocyte and nonmyocyte mitosis. These effects were receptor-mediated and occurred without changes in circulating growth hormone and insulin-like growth factor I levels. GHRH-A also stimulated CSC proliferation, which was offset by MIA-602. The findings suggest that the GHRH signaling pathway within the heart plays an important role, and therapy with GHRH-A initiated after MI can substantially improve cardiac performance and promote a regenerative process, making it a potential therapeutic approach to reverse remodeling after MI.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3258609/
- Cai R, et al. (2014) Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities. Peptides 52:104–112.
Synthesis of new potent agonistic analogs of growth hormone-releasing hormone (GHRH) and evaluation of their endocrine and cardiac activities
The study synthesized and evaluated new analogs of Growth Hormone-Releasing Hormone (GHRH) to produce more potent compounds. Three series of GHRH analogs were tested, including “agmatine analogs,” analogs with modifications at the N-terminus, and analogs with modifications at the C-terminus. The modified analogs showed improved potency in stimulating growth hormone release in vivo and binding in vitro. Some analogs exhibited dramatically increased endocrine activities, making them the most potent GHRH agonists developed so far. Several analogs, including MR-409 and MR-356, showed higher potency than the original JI-38 in activating myocardial repair in rats with induced myocardial infarction. These more potent GHRH agonists have the potential for various medical applications, including in cardiology, diabetes, and wound healing.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4745889/
- Ma Y, Zhang L, Launikonis BS, Chen C. Growth hormone secretagogues preserve the electrophysiological properties of mouse cardiomyocytes isolated from in vitro ischemia/reperfusion heart. Endocrinology. 2012;153(11):5480-90.
Growth hormone secretagogues preserve the electrophysiological properties of mouse cardiomyocytes isolated from in vitro ischemia/reperfusion heart
This study investigated the effects of growth hormone secretagogues (GHS) ghrelin and hexarelin on cardiomyocytes during ischemia/reperfusion (I/R) in the context of ischemic heart diseases. The study used isolated hearts from mice subjected to I/R and treated with ghrelin or hexarelin before or after ischemia. GHS treatments preserved the electrophysiological properties of cardiomyocytes, including action potential amplitude and duration, which were otherwise affected by I/R. GHS treatments also prevented the decrease in L-type calcium current (I(CaL)) and sodium current (I(Na)) after I/R, helping to maintain action potential amplitude. Additionally, GHS treatments normalized transient outward potassium current (I(to)), contributing to the preservation of action potential duration. The study also revealed that GHS treatments inhibited cardiomyocyte apoptosis and promoted cell survival by modifying MAPK pathways through activating GHS receptor type 1a. These findings suggest that GHS can protect cardiomyocytes from the detrimental effects of I/R and may have therapeutic potential in ischemic heart diseases.
You can read the full article at https://academic.oup.com/endo/article/153/11/5480/2424502?login=false
- Frascarelli S, Ghelardoni S, Ronca-Testoni S, Zucchi R 2003 Effect of ghrelin and synthetic growth hormone secretagogues in normal and ischemic rat heart. Basic Res Cardiol 98:401–405.
Effect of ghrelin and synthetic growth hormone secretagogues in normal and ischemic rat heart
This study investigated the effects of growth hormone secretagogues (ghrelin, hexarelin, and MK-0677) on contractile performance and susceptibility to ischemic injury in isolated working rat hearts. Under normal conditions, none of the secretagogues had significant hemodynamic effects. However, during ischemia followed by reperfusion, both ghrelin and hexarelin demonstrated cardioprotective effects, reducing infarct size. In contrast, MK-0677 was ineffective in providing protection. The cardioprotective effect of hexarelin was partly dependent on protein kinase C activation. These findings suggest that ghrelin and hexarelin have specific cardioprotective effects independent of growth hormone secretion, potentially involving protein kinase C activation.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/14556085/
- Chang L, Ren Y, Liu X, Li WG, Yang J, Geng B, Weintraub NL, Tang C 2004 Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart. J Cardiovasc Pharmacol 43:165–170.
Protective effects of ghrelin on ischemia/reperfusion injury in the isolated rat heart
This study investigated the cardioprotective effects of ghrelin, an endogenous ligand of the growth hormone secretagogue receptor, using an isolated rat heart model of ischemia/reperfusion (I/R) injury. When administered during reperfusion, ghrelin improved coronary flow, heart rate, left ventricular systolic pressure, left ventricular end-diastolic pressure, and rates of left ventricular contraction and relaxation. Ghrelin also reduced myocardial release of lactate dehydrogenase and myoglobin, indicating protection against cardiomyocyte injury, and attenuated the depletion of myocardial ATP resulting from I/R. Ghrelin was found to bind to sarcolemmal membranes, and its binding capacity increased after ischemia and reperfusion. The cardioprotective effects of ghrelin were independent of growth hormone release and likely involved binding to cardiovascular receptors, a process that was upregulated during I/R.
You can read the full article at https://journals.lww.com/cardiovascularpharm/Fulltext/2004/02000/Protective_Effects_of_Ghrelin_on.1.aspx
- Zhang GG, Teng X, Liu Y, Cai Y, Zhou YB, Duan XH, Song JQ, Shi Y, Tang CS, Yin XH, Qi YF 2009 Inhibition of endoplasm reticulum stress by ghrelin protects against ischemia/reperfusion injury in rat heart. Peptides 30:1109–1116.
Inhibition of endoplasm reticulum stress by ghrelin protects against ischemia/reperfusion injury in rat heart
This study investigated the cardioprotective effects of ghrelin and its potential mechanism of action in inhibiting myocardial endoplasmic reticulum stress (ERS). Using an isolated rat heart model of ischemia/reperfusion (I/R) injury, the researchers found that ghrelin administration significantly improved cardiac function, reduced myocardial injury, and decreased the number of apoptotic cardiomyocytes. They observed that I/R injury led to increased expression of ERS markers in the heart, but preadministration of ghrelin attenuated this response. In both in vivo and in vitro experiments, ghrelin inhibited the expression of glucose-regulated protein78 (GRP78), C/EBP homologous protein (CHOP), and caspase-12, which are involved in the ERS pathway. These findings suggest that ghrelin’s cardioprotective effect may be mediated, at least in part, by inhibiting myocardial endoplasmic reticulum stress.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0196978109000965?via%3Dihub
- Torsello A, Bresciani E, Rossoni G, Avallone R, Tulipano G, Cocchi D, Bulgarelli I, Deghenghi R, Berti F, Locatelli V 2003 Ghrelin plays a minor role in the physiological control of cardiac function in the rat. Endocrinology 144:1787–1792.
Ghrelin plays a minor role in the physiological control of cardiac function in the rat
In this study, the researchers investigated the cardioprotective effects of ghrelin, an endogenous ligand of the GH secretagogue receptor (GHS-R), compared to hexarelin, a synthetic ligand of the same receptor. They used hypophysectomized rats treated with either ghrelin or hexarelin and subjected their hearts to ischemia and reperfusion in vitro. The results showed that hexarelin was much more effective than ghrelin in preventing increases in left ventricular end-diastolic pressure, coronary perfusion pressure, and release of creatine kinase in the heart perfusate. In another experiment, normal rats were passively immunized against ghrelin, but the ischemia-reperfusion damage in their hearts was not significantly increased compared to control rats. The study suggests that ghrelin plays a minor role in controlling heart function, while hexarelin’s effects are mediated in part by GHS-R and largely by interactions with the CD36 receptor.
You can read the full article at https://academic.oup.com/endo/article/144/5/1787/2502093?login=false
- Cittadini A, Grossman JD, Strömer H, Katz SE, Morgan JP, Douglas PS. Importance of an intact growth hormone/insulin-like growth factor 1 axis for normal post-infarction healing: studies in dwarf rats. Endocrinology. 2001;142(1):332-8.
Importance of an intact growth hormone/insulin-like growth factor 1 axis for normal post-infarction healing: studies in dwarf rats
In simple terms, this study looked at the effects of growth hormone (GH) on the healing of the heart after a heart attack. They used rats with a GH deficiency and compared them to normal rats. Both groups of rats had a heart attack induced, and they measured how their hearts healed over time. The rats with GH deficiency had more severe damage to their hearts and worse heart function after the heart attack compared to the normal rats. This suggests that GH is important for the normal healing of the heart after a heart attack.
You can read the full article at https://academic.oup.com/endo/article/142/1/332/2988995?login=false
- Waters MJ, Blackmore DG (2011) Growth hormone (GH), brain development and neural stem cells. Pediatr Endocrinol Rev 9(2):549–553.
Growth hormone (GH), brain development and neural stem cells
In summary, there is evidence suggesting that growth hormone (GH) plays a role in the development and function of the brain. Studies have shown altered brain structure in mice lacking GH receptors, and impaired cognition in GH-deficient rodents and some human patients. GH affects processes like neurogenesis, myelin synthesis, and dendritic branching. There are neural stem cells activated by GH, which give rise to neurons, and this activation can also be influenced by voluntary exercise. Additionally, GH is locally produced in the hippocampus during memory tasks, and GH replacement has been found to improve memory and cognition in both animals and humans. These findings indicate that GH replacement in GH-deficient conditions may have significant clinical benefits.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/22397139/
- Khorram, M. Garthwaite, T. Golos. The influence of aging and sex hormones on expression of growth hormone-releasing hormone in the human immune system. J. Clin. Endocrinol. Metab., 86 (2001), pp. 3157-3161.
The influence of aging and sex hormones on expression of growth hormone-releasing hormone in the human immune system
In summary, GHRH (growth hormone-releasing hormone) is a neuropeptide that has been found in the immune system. This study aimed to explore the expression of GHRH in immune cells in various conditions. GHRH protein and mRNA were detected in a small percentage of peripheral blood mononuclear cells (PBMC), with higher expression in monocytes and certain immune cell-derived tumors. The study also observed changes in GHRH expression related to age, gender, and hormonal status. Older individuals showed a decrease in GHRH-expressing lymphocytes, while postmenopausal women had lower GHRH mRNA expression in PBMC compared to premenopausal women. However, hormone replacement therapy (HRT) seemed to affect GHRH secretion. The study suggests that GHRH may have a role as a local immune modulator and could be involved in the development of certain immune cell tumors and immunosenescence
You can read the full article at https://academic.oup.com/jcem/article/86/7/3157/2848799?login=false
- Dialynas, H. Brown-Borg, A. Bartke. Immune function in transgenic mice overexpressing growth hormone (GH) releasing hormone, GH or GH antagonist. Proc. Soc. Exp. Biol. Med., 221 (1999), pp. 178-183.
Immune function in transgenic mice overexpressing growth hormone (GH) releasing hormone, GH or GH antagonist
In this study, the effects of lifelong exposure to high levels of growth hormone (GH) or GH resistance on selected parameters of immune function were investigated in transgenic mice. Mice were genetically modified to overexpress GH-releasing hormone (GHRH), bovine GH, or an antagonistic bovine GH analog. The mice with high levels of GH showed increased thymus and spleen weight and enhanced immune responses to certain mitogens. Mice with elevated homologous (mouse) GH also exhibited similar immune stimulatory effects. However, mice with an antagonistic GH analog, which interfered with GH actions, showed reduced spleen weight but did not have significant effects on immune responses to mitogens. Overall, the study suggests that altered GH levels can influence immune function in transgenic mice
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10404033/
- Dorshkind, N.D. Horseman. Anterior pituitary hormones, stress, and immune system homeostasis. Bioessays, 23 (2001), pp. 288-294.
Anterior pituitary hormones, stress, and immune system homeostasis
The literature on prolactin (PRL), growth hormone (GH), insulin-like growth factor-I (IGF-I), and thyroid hormones and their role in immunoregulation is extensive and controversial. Studies on mice deficient in these hormones or their receptors suggest that they may not be essential for lymphocyte development or antigen responsiveness. Instead, their primary function may be to counteract the negative effects of immunoregulatory factors like glucocorticoids, which are produced during major stressors. These hormones may protect the immune system and maintain homeostasis, reducing susceptibility to stress-induced diseases. This immune-enhancing potential could be beneficial in clinical situations where the immune system is compromised due to illness or treatments.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11223886/
- Burgess, et al. The immune–endocrine loop during aging: role of growth hormone and insulin-like growth factor-I. Neuroimmunomodulation, 6 (1999), pp. 56-68.
The immune–endocrine loop during aging: role of growth hormone and insulin-like growth factor-I
The process of thymus involution during aging is a key question in immunology. Aging is associated with reduced levels of growth hormone (somatotropin) and IGF-I, suggesting a connection between the neuroendocrine and immune systems. Studies have shown that treatment with growth hormone or IGF-I can reverse thymus involution by restoring immature thymocytes and preventing thymulin synthesis decline in old or GH-deficient individuals. Similarly, the age-related decline in various components of the immune system, such as T and B cells, macrophages, and neutrophils, can be restored by IGF-I.
Recent research has demonstrated that IGF-I prevents apoptosis in promyeloid cells, allowing them to differentiate into neutrophils. IL-4 also promotes survival of promyeloid cells and activates a specific protein kinase pathway called phosphatidylinositol 3′-kinase (PI 3-kinase). Despite their different receptor structures, both IGF-I and IL-4 receptors share some components of the PI 3-kinase pathway, leading to the maintenance of high levels of the anti-apoptotic protein Bcl-2 and inhibiting cell death. This shared activation pathway suggests the possibility of cross talk between IL-4 and IGF-I in regulating various hematopoietic events, including differentiation, proliferation, and cell survival. Understanding these mechanisms may offer insights to improve the health of aging individuals.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/9876236/
- C. Gelato. Aging and immune function: a possible role for growth hormone. Horm. Res., 45 (1996), pp. 46-49.
Aging and immune function: a possible role for growth hormone
Elderly individuals have higher rates of certain diseases like cancer, tuberculosis, herpes zoster, and pneumonia compared to young adults. Two changes associated with aging are decreased production of growth hormone (GH) and insulin-like growth factor-1 (IGF-1), as well as decreased immune function. Research has shown that about 40% of adults aged 60 and older are GH deficient based on GH secretory dynamics or IGF-1 levels. Additionally, immune function declines with age, leading to reduced cell-mediated and humoral immune responses. Some of these immune deficits can be reversed in humans and primates through treatment with GH and/or IGF-1. This paper reviews the available data on these topics
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8742118/
- L. Marshall, B. Perras, H.L. Fehm, J. Born. Changes in immune cell counts and interleukin (IL)-1beta production in humans after a somnogenically active growth hormone-releasing hormone (GHRH) administration. Brain Behav. Immun., 15 (2001), pp. 227-234.
Changes in immune cell counts and interleukin (IL)-1beta production in humans after a somnogenically active growth hormone-releasing hormone (GHRH) administration
The study investigated the effects of growth hormone-releasing hormone (GHRH) administration on sleep and immune parameters in humans. When a single dose of GHRH was given, it enhanced slow-wave sleep (SWS) and reduced the numbers of circulating suppressor T cells (CD3+/CD8+), with a similar trend observed for B cells (CD19+) and suppressed interleukin-1beta (IL-1beta) production. However, when the same total dose of GHRH was administered in smaller repetitive doses, there were no significant changes in sleep or immune parameters. The findings suggest that GHRH can modulate immune functions through brain mechanisms that are also involved in the regulation of sleep.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11566047/.
- Hattori N. Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system. Growth Horm IGF Res. 2009;19(3):187-97.
Expression, regulation and biological actions of growth hormone (GH) and ghrelin in the immune system.
The immune and neuroendocrine systems have bidirectional communications. Growth hormone (GH) and ghrelin, an orexigenic hormone, are expressed in immune cells and have receptors in these cells. Ghrelin stimulates GH expression in immune cells similar to the anterior pituitary gland. Immune cells secrete GH in response to cytokines and mitogens. GH and ghrelin have various biological actions in the immune system, including enhancing T and B cell development, modulating cytokine production, promoting antibody production, and supporting neutrophil and monocyte functions. Ghrelin also has anti-inflammatory effects and can enhance thymopoiesis. These hormones play essential roles in immune system regulation and may hold potential for treating immunological disorders such as AIDS and inflammation-related conditions associated with aging and obesity.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1096637408001457?via%3Dihub
- Masternak MM, Bartke A. Growth hormone, inflammation and aging. Pathobiology of Aging & Age Related Diseases. 2012;2:10.3402/pba.v2i0.17293. doi:10.3402/pba.v2i0.17293.
Growth hormone, inflammation and aging. Pathobiology of Aging & Age Related Diseases.
Mutant animals with extended longevity provide valuable insights into aging mechanisms. The growth hormone and insulin-like growth factor-1 (IGF-1) pathway plays a significant role in regulating aging and lifespan. Certain GH-deficient and GH-resistant mice, characterized by genetic mutations, live significantly longer than normal animals. During normal aging, rodents and humans experience increased insulin resistance, disrupted metabolism, and immune system decline, leading to chronic inflammation and tissue damage. However, long-living mutants and calorie-restricted animals show reduced pro-inflammatory activity and increased levels of anti-inflammatory adipokines like adiponectin. These long-lived animals also exhibit improved insulin signaling and carbohydrate balance, likely due to alterations in adipose tissue secretions that promote healthy metabolism and reduced inflammation, contributing to their extended longevity. This mechanism may also be relevant to human longevity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3417471/
- Meazza C, Pagani S, Travaglino P, Bozzola M. Effect of growth hormone (GH) on the immune system. Pediatr Endocrinol Rev. 2004;1 Suppl 3:490-5.
Effect of growth hormone (GH) on the immune system
The relationship between the neuroendocrine system and immune functions is bidirectional. Lymphoid organs like the thymus, spleen, and peripheral blood produce growth hormone (GH), and lymphocytes express GH receptors. In vitro and animal studies show that GH plays a crucial role in immunoregulation. It stimulates T and B cells proliferation, enhances immunoglobulin synthesis, promotes myeloid progenitor cell maturation, and modulates cytokine response. However, in humans with GH deficiency (GHD), immune function abnormalities are relatively minor compared to GHD animals. This suggests that locally produced GH in the immune system may compensate for the lack of endocrine GH. The review summarizes the main actions of GH on the immune system in vitro, animal models, and humans.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16444180/
- Rapaport R, Oleske J, Ahdieh H, Solomon S, Delfaus C, Denny T. Suppression of immune function in growth hormone-deficient children during treatment with human growth hormone. J Pediatr. 1986;109(3):434-9.
Suppression of immune function in growth hormone-deficient children during treatment with human growth hormone.
The study examined the impact of human growth hormone (HGH) treatment on immune functions in children with growth hormone deficiency (GHD). Before treatment, the immune functions of the children were normal. However, during 12 to 16 months of HGH treatment, certain immune parameters were affected. Percent B cells decreased to subnormal levels in most patients, T helper/suppressor ratios decreased in all patients, and mitogen responses decreased below normal in all patients. These changes were mostly transient and did not lead to increased infections during the observation period. The study suggests caution in the indiscriminate use of HGH, as its effects on immune function require further investigation.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/3489089/
- Koo GC, Huang C, Camacho R, et al. Immune enhancing effect of a growth hormone secretagogue. J Immunol. 2001;166(6):4195-201.
Immune enhancing effect of a growth hormone secretagogue
The study investigated the effects of a nonpeptidyl small molecule compound called Growth Hormone Secretagogue (GHS) on the immune system in mice of different age groups. In young mice, GHS treatment led to an increase in peripheral blood lymphocytes (PBLs), but T and B cell-proliferative responses were not consistently enhanced. However, in old mice treated with GHS for 3 weeks, there was a significant increase in thymic cellularity and differentiation. These treated old mice showed increased resistance to tumor initiation and metastases when inoculated with lymphoma cells. GHS also enhanced the generation of cytotoxic T lymphocytes (CTL) in response to the lymphoma cells. Additionally, GHS improved thymic engraftment in bone marrow transplant of SCID mice. The findings suggest that GHS has a considerable immune-enhancing effect, particularly in older mice, and may hold promise as a potential therapy for aging, AIDS, and transplant recipients with compromised immune function.
You can read the full article at https://journals.aai.org/jimmunol/article/166/6/4195/70382/Immune-Enhancing-Effect-of-a-Growth-Hormone
- Morrhaye G, Kermani H, Legros J-J, et al. Impact of Growth Hormone (GH) Deficiency and GH Replacement upon Thymus Function in Adult Patients. Unutmaz D, ed. PLoS ONE. 2009;4(5):e5668. doi:10.1371/journal.pone.0005668.
