Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Health Benefits of Valproic Acid
- Key Takeaways
- What is Valproic Acid?
- Valproic Acid Mechanism of Action
- Chemical Structure of Valproic Acid
- Research on Valproic Acid
- Valproic Acid Side Effects
- Depakene
- Valproic Acid Dosage
- Depakene vs Depakote
- Valproic Acid Dosage for Bipolar
- Valproic Acid Brand Name
- Valproic Acid Uses
- What Happens When Valproic Acid Level is Low?
- Valproate vs Valproic Acid
- Valproic Acid Drug Class
- FAQ
- Reference
Table of Contents
- Potential Health Benefits of Valproic Acid
- Key Takeaways
- What is Valproic Acid?
- Valproic Acid Mechanism of Action
- Chemical Structure of Valproic Acid
- Research on Valproic Acid
- Valproic Acid Side Effects
- Depakene
- Valproic Acid Dosage
- Depakene vs Depakote
- Valproic Acid Dosage for Bipolar
- Valproic Acid Brand Name
- Valproic Acid Uses
- What Happens When Valproic Acid Level is Low?
- Valproate vs Valproic Acid
- Valproic Acid Drug Class
- FAQ
- Reference
Potential Health Benefits of Valproic Acid
Valproic acid benefits include its effectiveness in treating seizure disorders, bipolar disorder, and preventing migraines. It also acts as a mood stabilizer, helping to manage manic episodes and reduce the frequency of seizures.
- Reduces seizures [1-12]
- Fights hair loss [13-20]
- Treats migraines [21-33]
- Improves mood [34-58]
- Improves cognitive function [59-70]
Key Takeaways
- Seizure Control: Valproic acid is commonly used to treat epilepsy by preventing and controlling seizures.
- Mood Stabilization: It serves as a mood stabilizer for individuals with bipolar disorder, helping to manage manic episodes.
- Migraine Prevention: Valproic acid is also prescribed to prevent migraines in individuals prone to frequent episodes.
- Liver and Side Effect Monitoring: Regular monitoring of liver function and potential side effects, such as gastrointestinal upset and drowsiness, is essential.
- Suicidal Thoughts Risk: Like many mood-stabilizing medications, valproic acid may be associated with an increased risk of suicidal thoughts, requiring careful monitoring by healthcare providers.
What is Valproic Acid?
Valproic acid is an anticonvulsant medication that is primarily used to treat epilepsy, bipolar disorder, neuropathic pain, migraine, attention deficit hyperactivity disorder (ADHD), and other psychological disorders. It exerts its therapeutic effect by affecting certain brain chemicals or neurotransmitters, thus improving overall brain health.
Valproic Acid Mechanism of Action
Valproic acid achieves its desired effects through the following important mechanisms:
- It increases the levels of gamma-aminobutyric acid (GABA), a neurotransmitter that reduces overstimulation in the brain.
- It restores communication between neurons (nerve cells) by reducing overactivity and blocking certain pathways in the brain.
- It protects new brain cells by blocking certain enzymes responsible for gene alteration.
Chemical Structure of Valproic Acid
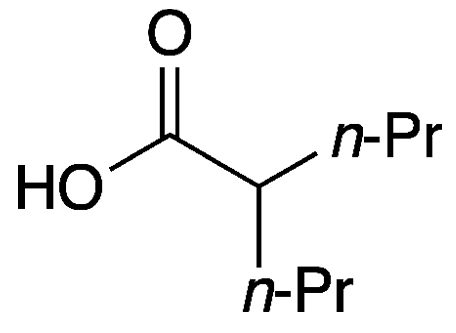
Research on Valproic Acid
A. Reduces Seizures
Valproic acid reduces seizures by increasing gamma-aminobutyric acid (GABA) levels, an inhibitory neurotransmitter that calms excessive neuronal activity in the brain. It also blocks voltage-gated sodium channels and T-type calcium channels, stabilizing neuronal membranes and preventing abnormal electrical discharges that cause seizures. These combined mechanisms make valproic acid effective for various types of seizures, including generalized and focal epilepsy.
- In epileptic children, valproic acid exhibited similar effectiveness to ethosuximide, the current gold standard for treating epileptic seizures. [1]
- In patients with glioblastoma multiforme, a deadly brain cancer, valproic acid administration reduced seizure attacks and resulted in a 2-month longer survival. [2]
- In late-onset post-stroke epilepsy patients, valproic acid administration was associated with better seizure control. [3]
- In patients with chronic seizures, valproic acid was found to be effective at reducing the incidence of attacks. [4]
- In patients with idiopathic generalized epilepsies, valproic acid provided superior control of epileptic seizures and was associated with fewer hospitalizations and emergency department visits. [5]
- In patients with hemorrhagic stroke (caused by blood vessel rupture), valproic acid reduced the occurrence of seizures in the early period. [6]
- In patients with uncontrolled seizures of a generalized or partial type, valproic acid administration was associated with a significant reduction in seizure frequency ranging from 25 to 100%. [7]
- In patients with poorly controlled complex partial seizures, valproic acid treatment significantly reduced seizures by 50%. [8]
- Valproic acid was found to be similar in efficacy to other antiepileptic drugs such as carbamazepine, phenytoin, phenobarbital, and ethosuximide in the treatment of generalized, partial, and absence seizures. [9-10]
- In patients with juvenile myoclonic epilepsy, valproic acid significantly reduced the incidence of seizure attacks. [11]
- In patients with generalized tonic-clonic seizures, valproic acid administration alone demonstrated greater efficacy at reducing attacks. [12]
B. Fights Hair Loss

Valproic acid may help fight hair loss by promoting hair follicle regeneration and stimulating hair growth. Research suggests that it activates the Wnt/β-catenin signaling pathway, which plays a key role in hair follicle development and the hair growth cycle. Topical application of valproic acid has shown potential in increasing hair density and thickness, particularly in conditions like androgenetic alopecia. However, more clinical studies are needed to confirm its long-term effectiveness and safety for treating hair loss.
- In male patients with androgenetic alopecia, a type of hair loss common in both genders, valproic acid spray treatment for 24 weeks significantly increased total hair count compared to placebo spray. [13]
- In participants with hair baldness, treatment with 7.2% valproic acid spray on the scalp twice a day (morning and evening) for 24 weeks increased linear hair growth rate and final hair density. [14]
- In patients with alopecia, administration of valproic acid induced hair growth in bald areas. [15]
- A study found that implantation of valproic acid-encapsulated dissolving microneedles stimulated hair follicle regrowth. [16]
- A cell study also found that valproic acid has the ability to inhibit glycogen synthase kinase 3ß and activation of the Wnt/βcatenin pathway, which in turn induces hair regeneration and hair growth phase (anagen). [17]
- In male patients with moderate androgenic alopecia, valproic acid spray treatment for 24 weeks significantly increased total hair count without any adverse side effects. [18]
- Treatment of human dermal papilla cells (hair follicles) with valproic acid induced hair regeneration. [19]
- Administration of valproic acid in patients with alopecia increased hair count by boosting the production of biotin, a protein that promotes hair growth. [20]
C. Treats Migraines

Valproic acid treats migraines by stabilizing electrical activity in the brain and enhancing gamma-aminobutyric acid (GABA) levels, an inhibitory neurotransmitter that helps calm nerve activity. It also reduces the release of excitatory neurotransmitters and modulates ion channels, which helps prevent the abnormal brain signaling that triggers migraines. This dual action decreases the frequency and severity of migraine attacks.
- In patients with chronic (recurring) migraines, valproic acid administration at daily doses of 500 to 600 mg reduced migraine frequency. [21]
- Larger clinical trials confirmed the benefit of valproic acid on migraine attacks and the FDA approved the use of this drug. [22-23]
- Valproic acid administration in patients with recurrent migraines was associated with better clinical response. [24]
- A study found that a low dose of valproic acid (10 mg/kg) for 6 months may be helpful in preventing migraine in pediatric patients. [25]
- In patients with severe migraine headaches, administration of intravenous valproic acid seems to be safe and rapidly effective at alleviating symptoms. [26]
- A study found that valproic acid treats migraine by increasing brain GABA levels. [27]
- In adult patients with episodic migraine, valproic acid reduced headache frequency and was reasonably well tolerated. [28]
- In patients with refractory migraine, a type of migraine not relieved or prevented by acute migraine therapies, valproic acid administration at a dose of 1,250 mg daily reduced headache severity. [29]
- In migraine sufferers, valproic acid administration at a dose of 100–600 mg/day was consistent in alleviating symptoms. [30]
- Administration of 400 mg valproic acid twice daily for 8 weeks in patients with migraine was effective in reducing the frequency, severity, and duration of the attacks. [31]
- Administration of 70 to 120 mg/L of valproic acid in patients with migraine headaches was associated with significantly lesser functional restriction and symptoms. [32]
- Valproic acid appeared to be equivalent in efficacy and safety to topiramate, an anti-epileptic drug, in reducing the duration, monthly frequency, and intensity of headaches. [33]
D. Improves Mood

Valproic acid improves mood by stabilizing neurotransmitter activity in the brain, particularly by increasing gamma-aminobutyric acid (GABA) levels, which has a calming effect on the nervous system. This helps regulate mood swings and reduce symptoms of mania in conditions like bipolar disorder. Additionally, valproic acid modulates glutamate, a neurotransmitter linked to excitatory signals, further balancing mood and preventing extreme emotional states.