Impact of Growth Hormone (GH) Deficiency and GH Replacement upon Thymus Function in Adult Patients
The study investigated thymic function in adults with adult growth hormone deficiency (AGHD) and the impact of growth hormone (GH) treatment on thymopoiesis. Twenty-two AGHD patients were studied. Thymic function was evaluated based on plasma IGF-1 concentrations and signal-joint T-cell receptor excision circle (sjTREC) frequency. One month after GH treatment withdrawal, both IGF-1 levels and sjTREC frequency decreased significantly, indicating reduced thymic T cell output and intrathymic T cell proliferation. Reintroduction of GH treatment restored IGF-1 levels and sjTREC frequency to the pre-withdrawal levels, suggesting a recovery of thymic function. However, the sj/beta TREC ratio, an indicator of thymic T cell proliferation, did not fully return to the pre-withdrawal level, indicating that some thymic functions may not be completely restored. The study highlights the importance of the somatotrope GH/IGF-1 axis for maintaining normal thymic function in adult humans.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2682582/
- Gelato MC. Aging and immune function: a possible role for growth hormone. Horm Res. 1996;45(1-2):46-9.
Gelato MC. Aging and immune function: a possible role for growth hormone
Elderly individuals have higher rates of certain diseases, such as cancer, tuberculosis, herpes zoster, and pneumonia, which may be linked to age-related changes in growth hormone (GH) and insulin-like growth factor-1 (IGF-1) production, as well as decreased immune function. Studies have shown that around 40% of adults aged 60 and older are GH deficient, and their immune function also declines, affecting cell-mediated and humoral immune responses. Some of these immune deficits have been reversed in humans and primates through treatment with GH and/or IGF-1. The paper reviews these findings and their potential implications for improving the health of elderly individuals.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8742118/
- Available at https://www.nature.com/articles/pr1984567.
Effect Of Human Growth Hormone (Hgh) On The Immune System (Is) Of Gh Deficient Children
In a study involving 8 growth hormone (GH) deficient children aged 1-17 years, the effects of GH treatment on various immune parameters were assessed. Serum immunoglobulins, B cells, T cells (suppressor and helper), and lymphocyte and polymorphonuclear leukocyte (PMN) function were measured before and at 1-3 month intervals for 1 year of GH treatment. GH treatment resulted in increased growth rates and a significant decrease in B cell percentage in 7 out of 8 patients. However, B cell values returned to pre-treatment levels or higher by 12 months. Lymphoblast responses to phytohemagglutinin (PHA) decreased in all patients, with complete suppression observed in 4 tested at 12 months. A temporary decrease in the T helper (TH) to T suppressor (TS) cell ratio was seen in 4 patients. Other immune parameters remained unchanged, and there was no increase in infections during GH treatment. These findings indicate a significant impact of GH treatment on the immune system, affecting B cell percentage, PHA response, and TH/TS ratio.
You can read the abstract of this article at https://www.nature.com/articles/pr1984567
- Available at https://clinicaltrials.gov/ct2/show/NCT00071240.
Growth Hormone to Increase Immune Function in People With HIV
This study aims to investigate thymic function in HIV-infected adults and its potential induction by growth hormone. The participants, totaling 24 volunteers, will undergo a 2-year study with 12 months of human growth hormone treatment. They will be randomly assigned to two study arms: Arm 1 receiving growth hormone for the first year and Arm 2 serving as an observational control arm. Participants will have up to 24 scheduled study visits, including physical exams, blood tests, and various scans. The goal is to assess whether true thymic function can be induced, whether growth hormone plays a role in this induction, and if thymic function contributes to sustaining the T cell compartment in the context of peripheral T cell depletion in HIV disease.
You can read the full article at https://classic.clinicaltrials.gov/ct2/show/NCT00071240
- Alzaid A, Kim JH, Devlin RH, Martin SAM, Macqueen DJ. Growth hormone transgenesis in coho salmon disrupts muscle immune function impacting cross-talk with growth systems. J Exp Biol. 2018;221(Pt 13).
Growth hormone transgenesis in coho salmon disrupts muscle immune function impacting cross-talk with growth systems.
The study investigated the regulation of immune responses in salmonid fish during different growth rates. It found that immune function was suppressed in fish with enhanced growth hormone (GH) and rapid growth, with no detectable antiviral response and evidence of a constitutive inflammatory state. The GH and insulin-like growth factor (IGF) system, which plays a role in growth regulation, showed significant alterations in expression patterns due to GH transgenesis and fast growth. These findings suggest that growth suppression during infection may allow for resource allocation towards effective immune function in fish.
You can read the full article at https://journals.biologists.com/jeb/article/221/13/jeb173146/257/Growth-hormone-transgenesis-in-coho-salmon
- Carroll PV, Christ ER, Bengtsson BA, et al. Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee. J Clin Endocrinol Metab. 1998;83(2):382-95.
Growth hormone deficiency in adulthood and the effects of growth hormone replacement: a review. Growth Hormone Research Society Scientific Committee
GH deficiency in adults is most commonly caused by pituitary or peripituitary tumors and their treatment. The incidence of adult-onset GH deficiency is estimated to be around 10 people per million annually. Childhood-onset GH deficiency is usually idiopathic and may not be associated with other pituitary hormone deficiencies.
Research into the role of GH in adults was limited due to the scarce availability of pituitary-derived GH. However, with the development of recombinant GH, studies on the effects of GH replacement in GH-deficient adults have increased. Adults with long-standing GH deficiency exhibit a specific clinical syndrome characterized by various symptoms, signs, and investigative findings. GH replacement has been shown to substantially improve physical and psychological health in adults with GH deficiency. Numerous studies, including randomized placebo-controlled trials, have consistently demonstrated the benefits of GH replacement in these individuals. This review summarizes the important features of GH deficiency in adults and the effects of GH replacement therapy up until 1997.You can read the full article at https://academic.oup.com/jcem/article/83/2/382/2865179?login=false
- Available at http://www.asja.eg.net/article.asp?issn=1687-7934;year=2016;volume=9;issue=2;spage=194;epage=200;aulast=Salem.
Effect of recombinant growth hormone on immune response in pediatric burn patients
The management of severely burned pediatric patients poses significant challenges, particularly in controlling the hypercatabolic state that influences patient survival. Immunocompromise and delayed wound healing often lead to severe sepsis, a primary cause of death in these cases. Despite its use to enhance burn patient recovery, the efficacy and safety of recombinant human growth hormone (rGH) remains debated. While the theoretical and serological roles of growth hormone in immunomodulation have been suggested, clinical validation is lacking. This study aims to assess the impact of rGH on immune status improvement and survival enhancement in burn patients.
Forty pediatric patients were enrolled and randomly assigned to either Group A (receiving rGH) or Group B (not receiving rGH). The two groups were compared based on overall mortality rate, hospital and ICU stay durations, serum transferrin levels, C-reactive protein levels, and blood culture results.
Group A exhibited a slightly lower overall mortality rate (20%) compared to Group B (25%). Hospital stay durations showed no significant difference between the groups. Notably, Group A experienced a significant increase in serum transferrin levels, particularly by day 14, and a significant decrease in C-reactive protein levels. Moreover, Group A demonstrated greater protection against bacteremia.
The administration of rGH in pediatric burn patients yields multiple benefits, including reduced overall mortality, enhanced immune status, improved wound and donor site healing, and optimization of hospital stay durationYou can read the full article at http://www.asja.eg.net/article.asp?issn=1687-7934;year=2016;volume=9;issue=2;spage=194;epage=200;aulast=Salem
- Villares R, Kakabadse D, Juarranz Y, Gomariz RP, Martínez-a C, Mellado M. Growth hormone prevents the development of autoimmune diabetes. Proc Natl Acad Sci USA. 2013;110(48):E4619-27.
Growth hormone prevents the development of autoimmune diabetes
Research indicates a connection between the neuroendocrine and immune systems, and growth hormone (GH) has been shown to have various effects on immune functions in mice, such as stimulating T and B cell proliferation and immunoglobulin synthesis. In a murine model of type 1 diabetes, which is a T-cell-mediated autoimmune disease, sustained GH expression reduced disease symptoms and prevented the progression to overt diabetes. The effects of GH involved altering the cytokine environment, promoting anti-inflammatory macrophages, maintaining suppressor T-cell activity, and limiting Th17 cell plasticity. Additionally, GH protected insulin-producing β-cells by reducing apoptosis and increasing their proliferation rate. These findings suggest that GH plays a role in immune response regulation and could potentially be a therapeutic target for treating type 1 diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3845149/
- Mazzoccoli G, Vendemiale G, De Cata A, Carughi S, Tarquini R. Altered time structure of neuro-endocrine-immune system function in lung cancer patients. BMC Cancer. 2010;10:314. doi:10.1186/1471-2407-10-314.
Altered time structure of neuro-endocrine-immune system function in lung cancer patients
This study investigated differences in hormone secretion and lymphocyte subpopulations between healthy individuals and those with lung cancer. The neuro-immune-endocrine system, including the hypothalamus, pituitary, thyroid, adrenal, pineal gland, and immune system, was evaluated. The healthy group showed clear circadian rhythms in hormone levels and lymphocyte subsets, with different hormones and lymphocytes peaking at specific times. However, in lung cancer patients, several hormones and lymphocyte subsets lost their circadian rhythmicity, indicating an altered integrated function of the neuro-immune-endocrine system. Some factors, such as cortisol, TRH, GH, IL-2, and CD16, had increased average levels in cancer patients, while others like TSH, IGF-1, CD8, and deltaTcS1 had decreased average levels. These findings suggest disruptions in the neuro-immune-endocrine system in individuals with neoplastic disease.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2910689/
- Smith TJ. Insulin-Like Growth Factor-I Regulation of Immune Function: A Potential Therapeutic Target in Autoimmune Diseases? Pharmacological Reviews. 2010;62(2):199-236. doi:10.1124/pr.109.002469.
Insulin-Like Growth Factor-I Regulation of Immune Function: A Potential Therapeutic Target in Autoimmune Diseases?
This review discusses the relationship between the immune system and insulin-like growth factors (IGF-I and IGF-II) and their receptors (IGF-IR). The IGF/IGF-IR pathway plays diverse roles in tissue development and function, regulating cell cycle progression, apoptosis, and protein translation. Recently, it has been recognized that IGF-I/IGF-IR signaling also regulates immune function, potentially impacting the quality and amplitude of immune responses. There are indications that IGF-I and IGF-IR may be involved in the pathogenesis of autoimmune diseases. While IGF-I appears to protect against certain types of diabetes, IGF-IR overexpression has been linked to Graves’ disease. Targeting IGF-IR with drugs has been explored for cancer treatment, and the broader role of IGF-IR in immune responses may offer therapeutic opportunities in difficult-to-treat autoimmune diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2879913/
- Ge R-T, Mo L-H, Wu R, et al. Insulin-like growth factor-1 endues monocytes with immune suppressive ability to inhibit inflammation in the intestine. Scientific Reports. 2015;5:7735. doi:10.1038/srep07735.
Insulin-like growth factor-1 endues monocytes with immune suppressive ability to inhibit inflammation in the intestine.
This study investigates the mechanism by which insulin-like growth factor-1 (IGF1) modulates monocyte properties to inhibit immune inflammation in the intestine, particularly in the context of inflammatory bowel disease. The study found that intestinal epithelial cells produce IGF1, which can be upregulated when exposed to CpG-ODN. When monocytes were cultured with CpG-ODN-primed intestinal epithelial cells or exposed to IGF1 directly, they expressed the anti-inflammatory cytokine IL-10. These IGF1-primed monocytes demonstrated immune suppressive effects and were capable of inhibiting immune inflammation in the mouse colon. This suggests that IGF1-primed monocytes play a role in suppressing immune inflammation in the intestine.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4295102/
- Bilbao D, Luciani L, Johannesson B, Piszczek A, Rosenthal N. Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease. EMBO Molecular Medicine. 2014;6(11):1423-1435. doi:10.15252/emmm.201303376.
Insulin-like growth factor-1 stimulates regulatory T cells and suppresses autoimmune disease.
The study demonstrates that recombinant human insulin-like growth factor-1 (rhIGF-1) can stimulate the proliferation of regulatory T (Treg) cells both in vitro and in vivo. When administered via continuous minipump in mouse models of type 1 diabetes and multiple sclerosis, rhIGF-1 halts the progression of autoimmune disease. The treatment increases Treg cells in affected tissues while maintaining their suppressive properties. Genetically, removing the IGF-1 receptor specifically from Treg cells abolishes the beneficial effects of rhIGF-1 administration in the multiple sclerosis model, indicating that IGF-1 directly affects Treg cell proliferation. These findings suggest that systemically delivered rhIGF-1 can effectively stimulate Treg cell action and provide a potential therapeutic approach for suppressing autoimmune diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4237469/
- Franz A-C, Faass O, Köllner B, et al. Endocrine and Local IGF-I in the Bony Fish Immune System. Dixon B, ed. Biology. 2016;5(1):9. doi:10.3390/biology5010009.
Endocrine and Local IGF-I in the Bony Fish Immune System
The role of growth hormone (GH) and insulin-like growth factor-I (IGF-I) in modulating the immune system has been discussed for some time. GH is generally considered a stimulator of innate immune parameters in mammals and teleost fish. The stimulatory effects are often linked to elevated endocrine IGF-I, which can be suppressed during infection. However, the data on this topic are still limited and fragmented. Some studies suggest that GH and IGF-I play a significant role during immune organ development and constitution. The localization of IGF-I in immune cells and tissues in mammals and fish is not well-defined, but it has been found in phagocytic cells of rainbow trout head kidney and T-cells of a channel catfish cell line. While there are analogies between mammals and teleosts in the GH/IGF-system and immune system, there are also differences that warrant further investigation. The predominantly reported role of GH/IGF-I in the innate immune response may be due to a lack of studies focusing on the adaptive immune system. Moreover, the influence of infectious challenges combined with GH/IGF-I manipulations on fish growth and health, particularly considering developmental and environmental factors, remains an important area for further research.
You can read the full article at You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4810166/
- Spadaro O, Camell C, Bosurgi L, et al. IGF1 shapes the macrophage activation in response to immunometabolic challenge. Cell reports. 2017;19(2):225-234. doi:10.1016/j.celrep.2017.03.046.
IGF1 shapes the macrophage activation in response to immunometabolic challenge.
Macrophages, in addition to their phagocytic activity, play a role in regulating the host’s immunometabolic responses by producing specific cytokines and metabolites. In this study, it was found that IL-4-differentiated, M2-like macrophages secrete insulin-like growth factor-1 (IGF1), a hormone previously believed to be produced only by the liver. Removing IGF1 receptors from myeloid cells in mice resulted in reduced phagocytosis, increased adipose tissue macrophages, higher adiposity, decreased energy expenditure, and insulin resistance when fed a high-fat diet. The absence of myeloid IGF1 receptors also led to altered activation of M2-like macrophages in adipose tissue, impacting their ability to resolve infections and maintain insulin sensitivity. The study highlights the role of IGF1 signaling in shaping the activation phenotype of macrophages.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5513500/
- Alzaid A, Castro R, Wang T, et al. Cross Talk Between Growth and Immunity: Coupling of the IGF Axis to Conserved Cytokine Pathways in Rainbow Trout. Endocrinology. 2016;157(5):1942-55.
Cross Talk Between Growth and Immunity: Coupling of the IGF Axis to Conserved Cytokine Pathways in Rainbow Trout.
In this study using rainbow trout, the researchers investigated the potential relationship between the IGF axis (a key regulator of growth) and infection. They hypothesized that during infection, the IGF axis might be repressed to prioritize resources for the immune response. The study involved challenging the trout with bacterial and viral pathogens at different developmental stages. The results showed that the response of the IGF axis varied depending on the pathogen. The viral challenge led to significant down-regulation of many IGF-related genes. Interestingly, two IGFBP subtypes (IGFBP-1A1 and IGFBP-6A2), known to negatively regulate IGF signaling, were highly induced during infection, particularly in correlation with genes involved in host defense regulated by cytokine pathways. Additional experiments demonstrated a strong correlation between these IGFBP subtypes and proinflammatory cytokine genes in primary immune tissues. Based on these findings, the researchers propose a model where certain IGFBP subtypes are directly regulated by cytokine signaling, allowing for the immediate modulation of growth and immune system phenotypes depending on the level of immune system activation. This study sheds light on the interplay between growth regulation and immune response in teleosts.
You can read the full article at https://academic.oup.com/endo/article/157/5/1942/2422696?login=false
- Rodrigues LS, Hacker MA, Illarramendi X, et al. Circulating levels of insulin-like growth factor-I (IGF-I) correlate with disease status in leprosy. BMC Infect Dis. 2011;11:339.
Circulating levels of insulin-like growth factor-I (IGF-I) correlate with disease status in leprosy
In this retrospective study on leprosy patients, researchers investigated the interaction between the immune and neuroendocrine systems, focusing on the growth hormone/insulin-like growth factor-I (GH/IGF-I) pathway. They measured the serum levels of IGF-I and its major binding protein (IGFBP-3) in different clinical forms of leprosy (NR BT, NR BL, and NR LL) at diagnosis and during the occurrence of reactional episodes. Healthy controls were also included in the study.
The results showed significant differences in circulating IGF-I/IGFBP-3 levels based on the disease status and occurrence of reactional episodes. At the time of leprosy diagnosis, lower levels of IGF-I/IGFBP-3 were found in NR BL and NR LL patients compared to NR BT patients and healthy controls. However, after treatment, IGF-I levels in BL/LL patients returned to normal. Interestingly, low levels of IGF-I at diagnosis were associated with patients who did not undergo erythema nodosum leprosum (ENL) during treatment (NR LL patients), while normal levels were observed in those who experienced ENL (R LL patients). During ENL episodes, IGF-I levels in R LL patients tended to decrease, approaching the levels found in NR LL patients. The behavior of IGF-I was different during reversal reaction episodes in R BL patients.
The findings suggest that alterations in the IGF system are related to the host’s immune-inflammatory response to Mycobacterium leprae infection, and circulating IGF-I/IGFBP-3 levels may serve as potential predictive biomarkers for ENL in LL patients at diagnosis. This study provides valuable insights into the role of the GH/IGF-I pathway in leprosy and its associated reactional episodes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3266221/
- Gelato MC. Aging and immune function: a possible role for growth hormone. Horm Res. 1996;45(1-2):46-9.
Aging and immune function: a possible role for growth hormone.
This paper discusses the increased susceptibility of elderly individuals to certain diseases like cancer, tuberculosis, herpes zoster, and pneumonia, which may be attributed to two age-related changes: decreased production of growth hormone (GH) and insulin-like growth factor-1 (IGF-1), and decreased immune function. Studies have shown that around 40% of adults aged 60 and older are GH deficient based on GH secretory dynamics or IGF-1 levels. Additionally, immune function declines with age, leading to reduced cell-mediated and humoral immune responsiveness. The paper reviews some data demonstrating that immune deficits in older adults can be partially reversed through GH and/or IGF-1 treatment in humans and primates.
You can read the full article at https://karger.com/hrp/article-abstract/45/1-2/46/370703/Aging-and-Immune-Function-A-Possible-Role-for?redirectedFrom=fulltext
- Van bilsen K, Driessen GJ, De paus RA, et al. Low level IGF-1 and common variable immune deficiency: an unusual combination. Neth J Med. 2008;66(9):368-72.
Low level IGF-1 and common variable immune deficiency: an unusual combination.
This case report discusses a patient with an insulin-like growth factor 1 (IGF-1) deficiency and common variable immune deficiency. IGF-1 is a downstream mediator of growth hormone (GH). The patient had low levels of both GH and IGF-1, and no IGF-1 response was observed after a GH-releasing hormone test. The authors also reviewed earlier publications that suggest a potential interaction between IGF-1 and the immune system. In a small cohort of 14 patients with hypogammaglobulinaemia (low levels of immunoglobulins), two patients had slightly decreased IGF-1 levels, and one patient with a thymoma had increased IGF-1 levels. The authors propose that there may be a common impairment in the IGF-1 and IgG pathways in this patient.