- In patients with neurosis, a mild mental illness characterized by stress, depression, anxiety, and obsessive behavior, valproic acid significantly improved scores in various tests assessing symptoms such as the Clinical Global Impression Scale for panic severity, the Hamilton Psychiatric Rating Scale for Anxiety, and the panic factor of the SCL-90. [34-35]
- In patients with panic disorder who failed to respond to conventional treatment, valproic acid reduced symptoms of anxiety and improved low and unstable mood. [36]
- In patients with social anxiety disorder, valproic acid administration at 500-2500 mg for 12 weeks improved scores on the Liebowitz Social Anxiety Scale (LSAS), a measure of anxiety symptoms. [37]
- In patients with a generalized anxiety disorder that is resistant to antidepressants, valproic acid improves symptoms by increasing the efficiency of gamma-aminobutyric acid. [38]
- In a human model of anxiety behavior, valproic acid treatment reduced subjective ratings of anxiety. [39]
- In bipolar patients, valproic acid administration at a dose range of 50-300 mg/day for 8 weeks appears to reduce anxiety. [40]
- In patients with major depressive disorder, valproic acid significantly improved scores on the Hamilton Rating Scale for Depression (HRSD). [41]
- In patients with major depressive disorder who are resistant to antidepressant medications, valproic acid administration provided substantial clinical improvement. [42]
- In patients with long-lasting depressive and anxiety symptoms, valproic acid administration at 100–1250 mg/day significantly reduced symptoms. [43-45]
- In patients with acute bipolar depression, valproic acid administration significantly reduced symptoms without any adverse effects. [46-50]
- In patients with acute mania, a mental illness characterized by great excitement, delusions, and overactivity, valproic acid was similar in efficacy to the antipsychotic drug haloperidol in treating manic episodes. [51]
- In patients with schizophrenia, a mental disorder characterized by cognitive impairment and detachment from reality, short-term valproic acid treatment improved psychotic symptoms. [52-55]
- In patients with schizoaffective disorder, a mental condition like schizophrenia but with depression and mania symptoms, valproic acid reduced symptoms and was found to be more effective than other antipsychotic drugs such as lithium. [56-57]
- Valproic acid treatment was also associated with reduced length and intensity of symptoms as well as fewer and shorter hospital stays. [58]
E. Improves Cognitive Function
Valproic acid may improve cognitive function by enhancing neuroprotection, promoting neurogenesis, and modulating neurotransmitter levels. It increases brain-derived neurotrophic factor (BDNF), which supports neuronal growth and synaptic plasticity, crucial for learning and memory. Valproic acid also inhibits histone deacetylase (HDAC), leading to changes in gene expression that improve cognitive processes. Additionally, it stabilizes mood and reduces neural hyperactivity, which can enhance focus and cognitive clarity in conditions like epilepsy and bipolar disorder.
- In patients with epilepsy, valproic acid treatment was associated with better scores on memory tasks. [59]
- In young patients with epilepsy, administration of valproic acid improved attention and memory. [60]
- In elderly patients, valproic acid treatment improved attention, concentration, psychomotor speed, and memory. [61]
- Valproic acid treatment was also associated with improved attention, memory, and visuomotor function among epileptic patients. [62]
- In patients with juvenile myoclonic epilepsy, valproic acid improved neuropsychological performance on different cognitive functions such as attention, short-term memory, processing speed, and verbal fluency. [63]
- In HIV-infected individuals with cognitive impairment, valproic acid improved mental performance. [64]
- In a rat model of convulsive status epilepticus, valproic acid significantly improved spatial cognitive dysfunction. [65]
- In rats, valproic acid administration after traumatic brain injury improved cognitive function by protecting brain nerve cells. [66]
- In a rat model of Parkinson’s disease, valproic acid prevented the death of brain neurons. [67]
- Animal and cell studies found that valproic acid may help reverse the progression of Alzheimer’s disease by stimulating the growth and development of new neurons. [68-69]
- In a rat model of stroke, valproic acid protected against the loss of cognitive function via inhibition of oxidative stress and inflammation. [70]
Valproic Acid Side Effects
Valproic acid side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on valproic acid. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of valproic acid. Despite this, it was listed as a side effect associated with valproic acid even though these associated side effects are very uncommon.
Side effects associated with valproic acid may include the following:
- Breathing problems
- Confusion
- Diarrhea
- Dizziness
- Dry or sore mouth
- Feeling tired or sleepy
- Headaches
- Irregular or delayed periods
- Muscle weakness
- Nausea
- Passing out
- Stomach pain
- Swollen gums
- Thinning hair or changes in the color or texture of the hair
- Tremors
- Unusual eye movements
- Vomiting
- Weight gain
Depakene
Depakene is a brand name for valproic acid, an anticonvulsant medication commonly prescribed to manage seizures, bipolar disorder, and migraines. It works by increasing gamma-aminobutyric acid (GABA) levels in the brain, which helps to stabilize nerve activity and prevent abnormal electrical discharges. Depakene is available in various forms, including capsules and liquid solutions, to accommodate individual patient needs.
Depakene is highly effective in controlling a wide range of seizure types, including absence seizures, generalized tonic-clonic seizures, and complex partial seizures. Beyond epilepsy, it is widely used as a mood stabilizer in patients with bipolar disorder, reducing the frequency and intensity of manic and depressive episodes. Additionally, it has been prescribed as a prophylactic treatment for migraines, helping individuals experience fewer and less severe headache episodes.
While Depakene is a valuable medication, it can cause side effects, such as nausea, dizziness, weight gain, and drowsiness. More severe risks include liver damage, pancreatitis, and teratogenic effects, making it unsuitable for use during pregnancy unless absolutely necessary. Patients taking Depakene require regular monitoring of liver function and blood levels to ensure safety and effectiveness. Careful dose adjustments by a healthcare provider are essential to minimize risks while maximizing therapeutic benefits.
Valproic Acid Dosage
Valproic acid dosage depends on the condition being treated, the patient’s age, weight, and individual response to the medication. For adults with epilepsy, the typical starting dose is around 10-15 mg/kg per day, which may be gradually increased by 5-10 mg/kg per week until seizure control is achieved, with a maximum dose of 60 mg/kg per day. In pediatric patients, dosing is similarly weight-based but adjusted according to age and tolerability. Regular blood tests are necessary to monitor valproic acid levels and ensure they remain within the therapeutic range, typically 50-100 µg/mL for seizure control.
In the treatment of bipolar disorder, initial dosages are generally lower, starting at 250-500 mg daily and titrated up based on clinical response. For acute mania, higher doses may be required, often adjusted until symptoms stabilize. Valproic acid can also be used for migraine prophylaxis, with typical doses ranging from 250-500 mg twice daily. Regardless of the indication, the goal is to use the lowest effective dose to minimize the risk of side effects while achieving therapeutic efficacy.
Dose adjustments may be necessary for patients with liver dysfunction, elderly individuals, or those taking other medications that interact with valproic acid. Side effects such as nausea, dizziness, weight gain, or hair thinning may require dose reductions or additional interventions. It is crucial to adhere strictly to the prescribed dosing schedule and to consult a healthcare provider before making any changes to the medication regimen.
Depakene vs Depakote
Depakene and Depakote are both medications derived from valproic acid, but they differ in formulation and use. Depakene contains pure valproic acid and is available in liquid and capsule forms. It is absorbed more rapidly in the body, leading to quicker onset of action but potentially higher rates of gastrointestinal side effects, such as nausea and stomach upset. This makes it suitable for patients who can tolerate its effects or require faster absorption for seizure control.
Depakote, on the other hand, is a delayed-release or extended-release formulation containing divalproex sodium, a compound of sodium valproate and valproic acid. The delayed-release design slows absorption, which helps reduce gastrointestinal side effects and provides steadier drug levels in the bloodstream. Depakote is commonly preferred for long-term management of epilepsy, bipolar disorder, and migraines due to its improved tolerability and convenience of dosing.
While both drugs share the same therapeutic purposes—primarily seizure control, mood stabilization, and migraine prevention—individual tolerances and clinical needs often guide the choice between Depakene and Depakote. Physicians consider factors such as side effect profiles, dosing schedules, and the speed of symptom relief needed when prescribing these medications.
Valproic Acid Dosage for Bipolar
Valproic acid is a commonly used mood stabilizer in the treatment of bipolar disorder, particularly for managing manic or mixed episodes. The typical starting dose for adults is 750 mg per day, divided into multiple doses. However, the dosage may vary based on individual factors, including the severity of symptoms, weight, age, and overall health. To minimize side effects, clinicians often start with a lower dose and gradually increase it to achieve therapeutic levels.
The therapeutic range for valproic acid in bipolar disorder is generally between 50-125 mcg/mL, measured through blood levels. Regular monitoring is crucial to ensure the drug is effective while avoiding toxicity. Dose adjustments are made based on both clinical response and serum valproate levels. Most patients achieve stabilization at daily doses ranging from 1,000 to 2,000 mg, though some may require higher doses depending on their metabolism and response.
Side effects such as drowsiness, nausea, weight gain, and tremors are common with valproic acid, especially at higher doses. Patients with bipolar disorder receiving this treatment need regular liver function and platelet count checks, as the medication can cause rare but serious complications like liver toxicity and thrombocytopenia. Close communication with healthcare providers ensures safe and effective use of valproic acid in managing bipolar symptoms.
Valproic Acid Brand Name
Valproic acid is marketed under various brand names worldwide, depending on the manufacturer and formulation. One of the most common brand names is Depakene, often used in the United States and other regions for oral and injectable formulations of valproic acid. Depakene is widely prescribed for managing epilepsy, bipolar disorder, and migraines, making it a cornerstone medication in the treatment of neurological and psychiatric conditions.