You can read the full article at https://www.njmonline.nl/getpdf.php?id=711
- Rodrigues LS, Hacker MA, Illarramendi X, et al. Circulating levels of insulin-like growth factor-I (IGF-I) correlate with disease status in leprosy. BMC Infectious Diseases. 2011;11:339. doi:10.1186/1471-2334-11-339.
Circulating levels of insulin-like growth factor-I (IGF-I) correlate with disease status in leprosy
This retrospective study investigated the interaction between the immune and neuroendocrine systems in leprosy patients. They measured serum levels of insulin-like growth factor-I (IGF-I) and its major binding protein (IGFBP-3) in different clinical forms of leprosy and healthy controls. Significant differences in circulating IGF-I/IGFBP-3 levels were observed based on disease status and occurrence of reactional episodes. Patients with lepromatous leprosy (NR LL) and borderline lepromatous leprosy (NR BL) had lower IGF-I/IGFBP-3 levels at diagnosis compared to nonreactional borderline tuberculoid leprosy (NR BT) patients and healthy controls. After treatment, IGF-I levels returned to normal in lepromatous and borderline lepromatous patients. During episodes of erythema nodosum leprosum (ENL), IGF-I levels tended to decrease in patients who previously had reactional leprosy (R LL). The study suggests that circulating IGF-I/IGFBP-3 levels could serve as predictive biomarkers for ENL in lepromatous leprosy patients at diagnosis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3266221/
- Ge RT, Mo LH, Wu R, et al. Insulin-like growth factor-1 endues monocytes with immune suppressive ability to inhibit inflammation in the intestine. Sci Rep. 2015;5:7735.
Insulin-like growth factor-1 endues monocytes with immune suppressive ability to inhibit inflammation in the intestine
This study aimed to explore how insulin-like growth factor-1 (IGF1) regulates immune inflammation in the intestine, particularly in the context of inflammatory bowel disease. The researchers found that intestinal epithelial cells (IECs) produced IGF1 and exposure to CpG-ODN (CpG-oligodeoxynucleotides) could increase IGF1 production in IECs. When monocytes (Mos) were cultured with CpG-ODN-primed IEC cells or exposed to IGF1 directly, the Mos expressed interleukin-10 (IL-10), which is known for its immune-suppressive properties. The study suggests that IGF1-primed Mos have the ability to suppress immune inflammation in the mouse colon, indicating a potential role for IGF1 in regulating the immune response in the intestine.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4295102/
- Puche JE, Castilla-Cortázar I. Human conditions of insulin-like growth factor-I (IGF-I) deficiency. Journal of Translational Medicine. 2012;10:224. doi:10.1186/1479-5876-10-224.
Human conditions of insulin-like growth factor-I (IGF-I) deficiency
Insulin-like growth factor I (IGF-I) is a hormone produced mainly by the liver in response to growth hormone (GH) stimulus, but it is also secreted by other tissues for various purposes. IGF-I has multiple properties, including anabolic, antioxidant, anti-inflammatory, and cytoprotective actions. It plays a role in systemic GH activities and is involved in various physiological and pathological conditions. Therapeutically, IGF-I is mainly used to restore physiological circulating levels in cases of IGF-I deficiency, such as Laron Syndrome in children, liver cirrhosis in adults, aging-related cardiovascular and neurological diseases, and intrauterine growth restriction. The review emphasizes that IGF-I treatment should be limited to states of proven IGF-I deficiency for replacement therapy, rather than increasing levels above the normal range. It also highlights that IGF-I treatment has not been associated with oncogenesis in the mentioned conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3543345/
- Quentien MH, Delemer B, Papadimitriou DT, et al. Deficit in anterior pituitary function and variable immune deficiency (DAVID) in children presenting with adrenocorticotropin deficiency and severe infections. J Clin Endocrinol Metab. 2012;97(1):E121-8.
Deficit in anterior pituitary function and variable immune deficiency (DAVID) in children presenting with adrenocorticotropin deficiency and severe infections.
In this study, researchers found a new association between two rare disorders in four patients from different families. The first disorder is ACTH deficiency, which affects the pituitary gland and can cause low levels of certain hormones. The second disorder is common variable immunodeficiency (CVID), which weakens the immune system’s ability to produce antibodies. The researchers investigated if these two disorders were linked by autoimmune reactions or a common genetic cause. However, they did not find any evidence of autoantibodies or mutations in specific genes associated with this link. Despite the lack of a clear cause, the study shows a strong connection between these two conditions, which they referred to as DAVID (Deficit in Anterior Pituitary Function and Variable Immune Deficiency).
You can read the full article at https://academic.oup.com/jcem/article/97/1/E121/2833839?login=false
- Available at https://www.tandfonline.com/doi/abs/10.1586/eem.10.73.
Role of the growth hormone–IGF-1 axis in cancer.
A significant body of evidence substantiates the involvement of the growth hormone (GH)–IGF-1 axis in the occurrence and advancement of cancer. This body of evidence encompasses epidemiological data linking elevated plasma IGF-1 levels to an increased incidence of cancer, as well as the absence of cancer cases in individuals with GH/IGF-1 deficiency. Notably, rodent models lacking GH or its receptor display remarkable resistance to the development of a diverse array of cancers. Additionally, the progression of tumors can be slowed down by administering the GH antagonist pegvisomant. While many cancers exhibit heightened expression of the GH receptor, several types also contain autocrine GH. Furthermore, the excessive expression of autocrine GH has the potential to trigger cellular transformation. Despite an unclear understanding of the precise mechanism underlying autocrine action, it is known to involve the activation of both STAT5 and STAT3, likely coupled with the nuclear translocation of the GH receptor. Considering these findings, there is a compelling rationale to explore the development of a more potent GH receptor antagonist or secretion inhibitor as a viable approach for cancer therapy.
You can read the full article at https://www.tandfonline.com/doi/full/10.1586/eem.10.73
- Tu W, Cheung PT, Lau YL. Insulin-like growth factor 1 promotes cord blood T cell maturation and inhibits its spontaneous and phytohemagglutinin-induced apoptosis through different mechanisms. J Immunol. 2000;165(3):1331-6
Insulin-like growth factor 1 promotes cord blood T cell maturation and inhibits its spontaneous and phytohemagglutinin-induced apoptosis through different mechanisms.
In this study, researchers investigated the role of insulin-like growth factor-I (IGF-I) in promoting the maturation and survival of T cells from cord blood (CB). They found that CB T cells matured more slowly than T cells in mixed cord blood mononuclear cell (CBMC) cultures. However, when autologous CD14+ monocytes were added to the T cell cultures, maturation was enhanced, and the effects of IGF-I were potentiated. The addition of IL-6, another immune system molecule, did not affect T cell maturation but reduced T cell apoptosis (cell death). They also observed that neutralizing IL-6 partially reversed the anti-apoptotic effect of IGF-I on T cells, suggesting that IL-6 plays a role in promoting T cell survival. These findings highlight the potential therapeutic use of IGF-I in enhancing the immune response of newborns.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2631546/
- Baatar D, Patel K, Taub DD. The effects of ghrelin on inflammation and the immune system. Mol Cell Endocrinol. 2011;340(1):44-58.
The effects of ghrelin on inflammation and the immune system.
The body’s energy status is monitored by various hormones and metabolic mediators, and the brain responds by controlling appetite and food consumption to maintain balance. In conditions of chronic inflammation and immune activation, there can be loss of body mass and appetite, indicating communication between the immune and neuroendocrine systems. Ghrelin is a hormone produced mainly in the stomach, which regulates food intake, energy expenditure, and growth hormone secretion. Recent research has shown that ghrelin has anti-inflammatory properties and can promote lymphocyte development, making it a potential therapeutic agent for inflammatory diseases and injuries. This review discusses the role of ghrelin as an anti-inflammatory mediator and its potential use in treating inflammatory conditions.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0303720711002279?via%3Dihub
- Brown PA, Davis WC, Draghia-akli R. Immune-enhancing effects of growth hormone-releasing hormone delivered by plasmid injection and electroporation. Mol Ther. 2004;10(4):644-51.
Immune-enhancing effects of growth hormone-releasing hormone delivered by plasmid injection and electroporation.
In this study, researchers investigated the effects of a growth hormone-releasing hormone (GHRH) treatment on immune function and health in Holstein heifers. The heifers were given a GHRH-expressing plasmid via intramuscular injection followed by electroporation. The GHRH-treated animals showed increased numbers of certain immune cells, which were maintained long-term and correlated with plasmid expression. They also exhibited improved health, including better body condition scores and reduced hoof pathology. The mortality of treated heifers was significantly decreased compared to the control group. The study suggests that the GHRH plasmid can be effectively transferred into large mammals and may have potential therapeutic applications and benefits for animal health.
You can read the full article at https://www.cell.com/molecular-therapy-family/molecular-therapy/fulltext/S1525-0016(04)01310-3?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS1525001604013103%3Fshowall%3Dtrue
- Available at https://www.researchgate.net/publication/326998293_Biological_activity_of_a_new_Growth_hormone_secretagogue_study_in_fish_and_murine_cell_line.
- Rubinek T, Rubinfeld H, Hadani M, Barkai G, Shimon I. Nitric oxide stimulates growth hormone secretion from human fetal pituitaries and cultured pituitary adenomas. Endocrine. 2005;28(2):209-16.
Nitric oxide stimulates growth hormone secretion from human fetal pituitaries and cultured pituitary adenomas.
In simple terms, this study looked at the effects of a chemical called nitric oxide (NO) on the secretion of growth hormone (GH) and prolactin in the human pituitary gland. They used human fetal pituitaries and hormone-secreting adenomas (abnormal growths) for their research. They found that NO increased GH secretion in both fetal pituitaries and adenomas, similar to the effect of a hormone called growth hormone-releasing hormone (GHRH). NO did not affect prolactin secretion. Another molecule called cGMP, which is involved in NO’s actions, also increased GH secretion. The study suggests that NO plays a role in regulating GH in the human pituitary gland.
You can read the abstract of this article at https://link.springer.com/article/10.1385/ENDO:28:2:209
- Valverde I, Peñalva A, Ghigo E, Casanueva FF, Dieguez C. Involvement of nitric oxide in the regulation of growth hormone secretion in dogs. Neuroendocrinology. 2001;74(4):213-9.
Involvement of nitric oxide in the regulation of growth hormone secretion in dogs.
In simple terms, this study looked at the role of a gas called nitric oxide (NO) in the secretion of growth hormone (GH) in dogs. They used different substances to manipulate NO levels and measured the effects on GH release. They found that increasing NO levels with L-arginine slightly increased GH secretion. Blocking NO with L-NAME completely suppressed the GH release induced by certain hormones (GHRH and GHRP-6). This suggests that NO is involved in regulating GH release in the body.
You can read the full article at https://karger.com/nen/article-abstract/74/4/213/225390/Involvement-of-Nitric-Oxide-in-the-Regulation-of?redirectedFrom=fulltext
- Rigamonti AE, Cella SG, Marazzi N, Müller EE. Nitric oxide modulation of the growth hormone-releasing activity of Hexarelin in young and old dogs. Metab Clin Exp. 1999;48(2):176-82.
Nitric oxide modulation of the growth hormone-releasing activity of Hexarelin in young and old dogs
In simple terms, this study looked at how a substance called Hexarelin, which can increase growth hormone (GH) release, interacts with nitric oxide (NO) in dogs. They found that giving dogs a NO donor along with Hexarelin significantly increased GH release. Blocking NO production had different effects in young and old dogs, suggesting changes in the way GH is regulated as dogs age. They also found that prostaglandins are involved in the process triggered by NO to stimulate GH release. Overall, this study helps us understand how different factors can affect GH levels in dogs.
You can read the abstract at https://www.metabolismjournal.com/article/S0026-0495(99)90030-6/pdf
- Doi SQ, Jacot TA, Sellitti DF, et al. Growth hormone increases inducible nitric oxide synthase expression in mesangial cells. J Am Soc Nephrol. 2000;11(8):1419-25.
Growth hormone increases inducible nitric oxide synthase expression in mesangial cells
In this study, researchers investigated the effects of bovine growth hormone (GH) on certain signaling events in mesangial cells, which are involved in glomerulosclerosis, a condition where the kidney’s filtering units are damaged. They focused on the L-arginine metabolic pathway, specifically on inducible nitric oxide (NO) synthase (iNOS), ornithine aminotransferase (OAT), and ornithine decarboxylase (ODC). They found that GH increased the expression of iNOS, leading to higher levels of nitrite, a product of NO, in the cell culture medium. The study also showed that GH did not affect the expression of OAT and ODC. This suggests that GH may directly regulate the L-arginine/NO pathway in mesangial cells, potentially contributing to glomerulosclerosis development.
You can read the full article at https://journals.lww.com/jasn/Fulltext/2000/08000/Growth_Hormone_Increases_Inducible_Nitric_Oxide.6.aspx
- Available at http://erj.ersjournals.com/content/31/4/815.
Effect of growth hormone therapy on nitric oxide formation in cystic fibrosis patients
This study investigated the effects of growth hormone therapy on nitric oxide production in patients with cystic fibrosis. Nitric oxide metabolites in serum and urine, amino acid concentrations in serum and sputum, and exhaled nitric oxide were measured before, during, and after 1 year of human growth hormone treatment. The results showed a significant increase in nitric oxide metabolite concentrations in serum and urine during treatment, along with increased serum amino acid concentrations. However, l-arginine concentrations in sputum decreased, leading to a reduction in exhaled nitric oxide levels. Therefore, growth hormone therapy in children with cystic fibrosis decreased exhaled nitric oxide by reducing l-arginine concentration in the airways.
You can read the full article at https://erj.ersjournals.com/content/31/4/815
- Deniz Tuncel, Fatma Inanc Tolun, and Ismail Toru, “Serum Insulin-Like Growth Factor-1 and Nitric Oxide Levels in Parkinson’s Disease,” Mediators of Inflammation, vol. 2009, Article ID 132464, 4 pages, 2009.
Serum Insulin-Like Growth Factor-1 and Nitric Oxide Levels in Parkinson’s Disease.
The purpose of this study was to investigate the levels of certain substances in patients with Parkinson’s disease (PD) compared to healthy individuals. The study included 25 PD patients and 25 matched healthy subjects as a control group. The researchers measured the concentrations of growth hormone (GH), insulin-like growth factor-1 (IGF-1), IGF binding protein-3 (IGFBP-3), and nitric oxide (NO) in the participants.
The results showed that the NO levels in PD patients were significantly lower (2.3 +/- 0.4 micromol/L) than in the control group (2.8 +/- 0.6 micromol/L). However, there were no significant differences in the GH, IGF-1, and IGFBP-3 levels between the two groups. Additionally, the researchers observed mildly elevated IGF-1 levels in PD patients.
These findings suggest that there may be changes in NO and IGF-1 levels in patients with PD, possibly as a response to the neurodegenerative processes occurring in the disease. However, this was a preliminary study, and further research is needed to confirm and better understand these results.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2655363/
- Available at https://clinicaltrials.gov/ct2/show/NCT00470002.
Effects of Growth Hormone on the Nitric Oxide Pathway.
This study aims to investigate the effects of growth hormone (GH) on the nitric oxide (NO) pathway and its role in cardiovascular functions. Impaired endothelial NO production is associated with endothelial dysfunction and atherosclerotic vascular disease. Recent studies have shown a significant relationship between insulin resistance and asymmetric dimethylarginine (ADMA), an endogenous NO synthase inhibitor. Higher levels of ADMA are associated with increased mortality and cardiovascular risk in haemodialysis patients. Patients with growth hormone deficiency have a higher risk of cardiovascular disease, and alterations in the NO pathway are implicated in this increased risk. Treatment with recombinant GH normalizes NO production. The effects of GH on NO may be mediated by insulin-like growth factor-I (IGF-I), which stimulates NO synthesis. The study aims to elucidate the in vivo effects of GH on the NO pathway and its role in cardiovascular functions.
You can read the full article at https://clinicaltrials.gov/ct2/show/NCT00470002
- Böger RH , Skamira C , Bode-Böger SM , Brabant G , von zur Muhlen A , Frolich JC. 1996. Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled
study. J Clin Invest 98:2706–2713.Nitric oxide may mediate the hemodynamic effects of recombinant growth hormone in patients with acquired growth hormone deficiency. A double-blind, placebo-controlled study.
In this double-blind, placebo-controlled trial, researchers investigated the effects of recombinant growth hormone (r-hGH) on systemic nitric oxide (NO) production and hemodynamics in adult patients with acquired growth hormone deficiency. The study included 30 patients who were randomly assigned to receive either r-hGH (2.0 IU/d) or a placebo for 12 months. In the following 12 months, both groups received r-hGH.
The results showed that r-hGH treatment led to a fourfold increase in plasma insulin-like growth factor-1 (IGF-1) concentrations within the first month. Patients with growth hormone deficiency had lower levels of urinary nitrate and cyclic GMP (indicators of NO production) at baseline compared to healthy individuals. However, r-hGH treatment significantly increased urinary nitrate and cyclic GMP excretion rates, indicating improved systemic NO production in the GH group during the first 12 months and in the placebo group during the second 12 months.
While blood pressure remained unchanged, cardiac output increased by 30-40%, and total peripheral resistance decreased by approximately 30% in both groups during r-hGH treatment. In the second year, when both groups received r-hGH, there were no significant differences in IGF-1 levels, urinary nitrate, cyclic GMP excretion, or hemodynamic parameters between the two groups.
In conclusion, untreated growth hormone-deficient patients showed decreased systemic NO production, which was normalized by r-hGH treatment, possibly through IGF-1 stimulation of endothelial NO formation. This increase in NO production may contribute to the improved cardiovascular performance observed in patients with acquired hypopituitarism during growth hormone therapy.You can read the abstract of this article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC507734/
- Thum T , Fleissner F , Klink I , Tsikas D , Jakob M , Bauersachs J , Stichtenoth DO. 2007. Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I. J Clin Endocrinol Metab 92:4172–4179.
Growth hormone treatment improves markers of systemic nitric oxide bioavailability via insulin-like growth factor-I
In this study, researchers investigated the effects of growth hormone (GH) treatment on nitric oxide (NO) bioavailability and endothelial progenitor cells (EPC) levels in healthy middle-aged volunteers. The participants were treated with recombinant human GH for 10 days, and markers of NO bioavailability and EPC levels were analyzed before and after the treatment.
The results showed that GH treatment led to significant increases in plasma insulin-like growth factor-1 (IGF-I) levels. After GH treatment, urinary cGMP levels (a marker of NO activity) increased, and diastolic blood pressure decreased. Plasma nitrate and nitrite levels (indicators of NO production) increased, while the NO synthase inhibitor asymmetric dimethylarginine was reduced. In cultured human endothelial cells, IGF-I treatment increased the expression of enzymes involved in metabolizing asymmetric dimethylarginine, further promoting NO bioavailability. IGF-I levels also correlated with cGMP concentrations.
EPC numbers increased after GH treatment and were correlated with markers of NO bioavailability. These findings were replicated in mice treated with GH for 7 days. GH treatment in mice also increased the expression of endothelial NO synthase in the aorta. Importantly, when the IGF-I receptor was blocked in vivo, the GH-mediated effects on markers of increased NO bioavailability were abolished.
In conclusion, GH treatment enhances systemic NO bioavailability through IGF-I, leading to increased EPC levels in healthy individuals. These results suggest that GH treatment may have potential benefits in certain cardiovascular diseases by improving NO bioavailability.You can read the full article at https://academic.oup.com/jcem/article/92/11/4172/2598165?login=false
- Mani maran RR, Sivakumar R, Ravisankar B, et al. Growth hormone directly stimulates testosterone and oestradiol secretion by rat Leydig cells in vitro and modulates the effects of LH and T3. Endocr J. 2000;47(2):111-8.
Growth hormone directly stimulates testosterone and oestradiol secretion by rat Leydig cells in vitro and modulates the effects of LH and T3.
In this study, researchers investigated the effects of growth hormone (GH) on the secretion of testosterone and estradiol by purified rat Leydig cells in vitro. They cultured the cells with different concentrations of rat GH and studied its modulatory effects on basal, luteinizing hormone (LH), and triiodothyronine (T3)-mediated secretion of testosterone and estradiol.