In addition to Depakene, Epival is a brand name for valproic acid used in countries like Canada, where it is primarily available as divalproex sodium, a related compound. Other popular brand names include Valparin and Convulex, which are frequently prescribed in parts of Europe, Asia, and Africa. These brands may come in various formulations such as tablets, capsules, or syrups, catering to patients with different therapeutic needs.
The specific choice of brand or formulation is often influenced by regional availability, cost, and patient-specific factors such as tolerability and dosing requirements. While the active ingredient remains consistent across brands, the pharmacokinetics and tolerability can differ slightly, making it essential for healthcare providers to select the most appropriate option for each individual patient.
Valproic Acid Uses
Valproic acid is a versatile medication primarily used to treat epilepsy by controlling seizures. It is effective in various seizure types, including absence seizures, complex partial seizures, and generalized tonic-clonic seizures. By stabilizing electrical activity in the brain, valproic acid helps reduce the frequency and severity of epileptic episodes, providing significant relief for individuals with seizure disorders.
In addition to its anticonvulsant properties, valproic acid is widely used as a mood stabilizer for managing bipolar disorder. It helps prevent manic and depressive episodes, making it particularly beneficial for individuals with rapid cycling or mixed episodes. Its mood-stabilizing effects have also led to its off-label use in other psychiatric conditions, such as borderline personality disorder and agitation in dementia, although these applications require careful monitoring.
Valproic acid is also prescribed for migraine prevention, particularly in individuals who experience frequent or debilitating migraines. By modulating neurotransmitter levels, the drug reduces the occurrence of migraines and their associated symptoms. Despite its effectiveness, valproic acid has a range of potential side effects, such as liver toxicity, weight gain, and teratogenic risks, requiring careful consideration of its benefits and risks in clinical practice.
What Happens When Valproic Acid Level is Low?
When valproic acid levels are low in the body, it may result in inadequate therapeutic effects, particularly in conditions like epilepsy, bipolar disorder, or migraine prevention. Insufficient levels may lead to a resurgence or worsening of seizures in individuals using the drug for epilepsy, increasing the risk of injury and compromised quality of life. Similarly, patients with bipolar disorder may experience a relapse into manic or depressive episodes, as the drug’s mood-stabilizing effect becomes less effective.
The reasons for low valproic acid levels can vary and include poor medication adherence, drug interactions, or increased metabolism of the drug in the liver. Some medications, such as carbamazepine or phenytoin, can induce liver enzymes and reduce valproic acid concentrations. Additionally, certain physiological conditions, such as rapid clearance of the drug due to genetic factors or altered liver function, may contribute to subtherapeutic levels.
Low levels of valproic acid can be detected through routine blood level monitoring, which is commonly recommended for patients on long-term therapy. If levels are found to be below the therapeutic range, healthcare providers may adjust the dosage or investigate potential causes, such as drug interactions or compliance issues. Timely intervention is crucial to restore the drug’s effectiveness and minimize the risks associated with uncontrolled symptoms.
Valproate vs Valproic Acid
Valproate and valproic acid are closely related compounds used primarily in the treatment of epilepsy, bipolar disorder, and migraines. Valproic acid is the parent compound, a fatty acid that exerts its therapeutic effects by modulating neurotransmitters like gamma-aminobutyric acid (GABA) and stabilizing neuronal activity. It is available in various formulations, including oral and injectable forms, to suit different clinical needs.
Valproate generally refers to the salt form of valproic acid, such as sodium valproate, which is used in medications for its better solubility and stability. Divalproex, a popular variant, is a compound that combines valproic acid and sodium valproate in a specific ratio, designed to minimize gastrointestinal side effects while maintaining efficacy. These formulations differ slightly in pharmacokinetics and tolerability but are all converted to the active form, valproic acid, in the body.
When choosing between valproate and valproic acid, clinicians consider factors like patient tolerance, absorption profiles, and side effect risks. While both are effective in managing similar conditions, divalproex and sodium valproate tend to have fewer gastrointestinal side effects than pure valproic acid. Despite these differences, all forms share a common mechanism of action, making them essential tools in the management of neurological and psychiatric disorders.
Valproic Acid Drug Class
Valproic acid belongs to the drug class of anticonvulsants and is also classified as a mood stabilizer. It is widely used to manage epilepsy by reducing the frequency and severity of seizures. This action is primarily achieved through its ability to increase gamma-aminobutyric acid (GABA) levels in the brain, an inhibitory neurotransmitter that helps calm overactive neuronal activity. By stabilizing the electrical activity in the brain, valproic acid effectively controls seizures in various types of epilepsy.
In addition to its role in seizure management, valproic acid is also a key medication in treating psychiatric conditions such as bipolar disorder. It helps stabilize mood swings, particularly in individuals experiencing manic episodes. Its mood-stabilizing effects are believed to result from the modulation of GABAergic activity and the regulation of intracellular signaling pathways involved in mood regulation.
Valproic acid’s broad therapeutic utility extends to the prevention of migraine headaches. While its precise mechanism in migraine prevention is not fully understood, it is thought to involve the stabilization of neuronal activity and modulation of excitatory and inhibitory neurotransmitter systems. Despite its efficacy, valproic acid carries risks of side effects such as liver toxicity, weight gain, and teratogenic effects, which necessitate careful monitoring during treatment.
FAQ
What is the difference between valproate and divalproex?
Valproate refers to the ionized form of valproic acid, commonly present as sodium valproate in medications. Taking valproic acid in the form of divalproex can offer better gastrointestinal tolerability. Valproic acid may cause gastrointestinal upset, but the combined form of divalproex helps to balance efficacy and side effects. Divalproex, on the other hand, is a compound containing both sodium valproate and valproic acid in a 1:1 molar relationship. Taking valproic acid in this combined form helps to balance efficacy and side effects. Valproic acid may cause liver toxicity, but divalproex is formulated to improve tolerability and reduce gastrointestinal side effects compared to taking valproic acid alone. Valproic acid may cause weight gain as well, which can be lessened by using divalproex.
How much valproic acid is equivalent to sodium valproate?
Sodium valproate is slightly heavier due to the sodium ion. Approximately 1 mg of sodium valproate is equivalent to 0.87 mg of valproic acid. Taking valproic acid into account, this ratio accounts for the molecular weight difference between the two compounds. When taking valproic acid, it is essential to consider its bioavailability compared to sodium valproate. Certain factors, such as drug interactions or dosage adjustments, can increase plasma concentrations of valproic acid, which may impact therapeutic outcomes. Understanding the differences is crucial for those taking valproic acid for medical conditions. Additionally, dosage changes can be implemented to increase plasma concentrations of the drug for better efficacy. It is important to monitor plasma concentrations to avoid potential toxicity while managing treatment with sodium valproate or valproic acid.
What class of drug is valproic acid?
Taking valproic acid, which is classified as an anticonvulsant (antiepileptic) and a mood stabilizer, requires careful monitoring due to the risk of CNS depression. Individuals taking valproic acid should be aware of potential side effects, including CNS depression, and necessary precautions. Taking valproic acid consistently helps in managing conditions like epilepsy and bipolar disorder effectively, while also being cautious of CNS depression.
Is valproic acid antipsychotic?
Valproic acid is primarily used to treat epilepsy, bipolar disorder, and migraines. Taking valproic acid helps control seizures, stabilize mood, and prevent migraines. However, an allergic reaction to valproic acid is possible, and patients should be aware of any unusual symptoms. Taking valproic acid is also sometimes used off-label for other neurological or psychiatric conditions. If an allergic reaction occurs, it is important to seek immediate medical attention. Patients should be aware of potential side effects, including the risk of an allergic reaction, and consult their healthcare provider before taking valproic acid.
Is valproic acid a GABA agonist?
No, taking valproic acid is not a direct GABA agonist. However, taking valproic acid increases the availability of gamma-aminobutyric acid (GABA) in the brain by inhibiting its breakdown and enhancing its synthesis, leading to an overall calming effect on neuronal activity. Pregnant women should be cautious, as taking valproic acid during pregnancy can increase the risk of birth defects. Many patients, including pregnant women under careful medical supervision, benefit from taking valproic acid to manage conditions like epilepsy and bipolar disorder. It is important for pregnant women to discuss the risks and benefits with their healthcare provider before starting treatment.
Is valproic acid also Depakote?
Yes, taking valproic acid is the active ingredient in Depakote, but Depakote is a formulation that contains divalproex sodium, which is a combination of valproic acid and sodium valproate. When taking valproic acid, it is important to follow the usual dose prescribed by your doctor to avoid side effects. Many patients experience benefits from taking valproic acid for conditions such as epilepsy and bipolar disorder. Be sure to stick to the usual dose and consult your healthcare provider for any adjustments or concerns regarding your treatment plan.
What is the use of valproic acid?
Taking valproic acid is primarily used to treat seizure disorders (epilepsy), bipolar disorder, and to prevent migraines. When taking valproic acid, it is important to follow the prescribed dosage to achieve optimal results. Many patients benefit from taking valproic acid regularly to manage their symptoms effectively. However, it is important to inform your healthcare provider about other medicines you are taking, as they may interact with valproic acid. Always ensure your doctor is aware of all other medicines in use to prevent potential drug interactions. Additionally, make sure to monitor for any adverse effects when combining valproic acid with other medicines.
Which is the major side effect of valproic acid?
The major side effect of taking valproic acid is liver toxicity (hepatotoxicity), which can be severe and potentially fatal, especially in young children and those with underlying liver disease. It is important to monitor liver function tests regularly while taking valproic acid to detect any early signs of liver damage. Patients should be aware of the symptoms of liver toxicity when taking valproic acid, such as jaundice, abdominal pain, and unexplained fatigue.
What happens when the valproic acid level is low?
When valproic acid levels are low, it may lead to inadequate seizure control, mood instability in bipolar disorder, or increased frequency of migraines. If you stop taking valproic acid, these issues may worsen. It is important not to stop taking valproic acid suddenly without medical supervision, especially if you are taking other medicines. Always consult your healthcare provider before making any changes to your medication regimen, as stopping valproic acid abruptly, especially when combined with other medicines, can have serious consequences. Remember to discuss the potential interactions with other medicines to ensure your treatment plan is safe.
What is a common side effect of valproic acid?
A common side effect of valproic acid is gastrointestinal upset, including nausea, vomiting, and abdominal pain. Healthcare providers may prescribe valproic acid for conditions such as epilepsy or bipolar disorder, but it’s important to be aware of potential side effects, especially when combined with other medicines. When doctors prescribe valproic acid, they carefully monitor for adverse reactions like gastrointestinal discomfort and interactions with other medicines to ensure patient safety. If you are taking other medicines alongside valproic acid, it’s crucial to discuss any potential risks with your healthcare provider.
What do you monitor when taking valproic acid?
Regular monitoring includes liver function tests (LFTs), blood levels of valproic acid, platelet counts, and ammonia levels to check for toxicity and ensure therapeutic levels. It is essential for healthcare providers to prescribe valproic acid in equal doses with careful consideration of these parameters. When prescribing valproic acid in equal doses, it’s important to regularly assess these factors to prevent potential side effects. This helps ensure that the patient remains within the therapeutic range while avoiding any harmful effects.
What happens if you stop taking valproic acid?
Abruptly stopping valproic acid can lead to rebound seizures, mood swings, or worsening of the condition it was prescribed for, including in individuals with urea cycle disorder. It should be tapered off gradually under medical supervision, especially in those with urea cycle disorder, as sudden discontinuation may exacerbate metabolic imbalances. Additionally, it is important to consider the potential effects on breast milk in breastfeeding patients, as valproic acid can pass into breast milk and affect the infant. Patients with urea cycle disorder should be closely monitored when adjusting valproic acid dosage to avoid complications, especially if they are breastfeeding, as the drug may be transmitted through breast milk.
What is the most common side effect of Depakote?
The most common side effect of Depakote is drowsiness, along with gastrointestinal symptoms like nausea and weight gain. However, it’s important to monitor for signs of valproic acid toxicity, which can lead to more serious side effects such as liver damage and blood clots. If you experience symptoms of valproic acid toxicity, including unusual tiredness, weakness, or yellowing of the skin or eyes, contact your healthcare provider. Additionally, blood clots may occur in some individuals, and regular blood tests can help detect valproic acid toxicity and blood clots before they become severe.
Are Depakote and Depakene the same thing?
No, Depakote (divalproex sodium) contains a combination of valproic acid and sodium valproate, whereas Depakene contains only valproic acid. When you start taking valproic acid, you may experience different side effects compared to when you start taking valproic acid in its Depakote form. The administered dose of Depakote may differ from the administered dose of Depakene, affecting how your body reacts to the medication. They have similar effects but different formulations. It’s important to consult your doctor before you start taking valproic acid to determine which form and administered dose is best suited for your condition.
What is Depakene used for?
Depakene is used to treat epilepsy, bipolar disorder, and to prevent migraines. The effects of valproic acid can help control seizures and stabilize mood in patients with bipolar disorder. Additionally, the effects of valproic acid are beneficial in preventing migraines, reducing the frequency and severity of attacks. However, it is important to be aware of the potential adverse effects of valproic acid, such as liver toxicity and gastrointestinal issues. Understanding the effects of valproic acid and its adverse effects is important for managing the treatment of these conditions effectively. Monitoring for adverse effects ensures that the treatment remains both safe and effective.
What is the most common side effect of valproic acid?
The most common side effect of valproic acid is gastrointestinal discomfort, such as nausea and vomiting. Individuals with liver disease may be at an increased risk for experiencing more severe adverse effects, including liver damage. A missed dose of valproic acid can lead to inadequate seizure control, mood instability, or an increased risk of side effects. It is important to monitor liver function closely in patients with liver disease when taking valproic acid to detect any potential adverse effects early. If a missed dose occurs, it is important to follow dosing instructions to maintain therapeutic levels. Understanding and managing the adverse effects of valproic acid, as well as addressing any missed dose, is essential to ensure patient safety and prevent serious complications.
Is Depakene still available?
Yes, Depakene is still available as a prescription medication, but there is an increased risk of side effects in certain populations, such as those with liver disease. Patients should be monitored regularly due to the increased risk of hepatotoxicity and CNS depressant effects. It is important not to forget doses and to consult a healthcare provider to assess the increased risk before starting treatment with Depakene, especially considering its potential CNS depressant effects. Additionally, caution should be exercised with other medications that may also have CNS depressant effects, as they can intensify the sedative effects of Depakene. If you forget doses, it is crucial to follow the healthcare provider’s instructions on how to proceed. Forgetting doses can lead to inadequate seizure control or other complications.
What is the main mechanism of action of valproate?
Valproate works by increasing the levels of gamma-aminobutyric acid (GABA) in the brain, which helps to calm excessive nerve activity and prevent multiple seizure types, including absence seizures. This mechanism is particularly effective in controlling absence seizures, as it stabilizes electrical activity in the brain. By enhancing GABAergic activity, valproate can reduce the frequency and severity of multiple seizure types, providing broad anticonvulsant effects. This ability to manage multiple seizure types is one of the reasons valproate is widely used in epilepsy treatment.
How does valproic acid work?
Valproic acid capsules enhance GABAergic activity, inhibit voltage-gated sodium channels, and modulate calcium channels to stabilize electrical activity in the brain. These effects make valproic acid capsules effective in treating conditions like epilepsy and bipolar disorder. When taking valproic acid capsules, it is important to monitor for potential side effects and adjust the dosage as needed to maintain therapeutic levels. If you experience any severe side effects, contact your doctor immediately. It is also important to follow your doctor’s guidance on dosage adjustments to prevent complications. If you notice any unusual symptoms, call your doctor immediately for advice.
Is valproic acid a P450 inhibitor?
Yes, valproic acid is a weak inhibitor of the cytochrome P450 enzyme system, particularly CYP2C9, which can affect the metabolism of other drugs. This interaction can be important when taking medications alongside valproic acid syrup, especially in children younger than 12. If you are using valproic acid syrup, it is crucial to monitor potential drug interactions in children younger, as they may be more susceptible to side effects. Additionally, the P450 inhibition by valproic acid syrup may alter the metabolism of other commonly prescribed drugs, which could be particularly significant for children younger in age.
What is the mechanism of action of valproic acid headache?
For migraine prevention, valproic acid increases GABA activity and modulates neurotransmitters that contribute to headache development. It is also effective in managing complex absence seizures by stabilizing electrical activity in the brain. Additionally, valproic acid’s ability to modulate neurotransmitters makes it useful for treating conditions like complex absence seizures, alongside its role in preventing migraines.
What is Depakene good for?
Depakene is effective in treating epilepsy, bipolar disorder, and migraine prevention. Valproic acid may also be used to manage mood stabilization in patients with bipolar disorder. Additionally, valproic acid may help reduce the frequency of seizures in patients with epilepsy. Valproic acid may provide preventive benefits for those who experience chronic migraines. However, in patients with a mitochondrial disorder, caution should be taken, as valproic acid may exacerbate symptoms. In individuals with a mitochondrial disorder, the use of valproic acid requires close monitoring due to potential metabolic effects.
What's the difference between valproic acid and divalproex?
Valproic acid is the pure form of the drug, while divalproex is a compound that consists of both valproic acid and sodium valproate, designed for slower absorption and better gastrointestinal tolerance. It is important to note that folic acid supplementation is often recommended for patients taking valproic acid, as it may help reduce the risk of birth defects. Additionally, folic acid can assist in counteracting the potential folate depletion caused by valproic acid use. When it comes to preventing migraine headaches, valproic acid has been shown to be effective, and it is commonly prescribed for this purpose. Preventing migraine headaches is an important consideration when determining the appropriate treatment plan, especially for those who suffer from frequent migraines. Furthermore, valproic acid may play a role in preventing migraine headaches, making it a valuable treatment option for patients who experience these debilitating attacks.
What is a safer alternative to Depakote?
Safer alternatives might include lamotrigine or carbamazepine for bipolar disorder or seizures, depending on the condition being treated. These alternatives may also have different plasma concentrations, which can affect their efficacy and safety profile, particularly in individuals with renal impairment. It’s important to monitor plasma concentrations of any medication to ensure it stays within the therapeutic range, especially when switching from valproic acid to other treatments, as renal impairment can alter drug metabolism and excretion. Adjustments to dosage may be necessary for those with renal impairment to avoid toxicity.
Is Depakene a mood stabilizer?
Yes, Depakene can act as a mood stabilizer for conditions like bipolar disorder. Monitoring plasma concentrations of Depakene, especially when taking extended release tablets, is important to ensure it stays within the therapeutic range. If plasma concentrations are too low or too high, it can affect the effectiveness and safety of the medication. For patients on extended release tablets, it is crucial to follow dosing instructions carefully to maintain steady plasma levels.
What is the brand name of valproic acid?