The results showed that GH increased the secretion of testosterone and estradiol in a dose-dependent manner. Testosterone secretion reached saturation with 50 ng of GH, while estradiol secretion reached saturation with 150 ng of GH, and then decreased with higher GH concentrations. When GH was co-administered with minimum or maximum effective doses of LH, it decreased testosterone secretion, but increased it when co-administered with a specific dose of GH and LH. Similarly, T3 inhibited GH-mediated testosterone secretion when administered at specific doses.
For estradiol secretion, co-administration of GH with minimum or maximum effective doses of LH increased its concentration in the culture medium. Additionally, T3 at a specific dose enhanced estradiol secretion by Leydig cells in the presence of GH.
Overall, GH acted as a gonadotrophin, stimulating testosterone and estradiol secretion by Leydig cells. It also modulated LH or T3-induced secretion of these steroids, depending on the intensity of their stimulation.You can read the abstract of this article at https://www.jstage.jst.go.jp/article/endocrj1993/47/2/47_2_111/_article
- Ho KY, Evans WS, Blizzard RM, et al. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64(1):51-8.
Effects of sex and age on the 24-hour profile of growth hormone secretion in man: importance of endogenous estradiol concentrations
In this study, researchers investigated the effects of age and sex on the pattern of growth hormone (GH) secretion. They studied four groups: young women, young men, postmenopausal women, and older men. GH secretion was assessed over a 24-hour period, looking at total GH secretion and pulsatile secretion.
They found that total GH secretion was higher in women than in men and higher in young individuals compared to older ones. Pulsatile secretion, which refers to the pattern of bursts of GH release, was greater in the young compared to the older individuals but did not differ significantly between sexes. The frequency of GH pulses was not affected by sex or age.
The study also found that the level of free estradiol (a form of estrogen) in the blood correlated with GH secretion and pulse characteristics. After accounting for the effects of estradiol, sex and age no longer had significant effects on GH secretion or pulse characteristics, indicating that estradiol plays a significant role in regulating GH secretion.
The results suggest that sex and age can independently influence GH secretion, but much of this effect is related to differences in estradiol levels. Estradiol seems to amplify the neuroendocrine regulation of pulsatile GH release.You can read the abstract of this article at https://academic.oup.com/jcem/article-abstract/64/1/51/2653487?redirectedFrom=fulltext&login=false
- Amitani M, Amitani H, Cheng K-C, et al. The Role of Ghrelin and Ghrelin Signaling in Aging. International Journal of Molecular Sciences. 2017;18(7):1511. doi:10.3390/ijms18071511.
The Role of Ghrelin and Ghrelin Signaling in Aging.
This review discusses the factors related to healthy longevity, focusing on the role of orexigenic peptides, particularly ghrelin. Calorie restriction is known to delay aging and extend life, and ghrelin, which is secreted during fasting, is an orexigenic peptide that increases during caloric restriction. The review suggests that the ghrelin-growth hormone secretagogue-R signaling pathway may be crucial in the mechanism of anti-aging mediated by calorie restriction.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5536001/
- Minor RK, Chang JW, de Cabo R. Hungry for Life: How the arcuate nucleus and neuropeptide Y may play a critical role in mediating the benefits of calorie restriction. Molecular and cellular endocrinology. 2009;299(1):79-88. doi:10.1016/j.mce.2008.10.044.
Hungry for Life: How the arcuate nucleus and neuropeptide Y may play a critical role in mediating the benefits of calorie restriction.
Calorie restriction (CR) has been consistently shown to extend lifespan and delay the onset of age-related diseases in laboratory animals. It also improves cognitive function and overall health in aging. The mechanisms through which CR works are not fully understood, but one theory suggests that the neuroendocrine response to low energy availability, particularly involving the arcuate nucleus in the hypothalamus, plays a key role. Neuropeptide Y (NPY), a neurotransmitter involved in the arcuate response to low energy levels, is affected by CR and may be a critical mechanism responsible for the extension of lifespan.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2668104/
- Babaei-Balderlou F, Khazali H. Effects of Ghrelin on Sexual Behavior and Luteinizing Hormone Beta-subunit Gene Expression in Male Rats . Journal of Reproduction & Infertility. 2016;17(2):88-96.
Effects of Ghrelin on Sexual Behavior and Luteinizing Hormone Beta-subunit Gene Expression in Male Rats
The study aimed to understand how the hormone ghrelin affects sexual behavior and the expression of a specific gene (LH beta-subunit) in male rats. Ghrelin, known as a hunger hormone, was found to inhibit the reproductive function of the hypothalamo-pituitary-gonadal (HPG) axis, which plays a role in reproductive behavior. When ghrelin was injected into male rats, it increased the time taken for the first sexual activities (mounting, intromission, and ejaculation) and reduced the number of ejaculations. Additionally, ghrelin decreased the expression of the LH beta-subunit gene. On the other hand, when the rats were given an antagonist of ghrelin called [D-Lys(3) ]-GHRP-6, it counteracted the effects of ghrelin on sexual behavior and gene expression. Overall, the study showed that ghrelin has inhibitory effects on sexual behavior and gene expression, which can be reversed by the antagonist [D-Lys(3) ]-GHRP-6.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4842239/
- https://www.sciencedaily.com/releases/2015/01/150127110955.htm.
Researchers find hormone that increases the sex drive of mice
Swedish research has revealed that mice receiving a supplement of the “appetite hormone” ghrelin display an increase in their sexual activity. The potential impact of this hormone on humans remains uncertain. However, if the same effect is observed, it could hold the key to future interventions for addressing issues related to sexual abuse.
Ghrelin, a gastrointestinal hormone originating from the stomach, stimulates our appetite by triggering the brain’s reward system. Given that the same reward system is responsible for driving our desire for a partner and engaging in sexual activity, a team of scientists at the Sahlgrenska Academy embarked on an exploration of whether ghrelin could influence sexual behaviors.Confirmed Findings
Their investigations have yielded affirmative results, at least in mice. The study demonstrates that when mice are administered a ghrelin supplement, their sexual activity and pursuit of a partner are heightened. This observation is further confirmed through a subsequent experiment where mice administered a ghrelin inhibitor exhibit a reduction in sexual activity.
Elisabet Jerlhag, a researcher at the Sahlgrenska Academy, states, “Ghrelin is already recognized to impact reward mechanisms triggered by food, alcohol, and other addictive substances. Our study now establishes, for the first time, that ghrelin also exerts an influence on innate reward systems like sexual behavior.”Dopamine Connection
The research indicates that the effects of ghrelin are mediated through dopamine, a well-known and significant messenger within the brain’s reward system. The researchers’ deduction is that both ghrelin and dopamine jointly regulate natural sexual behavior in mice.
Nonetheless, this does not automatically translate to the same function in humans. Confirming this necessitates extensive further investigation. Nonetheless, the potential for ghrelin inhibitors to become instrumental in future treatments for sex addiction and abuse remains a possibility, according to Elisabet Jerlhag.
Elisabet Jerlhag emphasizes, “Addictive behaviors, including sexual abuse, constitute significant societal challenges, warranting innovative treatment strategies. Hopefully, our findings can contribute an additional piece to this intricate puzzle.”
The article titled “The role of ghrelin signaling for sexual behavior in male mice” was published online in the journal Addiction Biology on December 4. - Brod M, Pohlman B, Højbjerre L, Adalsteinsson JE, Rasmussen MH. Impact of adult growth hormone deficiency on daily functioning and well-being. BMC Research Notes. 2014;7:813. doi:10.1186/1756-0500-7-813.
Impact of adult growth hormone deficiency on daily functioning and well-being.
Adult growth hormone (GH) deficiency occurs when the pituitary gland does not produce enough GH, often due to pituitary or peri-pituitary diseases or their treatments. This deficiency leads to a combination of psychological and physical symptoms known as the adult ‘GHD syndrome’. Psychological symptoms include reduced energy, feeling socially isolated, low mood, and increased anxiety. Physical symptoms include changes in body composition, with less muscle and more central fat, reduced bone density, weaker muscles, and increased cholesterol levels. Hypopituitarism and GHD are associated with higher mortality rates. Diagnosis is confirmed through specific tests, and treatment involves replacing the missing GH with synthetic growth hormone injections. However, whether all patients with GHD should receive GH replacement is still a topic of debate, with some centers adopting a selective approach based on the individual’s well-being and quality of life.
You can read the abstract of this article at https://www.tandfonline.com/doi/abs/10.1080/07853890310001320
- Maggi M, Buvat J, Corona G, Guay A, Torres LO. Hormonal causes of male sexual dysfunctions and their management (hyperprolactinemia,thyroid disorders, GH disorders, and DHEA). J Sex Med. 2013;10(3):661-77.
Hormonal causes of male sexual dysfunctions and their management (hyperprolactinemia, thyroid disorders, GH disorders, and DHEA).
The article reviews the role of several pituitary and endocrine hormones in male sexual dysfunction (MSD). Severe hyperprolactinemia, often related to a pituitary tumor, negatively affects sexual function, including desire and erectile function. Hyperthyroidism is associated with an increased risk of premature ejaculation and possible erectile dysfunction, while hypothyroidism affects sexual desire and ejaculation reflex. Acromegaly, a condition of excess growth hormone, may lead to decreased libido and erectile dysfunction, possibly due to hormonal effects or pituitary mass effects on gonadotropic cells. However, the relationship between growth hormone and sexual dysfunction needs further investigation. Dehydroepiandrosterone (DHEA) supplementation does not seem to improve male sexual function significantly. While the link between hyperprolactinemia and sexual desire is well-established, more research is needed to fully understand the role of other hormones in male sexual functioning.
You can read the full article at https://academic.oup.com/jsm/article-abstract/10/3/661/6940230?redirectedFrom=fulltext&login=false
- Ginzburg E, Lin A, Sigler M, Olsen D, Klimas N, Mintz A. Testosterone and growth hormone normalization: a retrospective study of health outcomes. Journal of multidisciplinary healthcare. 2008;1:79-86.
Testosterone and growth hormone normalization: a retrospective study of health outcomes.
The study investigated the effects of long-term testosterone and/or growth hormone (GH) supplementation on body composition and quality of life in men and women across different age groups. The researchers reviewed the records of 91 men and 97 women who received various treatments: dehydroepiandrosterone (DHEA) with no hormonal supplementation (control), testosterone only, GH only, and testosterone plus GH. After an average of 3 years of treatment, both men and women experienced improvements in body composition, with increases in lean mass, reductions in fat mass, and improvements in bone mineral density (BMD) with testosterone and/or GH supplementation. Quality of life and mood also improved in all treatment groups. The treatments were generally safe and well tolerated. Overall, the study suggests that testosterone and/or GH supplementation can have favorable effects on body composition and well-being in both men and women across different age ranges.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3004552/
- Brod M, Højbjerre L, Adalsteinsson JE, Rasmussen MH. Assessing the impact of growth hormone deficiency and treatment in adults: development of a new disease-specific measure. J Clin Endocrinol Metab. 2014;99(4):1204-12.
Assessing the impact of growth hormone deficiency and treatment in adults: development of a new disease-specific measure.
The researchers developed and validated a patient-reported outcome measure (PRO) called the Treatment-Related Impact Measure-Adult Growth Hormone Deficiency (TRIM-AGHD). This measure was designed to assess the impact of growth hormone deficiency and its treatment on adults’ cognitive functioning, psychological well-being, and quality of life. The development process included interviews with patients and clinical experts, as well as a literature review. The TRIM-AGHD consists of four domains: energy level, physical health, emotional health, and cognitive ability. Psychometric validation showed that the measure is reliable and valid, making it a useful tool for evaluating the effects of the disease and its treatment on patients with adult growth hormone deficiency.
You can read the full article at https://academic.oup.com/jcem/article/99/4/1204/2537260?login=false
- Available at https://www.researchgate.net/publication/12600670_Effects_of_growth_hormone_on_male_reproductive_functions.
Effects of Growth hormone on male reproductive function
This review highlights the physiological role of growth hormone (GH) in male reproductive development and function, as well as the negative effects of excessive GH release on male sexual behavior and fertility. It explores the interaction between the somatotropic axis and the hypothalamic-pituitary-testicular (H-P-T) axis, which has been studied since the early investigations on GH deficiency or resistance and its impact on testicular development and fertility. The review also discusses the synergistic interactions between GH and gonadotropins observed in animal studies. Recent advancements, such as the availability of recombinant GH and the use of GH-deficient mutants, transgenic models, and knock-out mouse models, have contributed to a better understanding of GH’s role in reproduction.
You can read the abstract of this article at https://www.researchgate.net/publication/12600670_Effects_of_growth_hormone_on_male_reproductive_functions
- Galdiero M, Pivonello R, Grasso LF, Cozzolino A, Colao A. Growth hormone, prolactin, and sexuality. J Endocrinol Invest. 2012;35(8):782-94.
Growth hormone, prolactin, and sexuality
GH (growth hormone) and PRL (prolactin), although not considered classical sexual hormones, may play a role in regulating sexual function in both men and women. PRL seems to be involved in the central control of sexual behavior and activity by influencing dopaminergic and serotoninergic systems. After orgasm, PRL levels increase, potentially impacting further sexual arousal. Chronic high levels of PRL (hyperprolactinemia) can lead to sexual dysfunction and hypogonadism in both sexes, but treatment can often restore normal sexual function.
The role of GH in sexual function is not fully understood, but it plays a part in regulating the hypothalamus-pituitary-gonadal axis. Both GH deficiency (GHD) and GH excess (as seen in acromegaly) have been associated with sexual issues in men and women. GHD and acromegaly patients may experience decreased desire, arousability, and erectile function. The effects may be related to the hormone imbalance or other factors like hypogonadism, clinical complications, physical disfigurement, or psychological issues associated with the diseases.
While some studies suggest beneficial effects of GH replacement therapy and specific approaches for acromegaly on sexual function, more research is needed to fully understand the impact of GH and PRL on sexual function in different contexts.You can read the abstract of this article at https://link.springer.com/article/10.1007/BF03345805
- Becker AJ, Uckert S, Stief CG, et al. Possible role of human growth hormone in penile erection. J Urol. 2000;164(6):2138-42.
Possible role of human growth hormone in penile erection.
The study aimed to understand how recombinant human growth hormone (GH) affects the tissues in the penis and its role in penile erection. In lab tests, they found that GH can relax the smooth muscle of the human corpus cavernosum (tissue in the penis) and increase the levels of cyclic guanosine monophosphate (cGMP), which is important for the erection process. In human volunteers, they observed that GH levels in the blood of the penis were similar to those in the rest of the body during different phases of penile function, with the highest levels occurring during penile tumescence (when the penis is becoming erect). This suggests that GH may play a role in inducing penile erection by stimulating cGMP in the smooth muscle of the penis.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11061943/
- Becker AJ, Uckert S, Stief CG, et al. Serum levels of human growth hormone during different penile conditions in the cavernous and systemic blood of healthy men and patients with erectile dysfunction. Urology. 2002;59(4):609-14.
Serum levels of human growth hormone during different penile conditions in the cavernous and systemic blood of healthy men and patients with erectile dysfunction
The study aimed to investigate the changes in growth hormone (GH) levels during different penile conditions in healthy men and men with erectile dysfunction (ED). They found that in healthy men, GH levels significantly increased during penile tumescence (erection), followed by a slight decrease during rigidity and detumescence (loss of erection). In men with ED, the GH levels during flaccidity (non-erect) were about sevenfold lower than in healthy men. However, during penile tumescence, the GH levels in men with psychogenic ED (caused by psychological factors) were similar to those in healthy men, while in men with organogenic ED (caused by physical factors), the increase in GH levels was negligible. This suggests that GH may play a significant role in maintaining erectile function, potentially by stimulating cyclic guanosine monophosphate in the smooth muscle of the penis, and a decrease in GH release may contribute to the development of erectile dysfunction.
You can read the abstract of this article at https://www.goldjournal.net/article/S0090-4295(01)01594-1/fulltext
- Khorram O, Laughlin GA, Yen SS. Endocrine and metabolic effects of long-term administration of [Nle27]growth hormone-releasing hormone-(1-29)-NH2 in age-advanced men and women. J Clin Endocrinol Metab. 1997;82(5):1472-9.Gades NM, Jacobson DJ, McGree ME, et al. The associations between serum sex hormones, erectile function, and sex drive: the Olmsted County study of urinary symptoms and health status among men. The journal of sexual medicine. 2008;5(9):2209-2220. doi:10.1111/j.1743-6109.2008.00924.x.
Endocrine and metabolic effects of long-term administration of [Nle27] growth hormone-releasing hormone-(1-29)-NH2 in age-advanced men and women.
The study aimed to investigate the effects of a growth hormone-releasing hormone (GHRH) analog in age-advanced men and women. The trial involved 10 women and 9 men aged between 55-71 years. The participants received nightly injections of the GHRH analog for 16 weeks. The results showed that the GHRH analog induced an acute release of growth hormone (GH) that lasted for 2 hours. Over the course of the study, it led to sustained increases in GH levels in both genders. The treatment also resulted in increased levels of insulin-like growth factor-I (IGF-I) and IGF binding protein-3 (IGFBP-3), which were maintained for several weeks. In women, GH binding protein (GHBP) concentrations were significantly increased, but not in men. The treatment led to increased skin thickness in both genders and increased lean body mass in men only. Men also experienced improved insulin sensitivity, general well-being, and libido, while women did not show these effects. Overall, the GHRH analog activated the somatotropic axis and had anabolic effects, particularly favoring men. However, further studies are needed to understand the gender differences observed in response to GHRH analog administration.
You can read the full article at https://academic.oup.com/jcem/article/82/5/1472/2823341?login=false
- Otunctemur A, Ozbek E, Sahin S, et al. Low serum insulin-like growth factor-1 in patients with erectile dysfunction. Basic and Clinical Andrology. 2016;26:1. doi:10.1186/s12610-015-0028-x.
Low serum insulin-like growth factor-1 in patients with erectile dysfunction. Basic and Clinical Andrology
The study aimed to investigate the association between insulin-like growth factor-1 (IGF-1) levels and erectile dysfunction (ED). Two groups were evaluated: 80 patients with ED for more than a year and 80 subjects without ED (control group). ED diagnosis was based on the International Index of Erectile Function Score-5. The results showed that the plasma IGF-1 levels were significantly lower in the ED group compared to the control group. There was a positive correlation between IGF-1 levels and the severity of ED. The findings suggest that serum IGF-1 levels are associated with endothelial dysfunction, which can predict ED. IGF-1 levels could potentially be used as a specific predictor for ED in males and aid in early prediction of the condition.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4730635/
- Rajfer J. Growth Factors and Gene Therapy for Erectile Dysfunction. Reviews in Urology. 2000;2(1):34.
Growth Factors and Gene Therapy for Erectile Dysfunction
Erectile Dysfunction (ED) is a common condition in middle-aged and older men, and oral medications like phosphodiesterase type 5 inhibitors have been used to treat it. However, these drugs have limitations and may not be effective for some patients. Gene therapy has emerged as a potential novel treatment for ED, using viral or non-viral vectors to deliver therapeutic genes to tissues. Preclinical studies have shown promising results, but safety concerns need to be addressed before widespread clinical application. This review summarizes the progress in gene therapy for ED, focusing on stem cell-based approaches and other combinational strategies. The challenges in implementing gene therapy for ED treatment in clinical settings are also discussed.
You can read the abstract of this article at https://www.eurekaselect.com/article/93440
- Pastuszak AW, Liu JS, Vij A. IGF-1 levels are significantly correlated with patient-reported measures of sexual function. International journal of impotence research. 2011; 23(5):220-6.
IGF-1 levels are significantly correlated with patient-reported measures of sexual function.
The study aimed to investigate the relationship between serum insulin-like growth factor 1 (IGF-1) levels, a marker for growth hormone (GH) levels, and sexual function in men. The researchers assessed 65 men using the Sexual Health Inventory for Men (SHIM) and Expanded Prostate Cancer Index Composite (EPIC) questionnaires, along with measuring serum IGF-1 and testosterone levels. They found that IGF-1 levels positively correlated with the total SHIM score and all individual SHIM question scores, as well as the sexual domain of the EPIC questionnaire. However, no significant correlations were observed between IGF-1 levels and Gleason score, IGF-1 levels and testosterone levels, or SHIM score and testosterone levels. The results suggest a potential role for the GH axis in maintaining erectile function.
You can read the abstract of this article at https://www.nature.com/articles/ijir201131
- Pastuszak AW, Liu JS, Vij A, et al. IGF-1 levels are significantly correlated with patient-reported measures of sexual function. Int J Impot Res. 2011;23(5):220-6.
IGF-1 levels are significantly correlated with patient-reported measures of sexual function.
The study investigated the relationship between serum insulin-like growth factor 1 (IGF-1) levels, a marker for growth hormone (GH) levels, and sexual function in 65 men. They completed questionnaires about sexual health (SHIM and EPIC) and had their IGF-1 and testosterone levels measured. The results showed that IGF-1 levels were positively correlated with sexual function scores, including the total SHIM score, individual SHIM question scores, and the sexual domain of the EPIC questionnaire. However, no significant correlations were found between IGF-1 levels and Gleason score, IGF-1 and testosterone levels, or SHIM score and testosterone levels. These findings suggest that GH supplementation may help preserve erectile function, and the GH axis may play a role in maintaining sexual health in men.
You can read the abstract of this article at https://www.nature.com/articles/ijir201131
- El-Sakka AI, Lin CS, Chui RM, Dahiya R, Lue TF. Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model. Int J Impot Res. 1999;11:123–32. doi: 10.1038/sj.ijir.3900392.
Effects of diabetes on nitric oxide synthase and growth factor genes and protein expression in an animal model.
The study aimed to investigate the effects of diabetes on penile erection and the expression of nitric oxide synthase (NOS) and growth factors in a rat model. Diabetic rats were induced using Streptozotocin (STZ), and their erectile function was assessed after eight weeks. The diabetic group showed a significant decrease in NOS-containing nerve fibers in the penile nerves and had lower maximal intracavernosal pressure, indicating impaired erectile function. Molecular analysis revealed down-regulation of nNOS (large form), iNOS, and ER-beta mRNA expression, as well as decreased nNOS protein expression in the diabetic group. These findings suggest that diabetes may lead to molecular changes associated with erectile dysfunction, providing a basis for further exploration of the link between diabetes and impotence.
You can read the abstract of this article at https://www.nature.com/articles/3900392
- Soh J, Katsuyama M, Ushijima S, Mizutani Y, Kawauchi A, Yabe-Nishimura C, et al. Localization of increased insulin-like growth factor binding protein-3 in diabetic rat penis: Implications for erectile dysfunction. Urology. 2007;70:1019–23. doi: 10.1016/j.urology.2007.07.057.
Localization of increased insulin-like growth factor binding protein-3 in diabetic rat penis: Implications for erectile dysfunction.
The study aimed to understand the molecular mechanisms underlying diabetes-induced erectile dysfunction (ED) in rats. Using cDNA array analysis, the researchers identified changes in gene expression in the penis of diabetic rats. They found that the insulin-like growth factor binding protein 3 (IGFBP-3) gene expression significantly increased after 12 weeks of diabetes induction. Other genes, including ErbB3, cyclin D2, and hepatic neutral cholesteryl ester hydrolase precursor, showed marked decreases in expression. The increased levels of IGFBP-3 mRNA were observed as early as 2 weeks after hyperglycemia induction. The IGFBP-3 protein was found to be localized in specific areas of the penis, including the urethra, penile endothelium, and smooth muscle in the corpus cavernosum. The study suggests that the increased expression of IGFBP-3 during hyperglycemia might play a significant role in the development of erectile dysfunction.
You can read the full article at https://www.goldjournal.net/article/S0090-4295(07)01913-9/fulltext
- Pu XY, Zheng XG, Zhang Y, Xiao HJ, Xu ZP, Liu JM, et al. Higher expression of mRNA and protein of insulin-like growth factor binding protein-3 in old rat penile tissues: implications for erectile dysfunction. J Sex Med. 2011;8:2181–90. doi: 10.1111/j.1743-6109.2011.02318.x.
Higher expression of mRNA and protein of insulin-like growth factor binding protein-3 in old rat penile tissues: implications for erectile dysfunction.
The study aimed to investigate the expression of insulin-like growth factor binding protein-3 (IGFBP-3) in young and old rat penile tissues and its association with erectile dysfunction (ED) in aging. The researchers found that the mRNA and protein expression of IGFBP-3 were significantly higher in the penile tissues of aging rats compared to young rats. The increased IGFBP-3 protein was localized in specific areas of the penis. Additionally, the aging rats showed significant depletion of smooth muscle density in the corpus cavernosum and lower activity of nitric oxide synthase (NOS) and concentration of cGMP in penile tissue, which are related to erectile responses. The findings suggest that the increased expression of IGFBP-3 in old rats may contribute to erectile dysfunction in aging.
You can read the abstract of this article at You can read the abstract of this article at https://academic.oup.com/jsm/article-abstract/8/8/2181/6843913?redirectedFrom=fulltext&login=false
- Veldhuis JD, Erickson D, Wigham J, Weist S, Miles JM, Bowers CY. Gender, Sex-Steroid, and Secretagogue-Selective Recovery from Growth Hormone-Induced Feedback in Older Women and Men. The Journal of Clinical Endocrinology and Metabolism. 2011;96(8):2540-2547.
doi:10.1210/jc.2011-0298.Gender, Sex-Steroid, and Secretagogue-Selective Recovery from Growth Hormone-Induced Feedback in Older Women and Men.
The study aimed to investigate how gender, sex steroids, and peptidyl secretagogues influence the negative regulation of growth hormone (GH) secretion. The research involved 10 healthy postmenopausal women and 10 men, and they received treatments of either placebo or testosterone (men) / estradiol (women) in a double-blind crossover design. GH feedback inhibition was induced by intravenous GH pulses, and the recovery of feedback inhibition was measured during constant infusions of saline, GHRH, or GH-releasing peptide-2 (GHRP-2).
The study found that the total GH recovery during negative feedback depended on gender, sex hormone treatment, and the type of secretagogue. The nadir GH concentrations during feedback were influenced by sex hormone treatment but not gender. The peak GH escape from feedback was influenced by both treatment and gender. During feedback, GHRH or GHRP-2 enhanced both the nadir and peak GH levels. The interactions between gender, sex-steroid treatment, and secretagogue type contributed to the regulation of GH recovery during feedback.
Overall, the study revealed that gender, sex steroids, and secretagogues have distinct effects on the regulation of pulsatile GH secretion, adding complexity to the control of GH regulation.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3146792/
- Khorram O, Laughlin GA, Yen SS. Endocrine and metabolic effects of long-term administration of [Nle27]growth hormone-releasing hormone-(1-29)-NH2 in age-advanced men and women. J Clin Endocrinol Metab. 1997;82(5):1472-9.
Endocrine and metabolic effects of long-term administration of [Nle27]growth hormone-releasing hormone-(1-29)-NH2 in age-advanced men and women.
The study investigated the effects of a growth hormone-releasing hormone (GHRH) analog on age-advanced men and women over a 5-month period. The participants received nightly injections of the GHRH analog or placebo. The GHRH analog led to an increase in growth hormone (GH) release in both men and women. It also resulted in increased serum levels of insulin-like growth factor I (IGF-I) and IGF binding protein-3 (IGFBP-3), indicating activation of the somatotropic axis.
In men, the GHRH analog administration led to positive effects such as increased lean body mass, improved insulin sensitivity, enhanced general well-being, and libido. However, these positive effects were not observed to the same extent in women. Both genders experienced an increase in skin thickness, but no other significant changes in body composition or bone mineral density were observed.
The GHRH analog treatment was generally well-tolerated, with the only reported side effect being transient hyperlipidemia, which resolved by the end of the study.
Overall, the study suggests that GHRH analog administration induces anabolic effects that favor men more than women. Further research is needed to better understand the gender differences in response to GHRH analog treatment.You can read the abstract of this article at https://academic.oup.com/jcem/article/82/5/1472/2823341?login=false
- Nass R, Pezzoli SS, Oliveri MC, et al. Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial. Ann Intern Med. 2008;149(9):601-11.
Effects of an oral ghrelin mimetic on body composition and clinical outcomes in healthy older adults: a randomized trial.
The study aimed to investigate the effects of MK-677, an oral ghrelin mimetic, on growth hormone secretion and body composition in healthy older adults. The 2-year clinical trial included 65 participants aged 60 to 81 years. The participants were randomly assigned to receive either MK-677 (25 mg) or a placebo once daily.
The results showed that MK-677 administration significantly increased growth hormone and insulin-like growth factor I levels, bringing them closer to the levels found in healthy young adults. Over the course of 1 year, the MK-677 group experienced an increase in fat-free mass and body cell mass, while the placebo group showed a decrease in fat-free mass. Body weight also increased more in the MK-677 group compared to the placebo group.
Although abdominal visceral fat and total fat mass did not show significant differences between the groups, limb fat increased more in the MK-677 group. Some side effects of MK-677 included increased appetite (which subsided after a few months), mild lower-extremity edema, and muscle pain. Insulin sensitivity decreased, and fasting blood glucose levels increased in the MK-677 group.
The study concluded that MK-677 effectively enhanced growth hormone secretion and increased fat-free mass in healthy older adults without serious adverse effects. Long-term studies are recommended to assess its functional and economic impact in elderly individuals.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2757071/
- Catalina PF, Mallo F, Andrade MA, García-mayor RV, Diéguez C. Growth hormone (GH) response to GH-releasing peptide-6 in type 1 diabetic patients with exaggerated GH-releasing hormone-stimulated GH secretion. J Clin Endocrinol Metab. 1998;83(10):3663-7.
Growth hormone (GH) response to GH-releasing peptide-6 in type 1 diabetic patients with exaggerated GH-releasing hormone-stimulated GH secretion.
The study investigated the effects of GH-releasing peptide-6 (GHRP-6) alone and in combination with growth hormone-releasing hormone (GHRH) on growth hormone (GH) secretion in type 1 diabetes mellitus (DM 1). Six male patients with type 1 diabetes and six age-, sex-, and body mass index-matched control volunteers participated in the study.
The results showed that GH peak values were higher in DM 1 patients compared to control volunteers after administration of GHRH, GHRP-6, and the combination of GHRH plus GHRP-6. The combined administration of GHRP-6 and GHRH resulted in an additive GH response in diabetic patients. Serum insulin-like growth factor (IGF)-1 levels were lower in DM 1 patients than in normal subjects, while IGF-binding protein-3 levels were not significantly different between the two groups.
Overall, GHRP-6 was found to be a potent stimulus for GH secretion in type 1 diabetes. The combined administration of GHRP-6 and GHRH resulted in the most significant GH secretion in these patients. The study suggests that individuals with type 1 diabetes have a greater GH secretory capacity, possibly due to diminished IGF-1 negative feedback control on somatotroph responsiveness.You can read the full article at https://academic.oup.com/jcem/article/83/10/3663/2865731?login=false
- Jacks T, Smith R, Judith F, et al. MK-0677, a potent, novel, orally active growth hormone (GH) secretagogue: GH, insulin-like growth factor I, and other hormonal responses in beagles. Endocrinology. 1996;137(12):5284-9.
MK-0677, a potent, novel, orally active growth hormone (GH) secretagogue: GH, insulin-like growth factor I, and other hormonal responses in beagles.
MK-0677 is a novel orally active growth hormone (GH) secretagogue. The effects of MK-0677 were evaluated in beagles after both oral and intravenous (IV) single dose administrations. After oral administration, MK-0677 significantly increased peak GH concentrations in a dose-dependent manner, with the highest dose causing a 15.8-fold increase in GH levels. IGF-I levels were also significantly increased by 30% at 480 minutes after treatment. Cortisol levels were moderately increased.
After IV administration, MK-0677 caused an even larger increase in GH levels, with a 20.4-fold rise in peak GH concentrations compared to the control group. IGF-I levels were significantly elevated by 25% at 360 minutes after administration. Cortisol levels showed a modest increase.
Overall, MK-0677 is a potent GH secretagogue, inducing a rapid and substantial increase in GH levels after both oral and IV administration. Unlike other secretagogues, the GH levels remained elevated for an extended period, and this was associated with a significant increase in IGF-I levels. Cortisol levels were also increased but to a lesser extent than GH.You can read the abstract of this article at https://academic.oup.com/endo/article/137/12/5284/3037390?login=false
- Hickey GJ, Jacks TM, Schleim KD, et al. Repeat administration of the GH secretagogue MK-0677 increases and maintains elevated IGF-I levels in beagles. J Endocrinol. 1997;152(2):183-92.
Repeat administration of the GH secretagogue MK-0677 increases and maintains elevated IGF-I levels in beagles.
MK-0677 is an orally active growth hormone (GH) secretagogue that induces immediate and long-lasting increases in GH levels in dogs. With repeated oral administration, the GH response is decreased but remains above control levels. Serum insulin-like growth factor I (IGF-I) levels are significantly increased and are regulated by the dosage regimen. The cortisol response to MK-0677 is also initially increased but becomes attenuated with repeated treatment. Circulating IGF-I concentrations play a role in regulating the GH and cortisol responses to MK-0677. Chronic oral administration of MK-0677 maintains elevated GH and IGF-I levels, and the GH profile consists of episodic increases above control. The feedback loop involving IGF-I prevents hyperstimulation of the GH axis by MK-0677. Overall, MK-0677 shows promise as an orally active GH secretagogue that can sustain elevated IGF-I levels with chronic administration.
You can read the abstract of this article at https://joe.bioscientifica.com/view/journals/joe/152/2/joe_152_2_004.xml
- Schleim KD, Jacks T, Cunningham P, et al. Increases in circulating insulin-like growth factor I levels by the oral growth hormone secretagogue MK-0677 in the beagle are dependent upon pituitary mediation. Endocrinology. 1999;140(4):1552-8.
Increases in circulating insulin-like growth factor I levels by the oral growth hormone secretagogue MK-0677 in the beagle are dependent upon pituitary mediation.
The spiroindoline sulfonamide MK-0677 stimulates growth hormone (GH) secretion and increases serum insulin-like growth factor I (IGF-I) and cortisol levels. To investigate whether these increases are due to a direct action of MK-0677 or depend on the presence of an intact pituitary, hypophysectomy (removal of the pituitary gland) was performed in dogs. Before surgery, MK-0677 increased GH, IGF-I, and cortisol levels in both sham-operated and intact dogs. However, after hypophysectomy, MK-0677 did not increase GH, IGF-I, or cortisol levels, indicating that the effects of MK-0677 on these hormones are dependent on the presence of a functional pituitary gland. This study demonstrates that MK-0677 does not directly stimulate increases in IGF-I and cortisol levels but rather relies on an intact pituitary for its actions.
You can read the full article at https://academic.oup.com/endo/article/140/4/1552/2990303?login=false
- Teppala S, Shankar A. Association Between Serum IGF-1 and Diabetes Among U.S. Adults. Diabetes Care. 2010;33(10):2257-2259. doi:10.2337/dc10-0770.
Association Between Serum IGF-1 and Diabetes Among U.S. Adults. Diabetes Care.
Teppala and Shankar (2010) investigated the association between serum insulin-like growth factor 1 (IGF-1) levels and diabetes among U.S. adults. Their study revealed that there is an association between lower serum IGF-1 levels and the presence of diabetes in the studied population. This finding suggests that IGF-1 may have a role in the development or progression of diabetes. The study contributes to our understanding of the relationship between IGF-1 and diabetes in the context of adult population health.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2945170/
- Simpson HL, Umpleby AM, Russell-jones DL. Insulin-like growth factor-I and diabetes. A review. Growth Horm IGF Res. 1998;8(2):83-95.
Insulin-like growth factor-I and diabetes. A review.
The abstract discusses the potential therapeutic use of insulin-like growth factor I (IGF-I) in both insulin-dependent diabetes mellitus (IDDM) and non-insulin-dependent diabetes mellitus (NIDDM).
In IDDM, IGF-I has been shown to improve glycaemic control and reduce insulin requirements by replacing low circulating IGF-I levels. This leads to a reduction in growth hormone (GH) secretion, improving insulin sensitivity and glycaemic control. The mechanism may involve both reduced GH secretion and direct effects on insulin sensitivity. The effects of IGF-I treatment on IGF binding proteins are not clear, but with a restoration of a more normal GH/IGF-I axis, these proteins may return to normal concentrations, influencing glucose metabolism.
In NIDDM, the mechanism of IGF-I’s action remains unclear. At high doses, IGF-I may mimic insulin, but this results in acromegalic IGF-I levels and side effects. More promising data exists with low doses of IGF-I, where it improves insulin sensitivity via an unknown mechanism. This improvement might be mediated via the IGF-I receptor, cross-reactivity with the insulin receptor, or activation of hybrid receptors, but further investigation is needed.
IGF-I treatment appears to be effective in severe insulin-resistant states, and it may become an important therapeutic measure in the future. Long-term safety studies and short-term studies to understand the mechanisms of action are necessary. The limited availability of IGF-I for scientific and therapeutic research necessitates careful exploration of its potential as a therapeutic agent for various types of diabetes.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10987675/
- Friedrich N, Thuesen B, Jørgensen T, et al. The association between IGF-I and insulin resistance: a general population study in Danish adults. Diabetes Care. 2012;35(4):768-73.
The association between IGF-I and insulin resistance: a general population study in Danish adults.
The objective of this study was to assess the association between insulin-like growth factor I (IGF-I) levels and insulin resistance in a Danish general population. The study included 3,354 adults aged 19 to 72 years. Insulin resistance was estimated using the homeostasis model assessment of insulin resistance (HOMA-IR) index. Serum IGF-I levels were grouped into quintiles (Q1 to Q5), and linear and logistic regression analyses were performed.
The results showed a U-shaped association between IGF-I levels and insulin resistance. Both low IGF-I levels (Q1) and high IGF-I levels (Q5) were associated with higher odds of increased HOMA-IR values compared to subjects with intermediate (Q3) IGF-I levels. This association remained significant even after excluding subjects with type 2 diabetes and using an updated computer model for HOMA-IR (HOMA2-IR).
The study suggests that both low and high-normal levels of IGF-I are related to insulin resistance. However, the biological mechanism underlying this association needs further investigation for future risk stratification in relation to insulin resistance and type 2 diabetesYou can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3308317/
- Palta M, LeCaire T, Sadek-Badawi M, Herrera V, Danielson KK. The trajectory of IGF-1 across age and duration of type 1 diabetes. Diabetes/metabolism research and reviews. 2014;30(8):777-783. doi:10.1002/dmrr.2554.
The trajectory of IGF-1 across age and duration of type 1 diabetes.
The study examined the pattern of insulin-like growth factor 1 (IGF-1) levels in individuals with type 1 diabetes over time. Participants from an incident cohort study were followed for up to 18 years. It was found that IGF-1 levels are lower in type 1 diabetes compared to normative samples. The age-related pattern of IGF-1 is similar to normative samples, with a peak in adolescence and a slow decline after age 20. However, women with type 1 diabetes experienced a delayed adolescent peak in IGF-1 compared to men.
IGF-1 levels showed low to moderate tracking within individuals over time, indicating variability in its levels. Higher insulin dose, puberty, and female gender were associated with higher IGF-1 levels. After adjusting for these factors, IGF-1 declined rapidly during early diabetes duration. Lower HbA1c (a marker of glycemic control) was strongly related to higher IGF-1 levels during Tanner stages 1 and 2 (early and pre-adolescence).
The study suggests that IGF-1 levels are influenced by glycemic control, gender, and pubertal status in individuals with type 1 diabetes. However, the high variability within individuals may present challenges in investigating the associations between IGF-1 and long-term outcomes, possibly explaining contradictory findings in previous research.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4236234/
- Aguirre GA, De Ita JR, de la Garza RG, Castilla-Cortazar I. Insulin-like growth factor-1 deficiency and metabolic syndrome. Journal of Translational Medicine. 2016;14:3. doi:10.1186/s12967-015-0762-z.
Insulin-like growth factor-1 deficiency and metabolic syndrome.