The brand names of valproic acid include Depakene and Depakote. It is important to note that valproic acid has been associated with an increased risk of birth defects, particularly when used during pregnancy. Women who are pregnant or planning to become pregnant should discuss the potential risks of birth defects with their healthcare provider before using Depakene or Depakote.
Is valproic acid and Depakote the same thing?
Not exactly; valproic acid is the active ingredient, whereas Depakote is a formulation containing divalproex sodium. Both are types of prescription medicines used for conditions like epilepsy and bipolar disorder. When these medications are taken, they interact with plasma proteins in the body, which can affect their distribution and effectiveness. It is important to understand the differences, including how plasma proteins play a role in their action, when considering prescription medicines to treat these conditions. Always consult with a healthcare provider when using prescription medicines like Depakote or valproic acid, especially considering their interaction with plasma proteins.
What are the generic names for Depakote?
Generic names for Depakote include divalproex sodium and valproic acid. In some cases, valproic acid may be used as adjunctive therapy for conditions such as epilepsy or bipolar disorder when primary treatments are insufficient. Additionally, divalproex sodium is often prescribed as adjunctive therapy to enhance the effectiveness of other mood stabilizers or anticonvulsants. The protein binding of valproic acid can influence its therapeutic levels, affecting its interaction with other medications. Monitoring protein binding is crucial, as changes in protein binding can alter the drug’s efficacy and risk of side effects. Therefore, understanding the protein binding characteristics of divalproex sodium is important in managing its use in combination therapies.
What is the main use of valproic acid?
The main use of valproic acid is to treat epilepsy, bipolar disorder, and migraines. However, it is important to monitor for elevated liver enzymes, as this can indicate potential liver damage. Patients taking valproic acid should regularly have their liver function tested to check for any signs of elevated liver enzymes, which could suggest hepatotoxicity. Dark urine can be a sign of liver issues, so any changes in urine color should be reported to a healthcare provider. If dark urine is observed, it could indicate liver damage and warrants further investigation. Dark urine, along with other symptoms, may suggest a need for dosage adjustments or discontinuation of the medication.
What does valproic acid do to the brain?
It increases GABA levels, which helps to calm hyperactive neurons and stabilize mood and seizure activity. This mechanism also works to treat mania by promoting mood stabilization. Some people may experience stomach pain as a side effect. If stomach pain persists or worsens, it’s important to consult a healthcare provider. Increasing GABA levels also helps to reduce the risk of discomfort, including stomach pain, by stabilizing overall brain function, which can be beneficial in treating mania. Additionally, stabilizing brain function through increased GABA levels may help prevent withdrawal seizures, which can occur when abruptly discontinuing medications. For those at risk, it’s essential to gradually taper off medications to avoid withdrawal seizures.
Is valproic acid used as a mood stabilizer?
Yes, valproic acid is commonly used as a mood stabilizer in bipolar disorder. Valproate sodium, a form of valproic acid, is often prescribed for its mood-stabilizing effects. However, it is important to be aware that valproic acid can sometimes be associated with suicidal thoughts, and patients should be monitored for any changes in behavior. Additionally, valproate sodium can help manage other conditions such as epilepsy and migraines, but anyone taking it should be cautious of potential side effects, including suicidal thoughts. Always consult a healthcare provider if you experience any concerning symptoms.
Is valproic acid used for schizophrenia?
It is not typically a first-line treatment for schizophrenia but may be used adjunctively for mood stabilization or aggression control. If you are considering using it for this purpose, tell your doctor to ensure it is the appropriate treatment for your condition. Additionally, tell your doctor if you have any history of liver disease or other medical issues before starting the medication. Always follow your healthcare provider’s guidance and tell your doctor about any other medications you are taking to avoid potential interactions.
What can cause valproic acid levels to drop?
Factors such as poor medication adherence, drug interactions (e.g., with enzyme inducers like carbamazepine), and metabolic changes, including those related to body weight, can lower levels. Additionally, significant fluctuations in body weight may affect drug absorption and metabolism. Monitoring body weight changes is important, as they can influence valproic acid levels.
What does valproic acid level tell you?
It indicates whether the drug is within the therapeutic range for effectiveness while avoiding toxicity. Understanding how valproic acid works helps ensure that the drug is dosed appropriately. Monitoring valproic acid levels provides insights into how valproic acid works in the body and whether adjustments are needed for optimal therapeutic outcomes.
How to increase valproic acid level?
Levels can be increased by adjusting the dosage, improving adherence, and avoiding interacting medications, which may help prevent liver problems. It is important to monitor liver function regularly, as liver problems can occur with increased levels of the medication. Always consult with a healthcare provider if there are concerns about liver problems while adjusting the dosage.
What is a serious side effect of valproic acid?
A serious side effect is hepatotoxicity (liver failure), pancreatitis, or hyperammonemia, which can be life-threatening, especially in individuals of childbearing age. Women of childbearing age should be closely monitored for these risks. It is important for those of childbearing age to discuss the potential risks with their healthcare provider before starting treatment.
Is valproate the same as valproic acid?
Yes, valproate refers to the salt form of valproic acid and is often used interchangeably. Therapeutic drug monitoring is important to ensure that valproate levels remain within the therapeutic range for effective treatment. By using therapeutic drug monitoring, healthcare providers can adjust dosages to maintain optimal levels of valproate, minimizing the risk of toxicity.
Are valproic acid and sodium valproate interchangeable?
Yes, but they have different pharmacokinetic profiles, and dose adjustments may be required. It’s important to understand how medicine affects the body in order to tailor treatment. In some cases, how medicine affects the metabolism can influence the overall effectiveness. Understanding how medicine affects the body’s processes ensures safe and effective use.
Reference
Glauser TA, Cnaan A, Shinnar S, et al. Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy. N Engl J Med. 2010;362(9):790-9.
Ethosuximide, valproic acid, and lamotrigine in childhood absence epilepsy
A clinical trial comparing ethosuximide, valproic acid, and lamotrigine for childhood absence epilepsy found that ethosuximide and valproic acid were more effective than lamotrigine, with ethosuximide causing fewer attentional side effects.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/20200383/.Kerkhof M, Dielemans JC, Van breemen MS, et al. Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme. Neuro-oncology. 2013;15(7):961-7.
Effect of valproic acid on seizure control and on survival in patients with glioblastoma multiforme
The study found that combining valproic acid (VPA) with levetiracetam (LEV) provides better seizure control in patients with glioblastoma multiforme (GBM) compared to monotherapy. Additionally, using VPA alongside chemoradiation with temozolomide significantly extends median survival by two months.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/23680820/.Huang YH, Chi NF, Kuan YC, et al. Efficacy of phenytoin, valproic acid, carbamazepine and new antiepileptic drugs on control of late-onset post-stroke epilepsy in Taiwan. Eur J Neurol. 2015;22(11):1459-68.
Efficacy of phenytoin, valproic acid, carbamazepine and new antiepileptic drugs on control of late-onset post-stroke epilepsy in Taiwan
This nationwide cohort study in Taiwan found that valproic acid (VPA) and newer antiepileptic drugs (AEDs) provide better seizure control in late-onset post-stroke epilepsy patients compared to phenytoin (PHT), as evidenced by lower rates of emergency room visits and hospitalizations.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/26148132/.Sasidaran K, Singhi S, Singhi P. Management of acute seizure and status epilepticus in pediatric emergency. Indian J Pediatr. 2012;79(4):510-7.
Management of acute seizure and status epilepticus in pediatric emergency
Acute seizures and status epilepticus are major pediatric emergencies, with higher incidence in developing countries due to infections. Immediate treatment with benzodiazepines, followed by Phenytoin, Valproate, or Levetiracetam, is crucial, and refractory cases may require pharmacological coma and intensive care.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/22120613/.Miró J, Aiguabella M, Veciana M, et al. Low-dose sodium valproate in the treatment of idiopathic generalized epilepsies. Acta Neurol Scand. 2014;129(5):e20-3.
Low-dose sodium valproate in the treatment of idiopathic generalized epilepsies
Low-dose sodium valproate (VPA) treatment (<1000 mg/day) was effective in controlling seizures in most patients with idiopathic generalized epilepsy (IGE), particularly in those with generalized tonic-clonic seizures (GTCS) and juvenile myoclonic epilepsy (JME), while patients with juvenile absence epilepsy (JAE) were more likely to require higher doses or additional therapies. The study also found a reduction in adverse events with low-dose VPA use.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/24372179/.Gilad R, Boaz M, Dabby R, Sadeh M, Lampl Y. Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment?. Epilepsy Res. 2011;95(3):227-31.
Are post intracerebral hemorrhage seizures prevented by anti-epileptic treatment?
A randomized, double-blind clinical trial evaluated the effects of valproic acid (VPA) versus placebo in patients with spontaneous intracerebral hemorrhage (SICH) over one year. While VPA did not prevent seizures overall, it reduced early seizures and improved neurological outcomes, suggesting a potential neuroprotective effect.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/21632213/.Mattson RH, Cramer JA, Williamson PD, Novelly RA. Valproic acid in epilepsy: clinical and pharmacological effects. Ann Neurol. 1978;3(1):20-5.
Valproic acid in epilepsy: clinical and pharmacological effects
An open clinical trial of valproic acid in 23 patients with uncontrolled seizures showed that two-thirds experienced a 25–100% reduction in seizure frequency without serious systemic toxicity. The drug required divided daily dosing due to its short half-life and was found to interact with phenytoin by increasing its free concentration while decreasing total serum levels.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/350128/.Bruni J, Albright P. Valproic acid therapy for complex partial seizures. Its efficacy and toxic effects. Arch Neurol. 1983;40(3):135-7.