This review focuses on the association between insulin-like growth factor 1 (IGF-1) deficiency and metabolic syndrome. The metabolic effects of IGF-1, as well as the clinical manifestations of metabolic syndrome (impaired lipid profile, insulin resistance, increased glucose levels, obesity, and cardiovascular disease), are discussed. The review explores the possibility of IGF-1 replacement therapy as a potential beneficial strategy for patients with metabolic syndrome. The review includes studies conducted in both animals and humans and highlights the actions of IGF-1 on metabolism and its implication in the development of metabolic syndrome. Multiple studies demonstrate a link between IGF-1 deficiency and disrupted lipid metabolism, cardiovascular disease, diabetes, and altered metabolic profiles in diabetic patients. The review proposes IGF-1 as a key hormone in the pathophysiology of metabolic syndrome, particularly in its involvement in carbohydrate and lipid metabolism. The data suggest that IGF-1 replacement therapy within physiological ranges may be a viable option for treating metabolic syndrome, but caution is emphasized not to exceed physiological levels.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4702316/
- Drogan D, Schulze MB, Boeing H, Pischon T. Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor-Binding Protein 3 in Relation to the Risk of Type 2 Diabetes Mellitus: Results From the EPIC-Potsdam Study. Am J Epidemiol. 2016;183(6):553-60.
Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor-Binding Protein 3 in Relation to the Risk of Type 2 Diabetes Mellitus: Results From the EPIC-Potsdam Study.
This study investigated the relationship between insulin-like growth factor 1 (IGF-1), insulin-like growth factor-binding protein 3 (IGFBP-3), and their molar ratio in relation to the incidence of type 2 diabetes mellitus (T2DM). The study was conducted within the European Prospective Investigation Into Cancer and Nutrition-Potsdam Study. A subcohort of individuals without T2DM at the time of blood sampling (n = 2,269) and 776 individuals with incident T2DM identified between 1994 and 2005 were included in the analysis.
The results showed that higher levels of IGFBP-3 were associated with an increased risk of T2DM, while IGF-1 levels were not significantly associated with T2DM risk in the general study population. However, when considering the ratio of IGF-1 to IGFBP-3, a lower ratio was related to an increased risk of T2DM. These associations were observed even after adjusting for IGF-1 concentrations, suggesting that IGFBP-3 levels may independently influence the risk of T2DM. The study did not find a significant association between total IGF-1 concentrations and T2DM risk.You can read the full article at https://academic.oup.com/aje/article/183/6/553/2195447?login=false
- Suda K, Matsumoto R, Fukuoka H, et al. The influence of type 2 diabetes on serum GH and IGF-I levels in hospitalized Japanese patients. Growth Horm IGF Res. 2016;29:4-10.
The influence of type 2 diabetes on serum GH and IGF-I levels in hospitalized Japanese patients.
The study aimed to investigate the influence of type 2 diabetes mellitus (T2DM) on serum growth hormone (GH) and insulin-like growth factor type 1 (IGF-I) levels in Japanese patients. They analyzed 315 hospitalized Japanese patients with T2DM, who had relatively lower body mass index (BMI) compared to previous studies. The study found that there was no overall correlation between GH or IGF-I levels and fasting plasma glucose (FPG) or hemoglobin A1c (HbA1c). However, when patients were grouped based on their FPG and HbA1c levels, those with higher FPG (≥200mg/dL) and higher HbA1c (≥12%) had significantly lower IGF-I levels. The analysis also revealed a positive correlation between fasting C-peptide levels (a marker of insulin secretion) and IGF-I levels. The study suggests that impaired insulin secretion may be a mechanism underlying the decreased IGF-I levels in patients with uncontrolled T2DM, and these factors should be considered when diagnosing other conditions related to GH and IGF-I levels in such patients.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1096637416300119?via%3Dihub
- Færch L, Juul A, Pedersen-Bjergaard U, Thorsteinsson B. Association of IGF1 with glycemic control and occurrence of severe hypoglycemia in patients with type 1 diabetes mellitus. Endocrine Connections. 2012;1(1):31-36. doi:10.1530/EC-12-0012.
Association of IGF1 with glycemic control and occurrence of severe hypoglycemia in patients with type 1 diabetes mellitus.
The study aimed to investigate whether insulin-like growth factor 1 (IGF1) levels are associated with severe hypoglycemic events in patients with type 1 diabetes and whether glycemic control influences IGF1 concentration. They analyzed 228 outpatients with type 1 diabetes and measured serum total IGF1 levels at the beginning of the study. The study found that IGF1 levels were negatively associated with glycemic control (as measured by HbA1c), suggesting that lower IGF1 levels were linked to poorer blood sugar control. However, IGF1 levels were not associated with the occurrence of severe hypoglycemia, mild symptomatic hypoglycemia, biochemical hypoglycemia, or hypoglycemia awareness in these patients. In summary, while IGF1 levels are influenced by glycemic control, they do not seem to play a significant role in predicting the occurrence of hypoglycemic events or hypoglycemia awareness in type 1 diabetes patients.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3682234/
- Chantelau E. Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy. Br J Ophthalmol. 1998;82(7):725-30.
Evidence that upregulation of serum IGF-1 concentration can trigger acceleration of diabetic retinopathy.
The study aimed to observe the effects of reducing chronic hyperglycemia in poorly controlled adult insulin-dependent (type 1) diabetic patients without Mauriac’s syndrome, a condition characterized by severe insulin deficiency in adolescent patients. Four patients with early micro-angiopathy were studied prospectively. Intensive insulin therapy was used to lower hyperglycemia from high levels to a more controlled range. The results showed that as hyperglycemia was reduced, serum insulin-like growth factor 1 (IGF-1) levels increased significantly by 70-220%. However, despite the improvement in proteinuria and symptomatic neuropathy, the retinopathy (damage to the blood vessels in the retina) progressed from a mild to severe non-proliferative stage with maculopathy and proliferative stage in one patient. This deterioration led to the initiation of laser coagulation therapy to prevent sight-threatening complications. The study suggests that the upregulation of serum IGF-1 may play a role in the progression of retinopathy in poorly controlled type 1 diabetes patients when hyperglycemia is rapidly reduced.
You can read the abstract of this article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1722687/
- Fridlyand LE, Tamarina NA, Schally AV, Philipson LH. Growth Hormone-Releasing Hormone in Diabetes. Frontiers in Endocrinology. 2016;7:129. doi:10.3389/fendo.2016.00129.
Growth Hormone-Releasing Hormone in Diabetes. Frontiers in Endocrinology.
This review discusses the role of Growth Hormone-Releasing Hormone (GHRH) and its receptor (GHRHR) in pancreatic beta-cells, which are responsible for insulin secretion. GHRH analogs have been found to increase and preserve insulin secretion in isolated pancreatic islets, making them potential candidates for diabetes treatment. The review explores the similarities between the signaling pathways activated by GHRHR in the pituitary gland and pancreatic beta-cells, and how GHRHR can interact with glucose and other substances to stimulate insulin secretion. The authors propose that novel GHRHR agonists could improve glucose metabolism in type 2 diabetes by protecting the function and survival of beta-cells. Additionally, these GHRH agonists may have benefits in wound healing and cardioprotection, potentially addressing certain diabetic complications. The review suggests that modulators of GHRHR activity hold promise for new therapeutic approaches in diabetes and its related complications.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5056186/
- Johansen PB, Segev Y, Landau D, Phillip M, Flyvbjerg A. Growth hormone (GH) hypersecretion and GH receptor resistance in streptozotocin diabetic mice in response to a GH secretagogue. Exp Diabesity Res. 2003;4(2):73-81.
Growth hormone (GH) hypersecretion and GH receptor resistance in streptozotocin diabetic mice in response to a GH secretagogue.
The study investigated the growth hormone (GH) and insulin-like growth factor I (IGF-I) axis in diabetic and nondiabetic female mice. They were given a GH secretagogue called ipamorelin or saline. After 14 days, blood samples were taken before and after the injection. The results showed that diabetic mice had higher GH levels compared to nondiabetic mice after ipamorelin injection. However, the IGF-I levels were lower in diabetic mice and only increased in nondiabetic mice. The study suggests that these diabetic mice show similar hormonal changes seen in type 1 diabetes in humans, making them a good model for studying diabetes-related hormone disruptions.
You can read the abstract of this article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2478601/
- Clark RG, Thomas GB, Mortensen DL, et al. Growth hormone secretagogues stimulate the hypothalamic-pituitary-adrenal axis and are diabetogenic in the Zucker diabetic fatty rat. Endocrinology. 1997;138(10):4316-23.
Growth hormone secretagogues stimulate the hypothalamic-pituitary-adrenal axis and are diabetogenic in the Zucker diabetic fatty rat.
The study investigated the effects of GH secretagogues on the release of corticosterone (a type of adrenal steroid hormone) in rats. The researchers found that certain GH secretagogues stimulated the release of both GH and corticosterone to varying degrees. They tested a potent GH secretagogue analog in obese diabetic rats and found that it increased weight gain and raised blood glucose levels, similar to human GH treatment. However, it also had adverse effects on serum triglycerides and fat depot weights, which were not observed with human GH treatment alone. These effects might be due to the additional activation of the hypothalamic-pituitary-adrenal axis by the secretagogue. The study suggests that caution is needed when administering GH secretagogues with cortisol-releasing activity to individuals with obesity or diabetes, as it may worsen the diabetic state.
You can read the full article at https://academic.oup.com/endo/article/138/10/4316/2987894?login=false
- Holly JM, Amiel SA, Sandhu RR, Rees LH, Wass JA. The role of growth hormone in diabetes mellitus. J Endocrinol. 1988;118(3):353-64.
The role of growth hormone in diabetes mellitus.
The insulin and growth hormone (GH)/insulin-like growth factor-I (IGF-I) systems are interconnected in various ways. GH is a hormone that increases in response to low blood sugar and has actions that raise blood sugar levels and cause insulin resistance. IGF-I and its receptor are structurally and functionally similar to insulin and its receptor. Insulin can regulate IGF-I production by acting on the GH receptor or at a post-receptor site, while IGF-I may influence the pancreatic insulin response to glucose. Poorly controlled diabetes can lead to disruptions in the GH/IGF-I axis, with raised GH levels and normal or low IGF-I levels. Altered levels of IGF-binding proteins are also found. These disruptions could be caused by various interactions at different levels, and the exact mechanisms are not fully understood. The abnormal GH/IGF-I levels can worsen the metabolic problems associated with diabetes, and they may also play a role in the development of long-term complications, such as proliferative retinopathy.
You can read the abstract of this article at https://joe.bioscientifica.com/view/journals/joe/118/3/joe_118_3_002.xml
- Nazaimoon WM, Ng ML, Khalid BA. Fasting growth hormone levels in diabetes mellitus. Ann Acad Med Singap. 1993;22(6):861-3.
Fasting growth hormone levels in diabetes mellitus.
In this study, researchers measured the fasting levels of growth hormone (GH) in different groups of diabetic patients and compared them to age-matched healthy individuals. They found that children with insulin-dependent diabetes mellitus (IDDM) had lower fasting GH levels compared to healthy children. However, in adults aged 18-44 and 45-76 years with IDDM, the fasting GH levels were higher than in age-matched healthy individuals and those with non-insulin-dependent diabetes mellitus (NIDDM). Among IDDM patients aged 18-44 years, there were significant correlations between fasting GH levels and markers of glycemic control, such as glycated hemoglobin and fasting blood sugar levels.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8129344/
- Wurzburger MI, Prelevic GM, Sonksen PH, Balint-peric L. Growth hormone levels in patients with type 1 diabetes are age related. Diabet Med. 1993;10(2):159-61.
Growth hormone levels in patients with type 1 diabetes are age related.
The researchers studied the effects of age on growth hormone (GH) levels in three groups: patients with Type 1 diabetes (with or without beta cell activity) and healthy individuals. They divided the participants into three age groups: A (21-30 years), B (31-40 years), and C (41-50 years). The results showed that GH levels decreased significantly with age in patients with Type 1 diabetes. Among all age groups, patients without beta cell activity had higher GH levels compared to those with preserved beta cell activity and healthy individuals. Additionally, patients with preserved beta cell activity had higher GH levels than healthy individuals in all age groups.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8458193/
- Kim S-H, Park M-J. Effects of growth hormone on glucose metabolism and insulin resistance in human. Annals of Pediatric Endocrinology & Metabolism. 2017;22(3):145-152. doi:10.6065/apem.2017.22.3.145.
Effects of growth hormone on glucose metabolism and insulin resistance in human.
In general, growth hormone (GH) has different effects on glucose and lipid metabolism compared to insulin. GH doesn’t directly affect total glucose turnover under normal conditions. However, it may decrease glucose oxidation and suppress muscle uptake of glucose, directing glucose into a non-oxidative pathway, possibly for glycogen storage. GH’s actions are more prominent during the postprandial or fasting state since its secretion is inhibited in the fed state.
In conditions of excess GH (e.g., acromegaly, poorly controlled type 1 diabetes, or high-dose GH treatment), GH can have diabetogenic effects. It leads to increased endogenous glucose production, reduced muscle glucose uptake, and elevated blood glucose levels. In patients with intact beta-cell function, the body tries to counterbalance these changes with hyperinsulinemia, which can have long-term implications on cardiovascular health.
In GH-deficient patients, there is limited research on GH’s effects on glucose metabolism. These patients tend to be more sensitive to insulin, and when given GH after being deprived of it, they may initially exhibit insulin-like effects. The relationship between GH and insulin-like growth factors in this context is not fully understood.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/1806481/
- Luger A, Mattsson AF, Koltowska-häggström M, et al. Incidence of diabetes mellitus and evolution of glucose parameters in growth hormone-deficient subjects during growth hormone replacement therapy: a long-term observational study. Diabetes Care.
2012;35(1):57-62.Incidence of diabetes mellitus and evolution of glucose parameters in growth hormone-deficient subjects during growth hormone replacement therapy: a long-term observational study.
The study aimed to investigate the incidence of diabetes in adult patients with growth hormone (GH) deficiency during GH replacement therapy (GHRT) and the effect of GHRT on fasting plasma glucose concentrations and HbA1c.
The study analyzed 5,143 GH-deficient patients over an average period of 3.9 years. Among them, 523 patients developed diabetes during the observation period. Those who developed diabetes were older and had higher BMI, waist circumference, triglyceride concentrations, and blood pressure, as well as lower HDL-cholesterol concentrations compared to those who did not develop diabetes.
The incidence of diabetes during GHRT was 2.6 per 100 patient-years and was higher than the reference rates from different populations. The risk of developing diabetes increased with BMI and decreased with the duration of GHRT but did not show a significant association with GH dose or IGF-I SDS.
In patients who did not develop diabetes, plasma glucose concentrations and HbA1c levels increased gradually over time during GHRT.
The study suggests that diabetes incidence may be higher in GH-deficient patients receiving GHRT, especially those with an adverse risk profile at baseline. Therefore, careful monitoring of glucose homeostasis parameters is essential for these patients during GHRT.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3241307/
- Del guercio MJ, Di natale B, Gargantini L, Garlaschi C, Chiumello G. Effect of somatostatin on blood sugar, plasma growth hormone, and glucagon levels in diabetic children. Diabetes. 1976;25(7):550-3.
Effect of somatostatin on blood sugar, plasma growth hormone, and glucagon levels in diabetic children.
In this study, nine children with insulin-dependent diabetes were given a somatostatin injection. When given rapidly, somatostatin did not cause significant changes in blood glucose, growth hormone, or glucagon levels. However, with a prolonged infusion, there was a significant reduction in blood glucose and plasma glucagon levels. The study suggests that somatostatin lowers blood glucose by inhibiting glucagon secretion. Although somatostatin is not suitable for diabetes therapy, the researchers believe that a similar substance with a more prolonged and specific action on glucagon could be valuable for treating diabetes mellitus in the future.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/1278605/
- Bonfig W, Molz K, Woelfle J, et al. Metabolic safety of growth hormone in type 1 diabetes and idiopathic growth hormone deficiency. J Pediatr. 2013;163(4):1095-8.e4.
Metabolic safety of growth hormone in type 1 diabetes and idiopathic growth hormone deficiency.
The study aimed to assess the effects of growth hormone (GH) treatment in children with type 1 diabetes and GH deficiency. They compared 37 patients with both conditions to a larger group of 48,856 adolescents with type 1 diabetes only. The results showed that children with GH deficiency and type 1 diabetes required a higher daily insulin dose (1.0 IU/kg/day) compared to the control group (0.85 IU/kg/day). Additionally, their height was lower, but there was no significant difference in hemoglobin A1c levels between the two groups. The study suggests that GH treatment may increase insulin requirements in these patients, but with proper insulin adjustments, metabolic control remains stable during GH treatment.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/23746867/
- Jeffcoate W. Growth hormone therapy and its relationship to insulin resistance, glucose intolerance and diabetes mellitus: a review of recent evidence. Drug Saf. 2002;25(3):199-212.
Jeffcoate W. Growth hormone therapy and its relationship to insulin resistance, glucose intolerance and diabetes mellitus: a review of recent evidence.
The use of growth hormone (GH) in adults with GH deficiency is recommended to reduce the excess cardiovascular risk associated with hypopituitarism. This excess risk may be linked to insulin resistance, which is common in hypopituitarism. However, it’s unclear if this insulin resistance is solely caused by GH deficiency. GH administration itself may also negatively impact insulin sensitivity, potentially worsening cardiovascular risk. Studies have shown that GH treatment in adults with GH deficiency can lead to a small deterioration in insulin sensitivity, with increases in fasting blood glucose and serum insulin levels. Though the changes are generally small, even minor reductions in insulin sensitivity could increase cardiovascular risk. More large-scale controlled trials are needed to establish the efficacy and safety of GH treatment in adults.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11945115/
- Nambam B, Schatz D. Growth hormone and insulin-like growth factor-I axis in type 1 diabetes. Growth Horm IGF Res. 2018;38:49-52.
Growth hormone and insulin-like growth factor-I axis in type 1 diabetes.
The relationship between type 1 diabetes (T1D) and the growth hormone (GH), insulin-like growth factor-I (IGF-I), and IGF binding protein-3 (IGFBP-3) axis is not fully understood. In T1D patients, there is often GH resistance with low IGF-I levels due to insufficient insulin in the portal system and lack of GH receptor upregulation. The impact of this dysregulated GH/IGF-I axis on height and chronic complications in children and adolescents with T1D is still unclear and has conflicting reports. This review discusses the interactions between the GH/IGF-I axis and T1D pathology, as well as how T1D may influence this hormonal axis.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1096637417301144?via%3Dihub
- Attanasio AF, Jung H, Mo D, et al. Prevalence and incidence of diabetes mellitus in adult patients on growth hormone replacement for growth hormone deficiency: a surveillance database analysis. J Clin Endocrinol Metab. 2011;96(7):2255-61.
Prevalence and incidence of diabetes mellitus in adult patients on growth hormone replacement for growth hormone deficiency: a surveillance database analysis.
The study aimed to assess the prevalence and incidence of diabetes mellitus (DM) in adult growth hormone (GH)-treated patients with GH deficiency. The study analyzed data from the Hypopituitary Control and Complications Study (HypoCCS) surveillance database, which included patients from the United States and Europe. The overall prevalence of DM was 8.2%, with a higher prevalence in the United States (11.3%) compared to Europe (5.7%). The overall incidence of DM was 9.7 cases per 1000 patient-years, with higher incidence rates in the United States (14.1 per 1000 patient-years) than in Europe (7.0 per 1000 patient-years). Obesity (BMI > 30 kg/m(2)) was more common in the United States than in Europe and was associated with a higher incidence of DM. However, there was no evidence to suggest that GH replacement therapy increased the risk of developing DM in adult GH-deficient patients. The study also found that GH dose was not correlated with DM incidence. Overall, the analysis indicated that GH treatment did not lead to a higher incidence of DM in the studied population.
You can read the full article at https://academic.oup.com/jcem/article/96/7/2255/2834501?login=false
- Bonfig W, Lindberg A, Carlsson M, et al. Efficacy of Growth Hormone Treatment in Children with Type 1 Diabetes Mellitus and Growth Hormone Deficiency-An Analysis of KIGS Data. J Pediatr. 2018;198:260-264.
Efficacy of Growth Hormone Treatment in Children with Type 1 Diabetes Mellitus and Growth Hormone Deficiency-An Analysis of KIGS Data.
The study aimed to analyze the first-year growth response and growth hormone (GH) dosage in prepubertal patients with both type 1 diabetes mellitus (T1DM) and growth hormone deficiency (GHD). The researchers enrolled 24 prepubertal patients who had developed T1DM before GHD and compared them with 15,024 prepubertal patients with GHD but without T1DM (controls). They found that patients with T1DM and GHD had similar characteristics and GH dosages compared to the GHD-alone group. The first-year catch-up growth was comparable between the two patient groups, and the height standard deviation score (SDS) of children with T1DM and GHD improved over one year of GH treatment. The study concluded that short-term response to GH therapy was similar in children with T1DM and GHD compared to those with GHD alone. Therefore, having T1DM does not compromise the GH response, and GH treatment is safe and effective in children with both T1DM and GHD.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/29656861/
- Granado M, García-cáceres C, Tuda M, Frago LM, Chowen JA, Argente J. Insulin and growth hormone-releasing peptide-6 (GHRP-6) have differential beneficial effects on cell turnover in the pituitary, hypothalamus and cerebellum of streptozotocin (STZ)-induced
diabetic rats. Mol Cell Endocrinol. 2011;337(1-2):101-13.Insulin and growth hormone-releasing peptide-6 (GHRP-6) have differential beneficial effects on cell turnover in the pituitary, hypothalamus and cerebellum of streptozotocin (STZ)-induced diabetic rats.
Poorly controlled type 1 diabetes can lead to hormonal imbalances and increased cell death in various tissues, such as the pituitary, hypothalamus, and cerebellum. Lactotrophs in the pituitary, astrocytes in the hypothalamus, and cerebellum are the cell populations most affected. Insulin treatment can delay but not fully prevent diabetic complications. Ghrelin and growth hormone (GH) secretagogues are known to prevent cell death and regulate glucose levels. To test the combined hormonal treatment’s potential benefits, the researchers studied the effects of insulin and GH-releasing peptide 6 (GHRP-6) on diabetes-induced cell death in rats.
They induced diabetes in adult male Wistar rats and divided them into four groups receiving daily injections of saline, GHRP-6, insulin, or a combination of insulin and GHRP-6 for eight weeks. They also had a control group of non-diabetic rats receiving saline injections. Diabetes increased cell death in the pituitary, hypothalamus, and cerebellum. Insulin treatment prevented apoptosis and the decline in certain hormone mRNA levels in the pituitary. In the hypothalamus, neither insulin nor GHRP-6 reduced cell death, but the combined insulin+GHRP-6 treatment prevented the decrease in a specific protein level. In the cerebellum, insulin increased a protein level, while the combined insulin+GHRP-6 treatment reduced cell death.
In conclusion, insulin and GHRP-6 had tissue-specific effects in diabetic rats, and the combined treatment showed some synergistic effects. Insulin alone was not fully effective in preventing some of the diabetes-induced changes in the central nervous system.You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0303720711000967?via%3Dihub
- Bailey CJ, Wilkes LC, Flatt PR, Conlon JM, Buchanan KD. Effects of growth hormone-releasing hormone on the secretion of islet hormones and on glucose homeostasis in lean and genetically obese-diabetic (ob/ob) mice and normal rats. J Endocrinol (1989)
123(1):19–24.10.1677/joe.0.1230019.Effects of growth hormone-releasing hormone on the secretion of islet hormones and on glucose homeostasis in lean and genetically obese-diabetic (ob/ob) mice and normal rats.
The researchers investigated the effects of synthetic human growth hormone-releasing hormone (hGHRH-40) on the endocrine pancreas and glucose regulation in lean and genetically obese-diabetic (ob/ob) mice and normal rats. In isolated islets from lean mice, hGHRH-40 increased insulin release at certain concentrations and also affected the release of other hormones like pancreatic polypeptide, glucagon, and somatostatin. However, in vivo administration of hGHRH-40 to lean and ob/ob mice did not significantly change glucose and insulin levels during fasting or after glucose challenge.
In rats, intravenous injection of hGHRH-40 increased insulin levels in the hepatic portal vein, but it did not affect plasma glucose levels. The study suggests that hGHRH-40 may have different effects on insulin and other pancreatic hormones in isolated islets compared to the in vivo setting. Further research is needed to fully understand the role of hGHRH-40 in glucose homeostasis.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/2572664/
- Ludwig B, Ziegler CG, Schally AV, Richter C, Steffen A, Jabs N, et al. Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets. Proc Natl Acad Sci U S A (2010) 107(28):12623–8.10.1073/pnas.1005098107.
Agonist of growth hormone-releasing hormone as a potential effector for survival and proliferation of pancreatic islets.
The researchers aimed to find therapeutic strategies to increase the mass of pancreatic islet cells for transplantation. They focused on the growth hormone-releasing hormone receptor variant-1 (GHRH-R SV-1) and its potential role in stimulating beta-cell growth. They found that GHRH-R SV-1 is expressed in rat insulinoma cells and both rat and human pancreatic islets.
In vitro studies using rat insulinoma cells showed that a GHRH agonist called JI-36 significantly increased cell proliferation and reduced cell apoptosis. Treatment with JI-36 also increased islet size and glucose-stimulated insulin secretion in isolated rat islets. Ultrastructural analysis of cells treated with JI-36 showed increased metabolic activity.
In animal experiments, transplantation of rat islets treated with JI-36 under the kidney capsule of diabetic mice led to earlier and more consistent normalization of blood glucose levels compared to untreated islets. Insulin response to a glucose tolerance test in mice receiving JI-36-treated islets was comparable to that of normal healthy mice.
These findings suggest that GHRH agonists may be a promising pharmacological therapy for promoting islet graft growth and proliferation in diabetic patients undergoing islet cell transplantation.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2906543/
- Ludwig B, Rotem A, Schmid J, Weir GC, Colton CK, Brendel MD, et al. Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist. Proc Natl Acad Sci U S A (2012) 109(13):5022–7.10.1073/pnas.1201868109.
Improvement of islet function in a bioartificial pancreas by enhanced oxygen supply and growth hormone releasing hormone agonist.
Islet transplantation is a potential treatment for type 1 diabetes patients aiming to achieve stable glycemic control and prevent complications. However, the shortage of donor organs, graft function decline, and the need for immunosuppression limit its applicability. To address these issues, researchers developed a macrochamber for islet transplantation. The implantable device provides controlled oxygen supply and immune protection for donor islets. When implanted in diabetic rodents, it normalized blood glucose levels for up to 3 months. The addition of a growth hormone-releasing hormone (GHRH) agonist, JI-36, improved graft function, reducing the required islet mass for metabolic control. This approach may offer a promising avenue for diabetes therapy and future xenotransplantation strategies.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3324017/
- Zhang X, Cui T, He J, Wang H, Cai R, Popovics P, et al. Beneficial effects of growth hormone-releasing hormone agonists on rat INS-1 cells and on streptozotocin-induced NOD/SCID mice. Proc Natl Acad Sci U S A (2015) 112(44):13651–6.10.1073/pnas.1518540112.
Beneficial effects of growth hormone-releasing hormone agonists on rat INS-1 cells and on streptozotocin-induced NOD/SCID mice
Growth hormone-releasing hormone (GHRH) agonists have been found to promote the growth and function of pancreatic beta cells. In this study, newly developed GHRH agonists were tested on rat pancreatic beta-cell line (INS-1) and islets. The treatment with GHRH agonists increased cell proliferation, insulin expression, insulin-like growth factor-1 (IGF1) expression, and insulin secretion in response to glucose. The agonists activated specific signaling pathways (ERK and AKT) in INS-1 cells. In rat islets, the GHRH agonist also increased cell proliferation and insulin expression. In vivo administration of the GHRH agonist in diabetic mice significantly improved diabetes severity and enhanced islet engraftment, leading to normoglycemia and increased insulin and IGF1 levels. This study highlights the potential therapeutic use of GHRH agonists in diabetes treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4640729/
- Schubert U, Schmid J, Lehmann S, Zhang XY, Morawietz H, Block NL, et al. Transplantation of pancreatic islets to adrenal gland is promoted by agonists of growth-hormone-releasing hormone. Proc Natl Acad Sci U S A (2013) 110(6):2288–93.10.1073/pnas.1221505110.
Transplantation of pancreatic islets to adrenal gland is promoted by agonists of growth-hormone-releasing hormone.
In this study, the researchers explored an alternative approach to improve pancreatic islet transplantation for diabetes treatment. They used a potent agonist of growth hormone-releasing hormone (GHRH) to precondition the islets before transplantation, aiming to enhance their viability and function. Additionally, they investigated the adrenal gland as an alternative site for islet engraftment, which offers unique advantages like high oxygen levels and local immunosuppressive properties. The GHRH agonist showed positive effects on cell viability and proliferation in insulinoma cells and isolated rat islets. It also promoted viability in adrenal beta-cell cocultures. Transplanting rat islets into diabetic mice at the intraadrenal site led to a rapid decrease in blood glucose levels, achieving normoglycemia. This study suggests that GHRH agonists and the adrenal gland may be promising for improving islet transplantation in diabetes treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3568317/
- Schmid J, Ludwig B, Schally AV, Steffen A, Ziegler CG, Block NL, et al. Modulation of pancreatic islets-stress axis by hypothalamic releasing hormones and 11beta-hydroxysteroid dehydrogenase. Proc Natl Acad Sci U S A (2011) 108(33):13722–7.10.1073/pnas.1110965108.
Modulation of pancreatic islets-stress axis by hypothalamic releasing hormones and 11beta-hydroxysteroid dehydrogenase.
In this study, the researchers investigated the expression and effects of corticotropin-releasing hormone (CRH) and growth hormone-releasing hormone (GHRH) in the endocrine pancreas. They found that pancreatic islets in rats and humans express mRNA for CRH, CRH-receptor type 1 (CRHR1), and protein for CRHR1. Activation of CRHR1 and GHRH-receptor led to increased cell proliferation and reduced cell apoptosis. CRH also stimulated insulin production and release in rat islet and insulinoma cells. Additionally, the study revealed the expression of mRNA for 11β-hydroxysteroid dehydrogenase (11β-HSD)-1 and 11β-HSD-2, enzymes that regulate glucocorticoids in pancreatic islets and insulinoma cells. Stimulation of CRHR1 and GHRH-receptor affected the insulinoma cell metabolism by down-regulating 11β-HSD-1 and up-regulating 11β-HSD-2, altering the glucocorticoid balance towards the inactive form. These findings suggest that CRH and GHRH receptor systems in the endocrine pancreas may have therapeutic potential for diabetes treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3158163/
- Svensson J, Jansson JO, Ottosson M, et al. Treatment of obese subjects with the oral growth hormone secretagogue MK-677 affects serum concentrations of several lipoproteins, but not lipoprotein(a). J Clin Endocrinol Metab. 1999;84(6):2028-33.
Treatment of obese subjects with the oral growth hormone secretagogue MK-677 affects serum concentrations of several lipoproteins, but not lipoprotein(a).
In this study, the researchers investigated the effects of the oral growth hormone secretagogue MK-677 on lipoproteins in healthy obese males. The study involved 24 obese males who were randomized and treated with either MK-677 or a placebo daily for 8 weeks. MK-677 treatment resulted in increased levels of serum apolipoprotein A-I and E (apoA-I and apoE) at 2 weeks, but these levels did not change at the end of the study. Total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels were not significantly affected by MK-677 treatment. However, high-density lipoprotein cholesterol (HDL-C) was increased at 2 weeks of treatment, but not at 8 weeks. The ratio of LDL-C to HDL-C was reduced after 8 weeks of MK-677 treatment. The size of LDL particles was decreased at 2 weeks but was unchanged compared to baseline at 8 weeks. Serum triglyceride levels were increased at 2 weeks but not at 8 weeks. Overall, MK-677 treatment had some transient effects on circulating lipoproteins, and it did not significantly affect lipoprotein(a) concentrations at the given dose and administration protocol in this study.
You can read the full article at https://academic.oup.com/jcem/article/84/6/2028/2864618?login=false
- Hersch EC, Merriam GR. Growth hormone (GH)–releasing hormone and GH secretagogues in normal aging: Fountain of Youth or Pool of Tantalus? Clinical Interventions in Aging. 2008;3(1):121-129.
Growth hormone (GH)–releasing hormone and GH secretagogues in normal aging: Fountain of Youth or Pool of Tantalus?
In adulthood, growth hormone (GH) has important metabolic functions beyond promoting linear growth in childhood. Adult GH deficiency (AGHD) is a distinct condition, and GH replacement therapy in AGHD can improve body composition, strength, aerobic capacity, and mood, and may reduce the risk of vascular disease. With aging, GH secretion decreases significantly, leading to age-related changes similar to partial AGHD. This suggests that replacing or stimulating GH in normal aging could have similar benefits, such as reducing muscle loss and frailty, allowing older adults to maintain independence. However, while GH studies have shown body composition effects similar to AGHD, functional changes have been inconsistent, and older adults are more sensitive to GH side effects. Preliminary reports suggest potential improvements in cognition, but the overall balance of benefits and risks of GH supplementation in normal aging is still uncertain.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2544358/
- Sigalos JT, Pastuszak AW. The Safety and Efficacy of Growth Hormone Secretagogues. Sex Med Rev. 2018;6(1):45-53.
The Safety and Efficacy of Growth Hormone Secretagogues. Sex Med Rev.
The review discusses growth hormone secretagogues (GHSs), which include GH-releasing peptides and the drug ibutamoren mesylate. GHSs promote pulsatile release of GH subject to negative feedback, preventing excessive GH levels. They have shown potential benefits in improving growth velocity in children, stimulating appetite, increasing lean mass, reducing bone turnover, and improving sleep. Few long-term studies on GHSs’ safety and efficacy exist, but they are generally well-tolerated, with some concerns about blood glucose levels. Further research is needed to understand their long-term impact on human anatomy and physiology and to evaluate safety in diverse clinical scenarios, including potential cancer risks.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5632578/
- O’neal DN, Hew FL, Best JD, Alford F. The effect of 24 months recombinant human growth hormone (rh-GH) on LDL cholesterol, triglyceride-rich lipoproteins and apo [a] in hypopituitary adults previously treated with conventional replacement therapy. Growth Horm IGF Res. 1999;9(3):165-73.
The effect of 24 months recombinant human growth hormone (rh-GH) on LDL cholesterol, triglyceride-rich lipoproteins and apo [a] in hypopituitary adults previously treated with conventional replacement therapy.
In simple terms, this study looked at the effects of 24 months of recombinant growth hormone (rh-GH) treatment on the cholesterol and lipoprotein levels in adults with growth hormone deficiency. They found that the treatment led to a decrease in total cholesterol and LDL cholesterol (commonly known as “bad” cholesterol), but did not significantly change triglyceride levels or HDL cholesterol (“good” cholesterol) levels. There was also an increase in a specific protein called apo [a], which is associated with cardiovascular disease risk. However, the overall impact of GH therapy on cardiovascular health is still not fully understood and may depend on the dosage used.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10502452/
- Leese GP, Wallymahmed M, Vanheyningen C, Tames F, Wieringa G, Macfarlane IA. HDL-cholesterol reductions associated with adult growth hormone replacement. Clin Endocrinol (Oxf). 1998;49(5):673-7.
HDL-cholesterol reductions associated with adult growth hormone replacement.
In simple terms, this study looked at the effects of human growth hormone (hGH) replacement on cholesterol and other lipids in patients with growth hormone deficiency. The participants were divided into two groups, with one group receiving hGH treatment and the other receiving a placebo for 6 months. The results showed that hGH treatment increased a hormone called IGF-1 but did not significantly change the levels of cholesterol and other lipids compared to the placebo group at 6 months. However, there was a decrease in HDL-cholesterol (the “good” cholesterol) levels in the hGH-treated group, which remained lower even after 12 months of treatment. The study suggests that more research is needed to understand the effects of hGH on cholesterol levels.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/10197085/
- Gács G, Romics L. Effect of growth hormone on serum lipoproteins in growth hormone deficiency. Exp Clin Endocrinol. 1987;90(2):227-31.
Effect of growth hormone on serum lipoproteins in growth hormone deficiency.
In this study, 13 children with growth hormone deficiency were treated with growth hormone. Before treatment, they had moderately higher levels of total cholesterol compared to normal. However, after growth hormone administration, their total cholesterol levels decreased significantly. This decrease was mainly due to a reduction in HDL-cholesterol during the first week of treatment and a decrease in LDL-cholesterol after one month. LDL-cholesterol levels returned to normal after one month of treatment. Additionally, the triglyceride level in the blood increased significantly during the first week of growth hormone treatment but returned to normal after one month. This increase was attributed to the rise in VLDL-triglycerides. There was no difference in cholesterol and triglyceride levels between growth hormone deficient children with normal or low plasma thyroxine concentrations.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/3428364/
- Leonsson M, Oscarsson J, Bosaeus I, et al. Growth hormone (GH) therapy in GH-deficient adults influences the response to a dietary load of cholesterol and saturated fat in terms of cholesterol synthesis, but not serum low density lipoprotein cholesterol levels. J Clin Endocrinol Metab. 1999;84(4):1296-303.
Growth hormone (GH) therapy in GH-deficient adults influences the response to a dietary load of cholesterol and saturated fat in terms of cholesterol synthesis, but not serum low density lipoprotein cholesterol levels.
In this study, GH-deficient adults were given a diet rich in cholesterol and saturated fat, with and without GH therapy. The diet increased serum cholesterol levels, LDL cholesterol, apolipoprotein B, and apolipoprotein A1, both with and without GH therapy. GH therapy did not affect the dietary effects on these lipoprotein levels. However, GH therapy did increase serum lipoprotein(a) levels.
Interestingly, GH therapy led to a lesser increase in a marker for cholesterol synthesis (total delta7-lathosterol/cholesterol ratio) compared to the diet without GH therapy. However, GH treatment did not have a significant impact on bile acid synthesis or sterol absorption.
In summary, GH therapy counteracted the increase in cholesterol synthesis induced by the high-fat diet without affecting other lipid parameters or the dietary effects on serum lipoprotein levels.You can read the full article at https://academic.oup.com/jcem/article/84/4/1296/2864237?login=false
- Zhu WF, Tang SJ, Shen Z, Wang YM, Liang L. Growth hormone reverses dyslipidemia in adult offspring after maternal undernutrition. Sci Rep. 2017;7(1):6038.
Growth hormone reverses dyslipidemia in adult offspring after maternal undernutrition.
In this study, researchers investigated the effects of growth hormone (GH) treatment on dyslipidemia in adult rats that were small for gestational age (SGA) due to a restricted intrauterine environment during pregnancy. SGA rats exhibited decreased body weight and length and increased serum triglycerides compared to rats of appropriate size for gestational age (AGA). The SGA rats also showed down-regulation of a metabolic regulator called AMPK-α1 and up-regulation of genes involved in lipid synthesis.
When GH was administered to the SGA rats during their adolescent period, it led to positive effects on lipid metabolism. GH treatment restored body weight and length and normalized serum triglyceride levels by reversing the expression of AMPK-α1 and its targeted genes involved in lipid synthesis. This improvement was achieved by increasing histone acetylation at the promoter of AMPK-α1, which likely contributed to the positive effects on lipid metabolism in the SGA rats during adulthood. Overall, the study suggests that short-term GH treatment during adolescence may help reverse dyslipidemia in adult SGA individuals.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5519748/
- Chen M, Gan D, Luo Y, et al. Effect of recombinant human growth hormone therapy on blood lipid and carotid intima-media thickness in children with growth hormone deficiency. Pediatr Res. 2018;83(5):954-960.
Effect of recombinant human growth hormone therapy on blood lipid and carotid intima-media thickness in children with growth hormone deficiency
In this study, researchers investigated the effect of different doses of recombinant human growth hormone (rhGH) therapy on blood lipid levels and carotid intima-media thickness (cIMT) in Chinese children with growth hormone deficiency (GHD). They enrolled 90 children, including 60 with isolated GHD and 30 healthy children. The GHD children were divided into two groups: Group A received a lower dose of rhGH (0.23 mg/kg/week) and Group B received a higher dose (0.35 mg/kg/week) for 12 months.
Before the treatment, the GHD children in both groups had higher levels of total cholesterol (TC), triglycerides (TG), low-density lipoprotein-cholesterol (LDL-C), and cIMT, and lower levels of high-density lipoprotein-cholesterol (HDL-C) compared to healthy children. After the 12-month rhGH therapy, the blood lipid levels and cIMT significantly improved in the GHD children, with the higher-dose group (Group B) showing even better results.