Valproic acid therapy for complex partial seizures
Valproic acid was tested in 24 adult outpatients with poorly controlled complex partial seizures, with initial seizure reduction in 12 patients, but only 5 maintained long-term benefit due to tolerance development. Side effects were generally mild, with weight gain in most patients and nausea being the most common complaint.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/6403001/.Davis R, Peters DH, Mctavish D. Valproic acid. A reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs. 1994;47(2):332-72.
A reappraisal of its pharmacological properties and clinical efficacy in epilepsy
Valproic acid is an effective and well-tolerated antiepileptic drug used as a first-line treatment for various seizure types, including generalized and partial seizures. It has comparable efficacy to other antiepileptic drugs, with fewer neurological side effects, though it requires monitoring for gastrointestinal issues, weight gain, rare liver toxicity, and potential drug interactions.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/7512905/.Löscher W. Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy. CNS Drugs. 2002;16(10):669-94.
Basic pharmacology of valproate: a review after 35 years of clinical use for the treatment of epilepsy
Valproate, widely used for treating generalized and partial seizures in both adults and children, exerts its broad antiepileptic effects through multiple mechanisms, including enhancing GABAergic activity and inhibiting NMDA-mediated excitation. While its precise mechanisms remain under investigation, its ability to modulate neuronal excitation and inhibition across various brain regions contributes to its effectiveness in treating a wide range of seizure types.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/12269861/.Prasad A, Kuzniecky RI, Knowlton RC, et al. Evolving antiepileptic drug treatment in juvenile myoclonic epilepsy. Arch Neurol. 2003;60(8):1100-5.
Evolving antiepileptic drug treatment in juvenile myoclonic epilepsy
A study comparing the efficacy of newer antiepileptic drugs (AEDs) like lamotrigine and topiramate with older AEDs like phenytoin and carbamazepine in treating juvenile myoclonic epilepsy (JME) found that lamotrigine and topiramate were more effective in controlling myoclonic seizures and could be viable alternatives to valproic acid. The study concluded that phenytoin and carbamazepine were less effective and may not be suitable for JME patients.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/12925366/.Wilder BJ, Rangel RJ. Review of valproate monotherapy in the treatment of generalized tonic-clonic seizures. Am J Med. 1988;84(1A):7-13.
Review of valproate monotherapy in the treatment of generalized tonic-clonic seizures
Studies show that converting epilepsy treatment from polytherapy to monotherapy, particularly with valproate, is beneficial, especially for patients with generalized tonic-clonic seizures. Valproate has proven to be as effective as phenytoin and carbamazepine for partial seizures and is considered the drug of choice for primary generalized tonic-clonic seizures.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/3146225/.Jo SJ, Shin H, Park YW, et al. Topical valproic acid increases the hair count in male patients with androgenetic alopecia: a randomized, comparative, clinical feasibility study using phototrichogram analysis. J Dermatol. 2014;41(4):285-91.
Topical valproic acid increases the hair count in male patients with androgenetic alopecia: a randomized, comparative, clinical feasibility study using phototrichogram analysis
A clinical trial assessed the efficacy of topical valproic acid (VPA) in treating androgenetic alopecia (AGA) and found that it significantly increased hair counts in patients compared to a placebo. The treatment was generally well-tolerated with mild side effects, suggesting that topical VPA could be a potential option for AGA treatment.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/24533507/.Available from https://clinicaltrials.gov/ct2/show/NCT01548066.
The Efficacy and Safety of Topical Valproic Acid in Preventing Hair Loss
Beta-catenin, a key player in Wnt signaling, regulates hair growth and regeneration, with its levels controlled by GSK-3-mediated degradation. Since GSK-3 inhibition increases nuclear β-catenin and may promote hair growth, valproic acid (VPA), a known GSK-3β inhibitor, could potentially influence hair growth, though its effects on hair have not yet been studied.
You can read the abstract of this article at https://clinicaltrials.gov/study/NCT01548066.Agrawal AK, Das S. Valproic acid and alopecia: A two-edged sword. Asian J Psychiatr. 2017;29:39-40.
Valproic acid and alopecia: A two-edged sword. Asian J Psychiatr
Valproic acid is used to treat various types of seizures and mood disorders but may cause adverse effects like tremor, weight gain, hair loss, gastrointestinal disturbances, liver dysfunction, and thrombocytopenia. Interestingly, recent studies have shown that valproic acid may promote hair re-growth through its action as a GSK3β inhibitor, activating the Wnt/β-catenin pathway.
You can read the full article at
https://www.sciencedirect.com/science/article/abs/pii/S1876201817302204?Fakhraei lahiji S, Seo SH, Kim S, et al. Transcutaneous implantation of valproic acid-encapsulated dissolving microneedles induces hair regrowth. Biomaterials. 2018;167:69-79.
Transcutaneous implantation of valproic acid-encapsulated dissolving microneedles induces hair regrowth
Topical application of valproic acid (VPA) has been shown to stimulate hair follicle regrowth by activating the Wnt/β-catenin pathway, and a novel dissolving microneedle (DMN-VPA) enhances VPA delivery efficiency by creating micro-wounds on the skin. In vivo studies reveal that DMN-VPA upregulates key markers involved in hair follicle regeneration more effectively than traditional topical applications.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29554482/.Kakunje A, Prabhu A, Sindhu Priya ES, et al. Valproate: It’s Effects on Hair. Int J Trichology. 2018;10(4):150–153. doi:10.4103/ijt.ijt_10_18.
Valproate: It’s Effects on Hair. Int J Trichology
Valproate is used to treat seizure disorders, bipolar disorder, and migraines, but it can cause adverse effects such as tremor, weight gain, gastrointestinal issues, liver dysfunction, and hair loss. Valproate-induced hair loss is typically diffuse, dose-related, and reversible, with some evidence suggesting topical valproic acid may promote hair regeneration.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/30386073/.Choi SY, Kim HD, Kim BJ, Kim MN, Han DH. A case of androgenetic alopecia treated with valproic acid. Int J Dermatol. 2014;53:e214–5.
A case of androgenetic alopecia treated with valproic acid. Int J Dermatol
No abstract is available for this article.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/23621357/.Lee SH, Yoon J, Shin SH, et al. Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells. PLoS One. ;7(4):e34152. doi:10.1371/journal.pone.0034152.
Valproic acid induces hair regeneration in murine model and activates alkaline phosphatase activity in human dermal papilla cells
Valproic acid (VPA), a GSK3β inhibitor, has been shown to promote hair re-growth by activating the Wnt/β-catenin pathway in both mice and human dermal papilla cells. Unlike minoxidil, VPA successfully stimulated hair growth and epidermal differentiation markers, suggesting that VPA and similar small molecules could be developed as potential treatments for alopecia.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/22506014/.Available from https://jddonline.com/articles/dermatology/S1545961614P0809X/1.
Evidence for Supplemental Treatments in Androgenetic Alopecia
Minoxidil and finasteride are the only FDA-approved treatments for female pattern hair loss and androgenetic alopecia, but due to their limitations, some patients turn to alternative therapies. This review examines the scientific evidence supporting alternatives like biotin, caffeine, melatonin, marine extracts, and zinc.
You can read the full article at https://jddonline.com/articles/dermatology/S1545961614P0809X/1.Kinze S, Clauss M, Reuter U, et al. Valproic acid is effective in migraine prophylaxis at low serum levels: a prospective open-label study. Headache. 2001;41(8):774-8.
Valproic acid is effective in migraine prophylaxis at low serum levels: a prospective open-label study
A study evaluating the efficacy of valproic acid for migraine prophylaxis found that lower serum levels (less than 50 microg/mL) resulted in a significant reduction in both the frequency of migraine attacks and headache days. The optimal dose for migraine prevention was found to be 500 to 600 mg daily, with minimal side effects and no additional benefit from higher doses.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/11576201/.Shelton CE, Connelly JF. Valproic acid: a migraine prophylaxis alternative. Ann Pharmacother. 1996;30(7-8):865-6.
Valproic acid: a migraine prophylaxis alternative
This study compared the efficacy of melatonin and sodium valproate in the prophylaxis of chronic migraines. Both melatonin and sodium valproate significantly reduced migraine frequency, severity, duration, and disability scores, with melatonin showing similar clinical effectiveness to sodium valproate but with fewer side effects, suggesting it could be a viable alternative for chronic migraine prevention.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/28800342/.Ichikawa M, Katoh H, Kurihara T, Ishii M. Clinical Response to Valproate in Patients with Migraine. J Clin Neurol. 2016;12(4):468–475. doi:10.3988/jcn.2016.12.4.468.
Clinical Response to Valproate in Patients with Migraine
This study compared the efficacy of melatonin and sodium valproate in the prophylaxis of chronic migraines. Both melatonin and sodium valproate significantly reduced migraine frequency, severity, duration, and disability scores, with melatonin showing similar clinical effectiveness to sodium valproate but with fewer side effects, suggesting it could be a viable alternative for chronic migraine prevention.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC5063874/#:~:text=There%20is%20also%20evidence%20that,of%20attacks%20in%2086.2%25%20patients.Fusco C, Pisani F, Capone C, Faienza C. Valproic acid in migraine prophylaxis of young patients. Three new reports. Acta Biomed. 2002;73(3-4):47-51.