Overall, the study concluded that rhGH replacement therapy in GHD children can lead to improvements in blood lipid profiles and carotid intima-media thickness, and higher doses of rhGH therapy may have superior effects.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6023698/
- Tonstad S, Sundt E, Ose L, et al. The effect of growth hormone on low-density lipoprotein cholesterol and lipoprotein (a) levels in familial hypercholesterolemia. Metab Clin Exp. 1996;45(11):1415-21.
The effect of growth hormone on low-density lipoprotein cholesterol and lipoprotein (a) levels in familial hypercholesterolemia.
In this study, researchers investigated the effect of growth hormone on low-density lipoprotein (LDL) cholesterol levels in individuals with familial hypercholesterolemia (FH), a condition characterized by high LDL cholesterol levels. Thirty-one men with FH participated in a randomized, double-blind, placebo-controlled study. They discontinued all lipid-lowering drugs and followed a stable diet before the study.
The participants were divided into two groups: one group received recombinant growth hormone daily for 12 weeks, and the other group received a placebo. The researchers found that the growth hormone treatment had a minimal effect on LDL cholesterol levels and did not significantly lower them as expected. However, they observed a significant increase in lipoprotein(a) [Lp(a)] levels in the growth hormone group, which could counteract the potential benefits on LDL cholesterol.
In conclusion, the study suggests that growth hormone is likely not useful as adjunctive therapy for reducing LDL cholesterol levels in individuals with familial hypercholesterolemia, as the increase in Lp(a) levels may offset its LDL-lowering effects.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8931648/
- Gács G, Romics L. Effect of growth hormone on serum lipoproteins in growth hormone deficiency. Exp Clin Endocrinol. 1987;90(2):227-31.
Effect of growth hormone on serum lipoproteins in growth hormone deficiency.
In this study, the researchers investigated the effect of growth hormone treatment on plasma lipoproteins in 13 children with growth hormone deficiency. Before the treatment, the children had moderately higher total cholesterol levels compared to normal levels. However, after the administration of growth hormone, the total cholesterol level decreased significantly. This reduction in total cholesterol was primarily due to a decrease in HDL-cholesterol levels during the first week and a decrease in LDL-cholesterol levels after one month of treatment. After one month, the LDL-cholesterol levels returned to normal.
Additionally, the study found that the plasma triglyceride level increased significantly during the first week of growth hormone treatment and then returned to normal levels after one month. This increase in triglycerides was attributed to the rise in VLDL-triglyceride levels.
Interestingly, the researchers observed no significant differences in cholesterol and triglyceride concentrations between growth hormone deficient children with normal or subnormal plasma thyroxine concentrations.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/3428364/
- Beentjes JA, Van tol A, Sluiter WJ, Dullaart RP. Effect of growth hormone replacement therapy on plasma lecithin:cholesterol acyltransferase and lipid transfer protein activities in growth hormone-deficient adults. J Lipid Res. 2000;41(6):925-32.
Effect of growth hormone replacement therapy on plasma lecithin:cholesterol acyltransferase and lipid transfer protein activities in growth hormone-deficient adults.
In this study, researchers investigated the effects of growth hormone (GH) replacement on factors involved in high-density lipoprotein (HDL) metabolism in GH-deficient adults. The participants were divided into three groups: placebo, low-dose GH, and high-dose GH for a period of 6 months, followed by an open extension study with high-dose GH for another 6 months.
After 6 months of GH treatment, the combined data showed a decrease in very low-density lipoprotein (VLDL) and low-density lipoprotein (LDL) cholesterol, as well as apolipoprotein B levels, while HDL cholesterol and HDL cholesteryl ester increased. Prolonged treatment showed similar effects.
Plasma lecithin:cholesterol acyltransferase (LCAT) and cholesteryl ester transfer protein (CETP) activities, as well as cholesterol esterification and cholesteryl ester transfer, decreased significantly after 12 months of GH treatment. However, phospholipid transfer protein (PLTP) activity did not significantly change.
The researchers concluded that GH replacement therapy improves the lipoprotein profile in GH-deficient adults. Chronic GH replacement lowers plasma LCAT and CETP activities, contributing to a decrease in cholesterol esterification and cholesteryl ester transfer. These effects may have implications for HDL metabolism and reverse cholesterol transport.You can read the full article at https://www.jlr.org/article/S0022-2275(20)32034-4/fulltext
- Xu L, Xu C, Yu C, et al. Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study. PLoS ONE. 2012;7(8):e44136.
Association between serum growth hormone levels and nonalcoholic fatty liver disease: a cross-sectional study
In this cross-sectional study, researchers investigated the association between growth hormone (GH) levels and nonalcoholic fatty liver disease (NAFLD), a condition linked to the metabolic syndrome. They analyzed data from 1,667 subjects with NAFLD and 5,479 control subjects.
The study found that individuals with NAFLD had significantly lower levels of serum GH compared to the control group. Those with lower GH levels were more likely to have NAFLD and the metabolic syndrome. The statistical analysis indicated that GH levels were significantly associated with the risk of developing NAFLD.
In conclusion, the study revealed a significant association between lower serum GH levels and the presence of NAFLD. This suggests that GH may play a role in the development or progression of NAFLD and its related metabolic abnormalities.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3432067/
- Succurro E, Arturi F, Grembiale A, et al. Positive association between plasma IGF1 and high-density lipoprotein cholesterol levels in adult nondiabetic subjects. Eur J Endocrinol. 2010;163(1):75-80.
Positive association between plasma IGF1 and high-density lipoprotein cholesterol levels in adult nondiabetic subjects.
In this cross-sectional study, researchers aimed to understand if insulin-like growth factor 1 (IGF1) plays a role in modulating high-density lipoprotein cholesterol (HDL-C) independently of other factors. They analyzed data from 1,004 nondiabetic participants aged 20-69 years.
The results showed that IGF1 levels were positively correlated with HDL-C and negatively correlated with body mass index (BMI), waist circumference, blood pressure, triglyceride levels, fasting insulin, and homeostasis model assessment (HOMA). After adjusting for age and gender, individuals with lower IGF1 levels (<125 ng/ml) had a higher risk of having low HDL-C compared to those with higher IGF1 levels (>186 ng/ml).
Even after accounting for other factors such as BMI, waist circumference, total cholesterol, triglyceride levels, and HOMA index, the association between low IGF1 levels and increased risk of low HDL-C remained significant. In a more comprehensive model, including multiple variables, IGF1 levels were independently associated with HDL-C, suggesting that IGF1 may be an independent modulator for HDL-C in nondiabetic individuals.
These findings provide evidence that IGF1 might play a role in regulating HDL-C levels and could be a relevant factor in the relationship between IGF1 and cardiovascular risk.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/20356932/
- Burchardt P, Tabaczewski P, Goździcka-józefiak A, et al. Association between insulin like growth factor-1 and lipoprotein metabolism in stable angina patients on statin therapy: a pilot study. Kardiol Pol. 2012;70(10):1017-22.
Association between insulin like growth factor-1 and lipoprotein metabolism in stable angina patients on statin therapy: a pilot study.
The study aimed to investigate the association between systemic levels of insulin-like growth factor-1 (IGF-1), IGF-1 binding protein-3 (IGFBP3), and selected lipid metabolism parameters in patients with coronary artery disease (CAD) who were on prolonged statin therapy to control serum lipids.
The study involved 140 patients who underwent coronary angiography. The researchers measured various lipid parameters, including LDL- and HDL-cholesterol, triglycerides, total cholesterol, apoB-100, apoA1, Lp(a), as well as IGF-1 and IGFBP3 levels. They also assessed the oxidation products of proteins and lipids.
The results showed that in patients with LDL target up to 100 mg/dL and using statins, as well as in the entire study population, IGF-1 and IGFBP3 levels were associated with protein oxidation products and Lp(a). Additionally, in the entire group, IGF-1 was associated with triglycerides and LDL cholesterol. Further analysis revealed that IGF-1 and IGFBP3 in the group with LDL up to 100 mg/dL and the entire group were associated with protein oxidation products, Lp(a), and a quantitative arteriosclerosis scale (Gensini score). These findings supported the previous observation that patients with advanced coronary atherosclerosis had higher systemic levels of IGF-1.
In summary, the study found associations between IGF-1, IGFBP3, and lipid metabolism parameters in patients with coronary artery disease, particularly in those using statins to control LDL cholesterol levels. The results suggest that IGF-1 may play a role in the pathogenesis of CAD and could be a potential marker for disease severity.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/23080092/
- Liang S, Hu Y, Liu C, Qi J, Li G. Low insulin-like growth factor 1 is associated with low high-density lipoprotein cholesterol and metabolic syndrome in Chinese nondiabetic obese children and adolescents: a cross-sectional study. Lipids in Health and Disease. 2016;15:112. doi:10.1186/s12944-016-0275-7.
Low insulin-like growth factor 1 is associated with low high-density lipoprotein cholesterol and metabolic syndrome in Chinese nondiabetic obese children and adolescents: a cross-sectional study.
The study aimed to investigate the relationship between insulin-like growth factor 1 (IGF-1), high-density lipoprotein cholesterol (HDL-C), and metabolic syndrome in Chinese nondiabetic obese children and adolescents.
The study included 120 obese Chinese children and adolescents and 120 healthy individuals. Obese subjects had lower IGF-1SDS (standard deviation score) and higher height SDS compared to the control group. Among the obese participants, 18.3% had low HDL-C levels (<1.03 mmol/L). Obese individuals with low HDL-C had significantly lower IGF-1SDS. Multivariate logistic regression analysis showed that IGF-1SDS was significantly associated with low HDL-C levels, even after adjusting for age, gender, pubertal status, BMI SDS (standard deviation score), blood pressure, insulin resistance, and other lipid markers. IGF-1SDS was also positively correlated with serum HDL-C levels in the study population. Furthermore, higher IGF-1SDS was associated with a decreased probability of metabolic syndrome and hypertriglyceridemia but did not show a significant correlation with hypertension. In conclusion, obese Chinese children and adolescents had lower IGF-1SDS and taller stature compared to healthy individuals. Low levels of IGF-1SDS were independently associated with low HDL-C levels in this population, as well as a decreased risk of metabolic syndrome and hypertriglyceridemia. The findings suggest that IGF-1 may play a role in lipid metabolism and cardiovascular risk in obese youths.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4919831/
- Eggert ML, Wallaschofski H, Grotevendt A, et al. Cross-sectional and longitudinal relation of IGF1 and IGF-binding protein 3 with lipid metabolism. Eur J Endocrinol. 2014;171(1):9-19.
Cross-sectional and longitudinal relation of IGF1 and IGF-binding protein 3 with lipid metabolism.
The study aimed to investigate the associations between insulin-like growth factor 1 (IGF1) and insulin-like growth factor-binding protein 3 (IGFBP3) serum levels with lipid metabolism in a large-scale population-based study.
Cross-sectional analysis of 2935 participants revealed that higher IGF1 and IGFBP3 levels were associated with increased total cholesterol and LDL cholesterol and decreased HDL cholesterol in both men and women. Additionally, IGFBP3 levels showed a positive relationship with triglycerides. Overall, IGFBP3 levels had a stronger association with lipids compared to IGF1.
However, in longitudinal analysis, no significant influence of baseline IGF1 or IGFBP3 levels on changes in lipid levels over time was observed. Only a potential link between IGFBP3 and incidentally elevated triglycerides in women was close to reaching statistical significance.
Based on these findings, IGF1 and IGFBP3 are considered risk markers rather than risk factors for lipid metabolism alterations. Further research is needed to understand the underlying mechanisms of the association between the growth hormone/IGF axis and lipid metabolism.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/24743393/
- Arturi F, Succurro E, Procopio C, et al. Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I. J Clin Endocrinol Metab. 2011;96(10):E1640-4.
Nonalcoholic fatty liver disease is associated with low circulating levels of insulin-like growth factor-I.
The study aimed to investigate the relationship between nonalcoholic fatty liver disease (NAFLD) and insulin-like growth factor I (IGF-I) levels. The researchers found that individuals with NAFLD had lower IGF-I levels compared to control subjects. Additionally, they hypothesized that insulin resistance induced by free fatty acids might impair the insulin-induced increase of GH receptor (GHR) expression in human liver cells.
In a cross-sectional study involving 503 nondiabetic Caucasians, those with NAFLD showed higher body mass index, waist circumference, fasting insulin, triglycerides, homeostasis model assessment index, liver enzymes, and lower high-density lipoprotein cholesterol after adjusting for age and gender. Furthermore, in vitro experiments with human hepatoma cells exposed to palmitate (a type of free fatty acid) showed a dose-dependent reduction in the insulin-induced increase of GHR expression.
These findings suggest that NAFLD is associated with lower IGF-I levels and that hepatic insulin resistance may play a role in this relationship by affecting the synthesis of hepatic IGF-I stimulated by growth hormone.You can read the full article at https://academic.oup.com/jcem/article/96/10/E1640/2834786?login=false
- Izumi S, Ribeiro-filho FF, Carneiro G, Togeiro SM, Tufik S, Zanella MT. IGF-1 Levels are Inversely Associated With Metabolic Syndrome in Obstructive Sleep Apnea. J Clin Sleep Med. 2016;12(4):487-93.
IGF-1 Levels are Inversely Associated With Metabolic Syndrome in Obstructive Sleep Apnea.
The study aimed to examine insulin-like growth factor-1 (IGF-1) production and its association with the metabolic syndrome (MS) in men with obstructive sleep apnea (OSA). The researchers evaluated 47 overweight and obese men with suspected OSA and classified them into three groups based on the severity of OSA. They measured IGF-1 levels and assessed the somatotropic axis function. MS was diagnosed according to specific guidelines.
The results showed that men with moderate-severe OSA had lower IGF-1 levels compared to those without OSA. IGF-1 levels were negatively correlated with body mass index, waist circumference, apnea-hypopnea index (AHI), and sleep duration with oxygen saturation below 90%, while positively correlated with average and minimum oxygen saturation. In multivariable analysis, minimum oxygen saturation was a predictor of low IGF-1 levels.
The proportion of patients with MS was higher in the moderate-severe OSA group compared to the other groups. Additionally, in the lowest tertile of IGF-1 levels, a higher percentage of patients had MS. Hemoglobin A1c (HbA1c), a marker of long-term blood sugar control, was negatively correlated with minimum oxygen saturation and IGF-1 levels. However, in multivariable analysis, only IGF-1 levels were a predictor of HbA1c levels.
In conclusion, OSA is associated with reduced IGF-1 levels, and alterations in IGF-1 levels in OSA may contribute to a higher prevalence of MSYou can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4795274/
- Wang WC, Chiu YF, Chung RH, et al. Gene Is Associated With Triglyceride Levels In Subjects With Family History Of Hypertension From The SAPPHIRe And TWB Projects. Int J Med Sci. 2018;15(10):1035-1042.
Gene Is Associated With Triglyceride Levels In Subjects With Family History Of Hypertension From The SAPPHIRe And TWB Projects.
The study investigated the association between the Insulin-like growth factor 1 (IGF1) gene and triglyceride (TG) levels in two independent samples from Taiwan. The first sample included 954 siblings from 397 families, and the second sample consisted of 13,193 unrelated subjects. The researchers found that a specific genetic variant (rs978458) of the IGF1 gene was associated with lower TG levels under a recessive genetic model. This association was observed in subjects with a family history of hypertension but not in those without such a family history. The results were replicated in both samples, indicating that IGF1 is linked to TG levels in individuals with a family history of hypertension in Taiwan.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6036157/
- Cappel M, Mauger D, Thiboutot D. Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women. ArchDermatol.2005;141(3):333-8.
Correlation between serum levels of insulin-like growth factor 1, dehydroepiandrosterone sulfate, and dihydrotestosterone and acne lesion counts in adult women.
The study aimed to investigate the relationship between insulin-like growth factor 1 (IGF-1) and androgen levels with the presence and severity of acne in adult men and women. The researchers conducted a case-control study with 34 participants (8 women and 8 men with clinical acne, 10 women and 8 men without clinical acne) and measured their serum hormone levels.
The results showed that in women, dehydroepiandrosterone (DHEAS), dihydrotestosterone (DHT), and IGF-1 levels correlated positively with acne lesion counts. Androstenedione and DHEAS levels correlated with acne lesion counts in men. While the age-adjusted mean serum levels of IGF-1 were higher in women with clinical acne, this difference did not reach statistical significance. There was no significant difference in IGF-1 levels in men based on the presence of clinical acne.
In women with clinical acne, IGF-1 correlated with DHT, and in men with clinical acne, IGF-1 correlated with DHEAS and androstenedione. The effects of androgens on increased acne lesion counts in both men and women depended on the influence of IGF-1.
The study concludes that increased IGF-1 levels, along with androgens, may play a role in influencing acne in adult men and women. IGF-1 seems to have a stronger effect on acne in women, while androgens may have a greater impact on acne in men. However, the hormones are interrelated in both men and women, possibly due to reciprocal effects on hormone production.You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/15781674/
- Sjögren K, Wallenius K, Liu JL, et al. Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism. Diabetes. 2001;50(7):1539-45.
Liver-derived IGF-I is of importance for normal carbohydrate and lipid metabolism.
The study focused on the role of liver-derived insulin-like growth factor 1 (IGF-I) in postnatal body growth and its effects on carbohydrate and lipid metabolism. Mice with their liver IGF-I gene inactivated at 24 days of age were used to investigate this. The results showed that liver-derived IGF-I is not required for postnatal body growth. However, mice lacking liver-derived IGF-I exhibited increased levels of leptin in the blood at 3 months of age and reduced fat mass at 13 months of age. They also developed marked hyperinsulinemia, indicating compensated insulin resistance, and had higher levels of serum cholesterol. The study concludes that liver-derived IGF-I plays an important role in regulating carbohydrate and lipid metabolism.
You can read the full article at https://diabetesjournals.org/diabetes/article/50/7/1539/11743/Liver-Derived-IGF-I-is-of-Importance-for-Normal
- Dichtel LE, Corey KE, Misdraji J, et al. The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clinical and Translational Gastroenterology. 2017;8(1):e217-. doi:10.1038/ctg.2016.72.
The Association Between IGF-1 Levels and the Histologic Severity of Nonalcoholic Fatty Liver Disease. Clinical and Translational Gastroenterology.
The study aimed to investigate the association between serum insulin-like growth factor-1 (IGF-1) levels and nonalcoholic fatty liver disease (NAFLD) in obese individuals with liver biopsies. The research involved 142 subjects undergoing NAFLD work-up or bariatric surgery. The results showed that lower serum IGF-1 levels were linked to increased liver fat accumulation, inflammation, and fibrosis. These associations remained significant even after controlling for age, body mass index (BMI), and diabetes, and were still observed when individuals with cirrhosis were excluded. Steatosis (fatty liver) was not significantly associated with IGF-1 levels. The study suggests that IGF-1 may play a role in the development and progression of NAFLD, and further investigation is needed to explore its potential therapeutic implications.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5288606/
- Romero MJ, Lucas R, Dou H, et al. Role of growth hormone-releasing hormone in dyslipidemia associated with experimental type 1 diabetes. Proc Natl Acad Sci USA. 2016;113(7):1895-900.
Role of growth hormone-releasing hormone in dyslipidemia associated with experimental type 1 diabetes.
In patients with type 1 diabetes (T1D), dyslipidemia associated with triglyceride-rich lipoproteins (TRLs) contributes to cardiovascular and chronic kidney disease risk. Elevated levels of growth hormone (GH) in T1D worsen hyperglycemia and dyslipidemia. Researchers investigated the role of the growth hormone-releasing hormone (GHRH) receptor in a rat model of T1D. They found increased GHRH receptor expression in the small intestine, involved in TRL synthesis. Treating T1D rats with a GHRH antagonist called MIA-602 reduced TRL levels, markers of renal injury, and improved blood vessel function without affecting GH levels. Additionally, T1D rats had impaired GLP-1 signaling, which regulates TRL synthesis. MIA-602 treatment normalized GLP-1 levels and decreased ApoB-48 secretion, a key component of TRLs, from intestinal cells. These findings suggest that blocking GHRH signaling in T1D could improve GLP-1 function in the intestine, leading to reduced TRLs and improved kidney and vascular health.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4763773/
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