Valproic acid in migraine prophylaxis of young patients Three new reports
Valproic acid is effective and well-tolerated for migraine prevention in adults, but its use in pediatric patients is limited. This study reports three cases of children with migraine successfully treated with low-dose sodium valproate (10 mg/kg/day) for six months after failing other preventive treatments. All showed good response, though one discontinued due to weight gain. The findings support valproic acid as a potential option for pediatric migraine prophylaxis.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/12596387/.Shahien R, Saleh SA, Bowirrat A. Intravenous sodium valproate aborts migraine headaches rapidly. Acta Neurol Scand. 2011;123(4):257-65.
Intravenous sodium valproate aborts migraine headaches rapidly
This preliminary study evaluated the efficacy and safety of intravenous sodium valproate (iVPA) for severe migraine attacks in 36 hospitalized patients. A single infusion (900–1200 mg) led to significant pain reduction within 60 minutes in 75% of cases, with no serious adverse effects. The results suggest iVPA is a safe and fast-acting treatment for intractable migraines, warranting further controlled studies.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/20569223/.Cutrer FM, Limmroth V, Moskowitz MA. Possible mechanisms of valproate in migraine prophylaxis. Cephalalgia. 1997;17(2):93-100.
Possible mechanisms of valproate in migraine prophylaxis
Valproate is an effective migraine prophylactic, though its mechanism remains unclear due to its broad biochemical effects. It increases brain GABA levels, potentially suppressing migraine-related activity in the cortex, trigeminal system, and perivascular pathways. Valproate also reduces neurogenic inflammation, modulates excitatory and inhibitory neurotransmitters, and affects neuronal membranes, suggesting its antimigraine action results from multiple mechanisms.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/9137844/#:~:text=Valproate%20increases%20brain%20GABA%20levels,and%20directly%20attenuates%20nociceptive%20neurotransmission..Linde M, Mulleners WM, Chronicle EP, Mccrory DC. Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults. Cochrane Database Syst Rev. 2013;(6):CD010611.
Valproate (valproic acid or sodium valproate or a combination of the two) for the prophylaxis of episodic migraine in adults
This Cochrane review assessed controlled trials on valproate for migraine prevention in adults. Analysis of ten trials showed that sodium valproate significantly reduced headache frequency, with divalproex sodium more than doubling the proportion of responders compared to placebo (NNT = 4). Valproate was comparable to flunarizine and propranolol but slightly less effective than topiramate. While generally well tolerated, adverse events led to NNHs ranging from 7 to 14. The findings confirm valproate as an effective option for migraine prophylaxis.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC10373438/.Erdemoglu AK, Ozbakir S. Valproic acid in prophylaxis of refractory migraine. Acta Neurol Scand. 2000;102(6):354-8.
Valproic acid in prophylaxis of refractory migraine
This study evaluated valproic acid for migraine prevention in 120 patients unresponsive to conventional treatments. A 50% or greater reduction in headache frequency was observed in 67% of patients, while severity improved in 60%, with an average daily dose of 1,250 mg. Side effects were generally mild and tolerable, supporting valproic acid as an effective and safe option for refractory migraine treatment.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11125749/.Takeshima T, Nishikawa W, Yoneda H, Kanki R, Yamashita S, Kikui S. Efficacy and safety of valproate in a series of Japanese migraine sufferers (in Japanese) Japanese J Headache. 2013;39:306–311.
Efficacy and safety of valproate in a series of Japanese migraine sufferers (in Japanese) Japanese J Headache
A large observational study in Japan evaluated the effectiveness and safety of an extended-release sodium valproate tablet for migraine prophylaxis in 1,222 patients. Migraine frequency significantly decreased from 10.2 to 5.0 days per month (P < 0.001), with 59% experiencing at least a 50% reduction. The drug was most effective in patients with more frequent migraines and well tolerated, with a 6.3% adverse reaction rate. These findings support its use as a safe and effective migraine prophylactic treatment in routine clinical practice.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC5074326/.Takeshima T, Nishikawa W, Yoneda H, Kanki R, Yamashita S, Kikui S. Efficacy and safety of valproate in a series of Japanese migraine sufferers (in Japanese) Japanese J Headache. 2013;39:306–311.
Efficacy and safety of valproate in a series of Japanese migraine sufferers (in Japanese) Japanese J Headache
A large observational study in Japan evaluated the effectiveness and safety of an extended-release sodium valproate tablet for migraine prophylaxis in 1,222 patients. Migraine frequency significantly decreased from 10.2 to 5.0 days per month (P < 0.001), with 59% experiencing at least a 50% reduction. The drug was most effective in patients with more frequent migraines and well tolerated, with a 6.3% adverse reaction rate. These findings support its use as a safe and effective migraine prophylactic treatment in routine clinical practice.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC5074326/.Hering R, Kuritzky A. Sodium valproate in the prophylactic treatment of migraine: a double-blind study versus placebo. Cephalalgia. 1992;12(2):81-4.
Sodium valproate in the prophylactic treatment of migraine: a double-blind study versus placebo
A double-blind, randomized crossover study in 29 patients compared sodium valproate (400 mg twice daily) to placebo for migraine prevention. Results showed that 86.2% of patients experienced reduced migraine frequency, severity, or duration with valproate. The drug was generally well tolerated, confirming its effectiveness as a migraine treatment.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/1576648/.Mathew NT, Saper JR, Silberstein SD, et al. Migraine prophylaxis with divalproex. Arch Neurol. 1995;52(3):281-6.
Migraine prophylaxis with divalproex
A multicenter, double-blind, placebo-controlled study evaluated divalproex sodium for migraine prevention in 107 patients. Over 12 weeks, divalproex reduced migraine frequency significantly (3.5 vs. 5.7 per 4 weeks; p ≤ 0.001), with 48% of patients achieving a 50% or greater reduction compared to 14% with placebo. Divalproex also reduced functional restriction and medication use, with no significant differences in migraine severity or duration. It was generally well tolerated, confirming its effectiveness for migraine prophylaxis.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/7872882/.Shaygannejad V, Janghorbani M, Ghorbani A, Ashtary F, Zakizade N, Nasr V. Comparison of the effect of topiramate and sodium valporate in migraine prevention: a randomized blinded crossover study. Headache. 2006;46(4):642-8.
Comparison of the effect of topiramate and sodium valporate in migraine prevention: a randomized blinded crossover study
A 24-week randomized, double-blind crossover trial compared topiramate and sodium valproate for migraine prevention in 64 patients. Both drugs significantly reduced migraine frequency, intensity, and duration (p < 0.001). While topiramate showed slightly greater reductions, the overall efficacy and safety profiles were comparable. These findings suggest that both medications are effective options for migraine prophylaxis.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16643559/.Primeau F, Fontaine R, Beauclair L. Valproic acid and panic disorder. Can J Psychiatry. 1990;35(3):248-50.
Valproic acid and panic disorder
This study evaluated valproic acid (VA) for treating recurrent panic attacks in ten patients. After a placebo washout, VA was administered at doses up to 2250 mg/day, leading to significant symptom improvement on multiple psychiatric scales (p < 0.001). The results suggest VA may be effective for panic disorder, warranting further research to confirm its efficacy and safety.
You can read the abstract of this article atRaevskiĭ KS, Aleksandrovskiĭ IuA, Poiurovskiĭ MV, Kharlamov AN, Neznamov GG. [Anxiolytic action of sodium valproate (possible role of gamma-aminobutyric acid in affective disorders)]. Zh Nevropatol Psikhiatr Im S S Korsakova. 1985;85(4):574-9.
Anxiolytic action of sodium valproate (possible role of gamma-aminobutyric acid in affective disorders)]
This study investigated the anxiolytic effects of sodium valproate, a GABAergic drug, in both experimental animals and patients with anxiety-related disorders. Results showed that valproate had a tranquilizing effect comparable to diazepam but without muscle relaxation, ataxia, or sedation. These findings support a link between the GABA system and anxiety, suggesting valproate as a potential treatment for anxiety disorders.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/2860764/.Baetz M, Bowen RC. Efficacy of divalproex sodium in patients with panic disorder and mood instability who have not responded to conventional therapy. Can J Psychiatry. 1998;43(1):73-7.
Efficacy of divalproex sodium in patients with panic disorder and mood instability who have not responded to conventional therapy
An 8-week open trial evaluated divalproex sodium for treatment-refractory panic disorder with comorbid mood instability. Among 13 participants, 10 completed the study, showing significant improvements in panic attacks, anxiety, depression, and mood instability. Two dropped out due to side effects. Results suggest divalproex sodium may benefit patients unresponsive to standard treatments, warranting further double-blind trials.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/9494751/.Kinrys G, Pollack MH, Simon NM, Worthington JJ, Nardi AE, Versiani M. Valproic acid for the treatment of social anxiety disorder. Int Clin Psychopharmacol. 2003;18(3):169-72.
Valproic acid for the treatment of social anxiety disorder
This 12-week open trial assessed valproic acid (500–2500 mg) for social anxiety disorder in 17 participants. Results showed significant improvement in social anxiety symptoms, with LSAS scores decreasing by 19.1–21.3 points and 41.1–46.6% achieving responder status. Findings suggest valproic acid may be effective for social anxiety, but placebo-controlled trials are needed for confirmation.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/12702897/.Available from https://www.tandfonline.com/doi/full/10.1080/24750573.2017.1317382.
Treatment response to valproate in case with generalized anxiety disorder resistant to antidepressants
This case report highlights the use of valproic acid in treating a patient with anxiety symptoms resistant to SSRIs and SNRIs. Given its anxiolytic effects via GABA modulation, valproate showed a favorable response, suggesting it may be a viable option for treatment-resistant anxiety disorders.
You can read the abstract of this article at https://www.tandfonline.com/doi/full/10.1080/24750573.2017.1317382#abstract.Bach DR, Korn CW, Vunder J, Bantel A. Effect of valproate and pregabalin on human anxiety-like behaviour in a randomised controlled trial. Transl Psychiatry. 2018;8(1):157. Published 2018 Aug 16. doi:10.1038/s41398-018-0206-7.
Effect of valproate and pregabalin on human anxiety-like behaviour in a randomised controlled trial
This study investigated valproate’s anxiolytic effects in a translational human model using a double-blind, placebo-controlled trial with 118 participants. Compared to placebo, both valproate (400 mg) and pregabalin (200 mg) reduced anxiety-like behavior in a computer-based conflict task, with no significant differences between them. Valproate was less sedative than pregabalin, and sedation did not account for the anxiolytic effects. These findings support the potential of valproate as an anxiolytic and suggest the need for clinical trials to evaluate its therapeutic use in anxiety disorders.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC6095858/.Sheehan DV, Harnett-sheehan K, Hidalgo RB, et al. Randomized, placebo-controlled trial of quetiapine XR and divalproex ER monotherapies in the treatment of the anxious bipolar patient. J Affect Disord. 2013;145(1):83-94.
Randomized, placebo-controlled trial of quetiapine XR and divalproex ER monotherapies in the treatment of the anxious bipolar patient
This study evaluated the anxiolytic effects of quetiapine XR (50-300 mg/day) versus divalproex ER (500-3000 mg/day) in 149 patients with bipolar disorder and co-occurring panic disorder or GAD. In an 8-week, double-blind, placebo-controlled trial, quetiapine XR showed significantly greater improvements in anxiety symptoms compared to divalproex ER and placebo, particularly on the HAM-A and SPS scales. Both medications were well tolerated, though weight gain was higher with quetiapine XR. The study suggests quetiapine XR may effectively reduce anxiety in this population, warranting further research on other mood stabilizers and antipsychotics.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0165032712005356?via%3Dihub.Davis LL, Kabel D, Patel D, et al. Valproate as an antidepressant in major depressive disorder. Psychopharmacol Bull. 1996;32(4):647-52.
Valproate as an antidepressant in major depressive disorder
In an 8-week open trial, 33 outpatients with major depressive disorder (MDD) were treated with valproate. By Week 4, 54% of completers showed a significant clinical response, and by Week 8, 86% demonstrated improvement, with mean HRSD scores dropping from 22 to 7 (p < .0001). An intent-to-treat analysis showed a 66% response rate and a 55% reduction in HRSD scores. These findings suggest valproate may be effective for MDD, but double-blind, placebo-controlled trials are needed to confirm its efficacy.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8993086/.Ghabrash MF, Comai S, Tabaka J, Saint-laurent M, Booij L, Gobbi G. Valproate augmentation in a subgroup of patients with treatment-resistant unipolar depression. World J Biol Psychiatry. 2016;17(2):165-70.
Valproate augmentation in a subgroup of patients with treatment-resistant unipolar depression
In a study of 14 patients with treatment-resistant depression (TRD), valproate (375-1000 mg/day) was added to their treatment regimen after failing multiple antidepressant trials. Significant reductions in MADRS and CGI-Severity scores were observed at 1, 4, and 7 months (p < 0.001), with MADRS scores approaching remission levels and no relapses reported. These findings suggest that valproate may provide substantial and sustained clinical improvement in TRD, warranting further investigation in larger double-blind trials.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/26444701/.Liu CC. Adjuvant valproate therapy for patients with suspected mixed-depressive features. Ther Adv Psychopharmacol. 2014;4(4):143–148. doi:10.1177/2045125314532868.
Adjuvant valproate therapy for patients with suspected mixed-depressive features
This retrospective study assessed the effectiveness of valproate as adjuvant therapy in 22 patients with suspected mixed-depressive features, a condition linked to antidepressant resistance and higher suicide risk. Valproate (100–1250 mg/day) led to moderate or significant improvement in most patients, though four showed limited response. Twelve continued long-term use, while some experienced symptom aggravation upon discontinuation. Adverse events were reported by 12 patients, with four stopping due to intolerability. These findings suggest that low-dose valproate may be a promising option for patients with subthreshold bipolarity, warranting further research.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4104709/#:~:text=Therefore%2C%20low%2Ddose%20adjuvant%20valproate,positive%20experiences%20with%20this%20approach.Vigo DV, Baldessarini RJ. Anticonvulsants in the treatment of major depressive disorder: an overview. Harv Rev Psychiatry. 2009;17(4):231-41.
Anticonvulsants in the treatment of major depressive disorder: an overview
This review examined 41 trials on anticonvulsants (ACs) for major depressive disorder (MDD), excluding studies focused on bipolar disorder. It identified potential benefits of carbamazepine, lamotrigine, and valproate, mainly as adjuncts to antidepressants, particularly for treating irritability and agitation. While some controlled trials suggest efficacy, most evidence remains preliminary. Further research is needed, especially on long-term adjunctive use in recurrent MDD with prominent agitation.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/19637072/.Kemp LI. Sodium valproate as an antidepressant. Br J Psychiatry. 1992;160:121-3.
Sodium valproate as an antidepressant
A patient with chronic depression experienced relief from major depressive episodes but remained dysthymic until treatment with anticonvulsants, specifically sodium valproate, which alleviated symptoms. This suggests valproate may be beneficial in a range of affective disorders.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/1543994/.Smith LA, Cornelius VR, Azorin JM, et al. Valproate for the treatment of acute bipolar depression: systematic review and meta-analysis. J Affect Disord. 2010;122(1-2):1-9.
Valproate for the treatment of acute bipolar depression: systematic review and meta-analysis
A meta-analysis of four randomized controlled trials (142 participants) found that valproate significantly reduced depressive symptoms in acute bipolar depression, with a standardized mean difference of -0.35 and a relative risk of 2.00 for achieving at least 50% symptom improvement. Effects on anxiety were small and inconclusive, and there was no significant difference in mania symptoms or adverse events, though nausea occurred more frequently with valproate. Despite small sample sizes, the findings suggest valproate is effective and well tolerated for acute bipolar depression.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0165032709004959?via%3Dihub.Haeberle A, Greil W, Russmann S, Grohmann R. Mono- and combination drug therapies in hospitalized patients with bipolar depression. Data from the European drug surveillance program AMSP. BMC Psychiatry. 2012;12:153.
Mono- and combination drug therapies in hospitalized patients with bipolar depression
An analysis of 2,246 inpatients with bipolar depression (1994–2009) found that 85% received combination therapy, with antidepressants (74%), antipsychotics (55%), anticonvulsants (48%), and lithium (33%) being commonly used. Lithium (33%) and valproic acid (23%) were frequently prescribed alongside SSRIs, mirtazapine, or venlafaxine. In 2010, quetiapine was the most prescribed drug (39%), with aripiprazole at 10%. Many treatment combinations, including multiple antidepressants, lack strong evidence, and the use of aripiprazole and certain antidepressant combinations deviates from guidelines.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3514299/.Bond DJ, Lam RW, Yatham LN. Divalproex sodium versus placebo in the treatment of acute bipolar depression: a systematic review and meta-analysis. J Affect Disord. 2010;124(3):228-34.
Divalproex sodium versus placebo in the treatment of acute bipolar depression: a systematic review and meta-analysis
A meta-analysis of four randomized, placebo-controlled trials (142 patients) found that divalproex significantly improved response (39.3% vs. 17.5%) and remission rates (40.6% vs. 24.3%) in acute bipolar depression. Despite its frequent use for maintenance treatment, divalproex is not commonly prescribed for acute depression due to perceived lack of evidence. These findings provide preliminary support for its efficacy, though larger studies are needed.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0165032709005011?via%3Dihub.Selle V, Schalkwijk S, Vázquez GH, Baldessarini RJ. Treatments for acute bipolar depression: meta-analyses of placebo-controlled, monotherapy trials of anticonvulsants, lithium and antipsychotics. Pharmacopsychiatry. 2014;47(2):43-52.
Treatments for acute bipolar depression: meta-analyses of placebo-controlled, monotherapy trials of anticonvulsants, lithium and antipsychotics
A meta-analysis of 24 placebo-controlled trials evaluating non-antidepressant treatments for bipolar depression found moderate overall efficacy, with quetiapine, valproate, and olanzapine-fluoxetine showing the strongest evidence. Valproate was effective in 2 of 4 trials, while lithium remains inadequately studied. Treatment-associated mania was rare. Findings highlight the need for more research, especially on lithium and other mood stabilizers.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/24549862/.Cipriani A, Reid K, Young AH, Macritchie K, Geddes J. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev. 2013;(10):CD003196.
Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder
Bipolar disorder is a recurrent and disabling illness with high healthcare costs. While lithium remains a first-line mood stabilizer, 20-40% of patients may not respond adequately. Valproate, effective in acute mania, is frequently used for maintenance treatment. Given the importance of episode prevention and minimizing adverse effects, this updated Cochrane review evaluates the long-term efficacy and tolerability of valproate in bipolar disorder management.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC6599863/Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306-15.
Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis
A multiple-treatments meta-analysis of 68 randomized controlled trials (16,073 participants) assessed the efficacy of antimanic drugs for acute mania. Haloperidol, risperidone, and olanzapine were the most effective, significantly outperforming mood stabilizers like valproate and lithium. Antipsychotics generally showed greater efficacy than mood stabilizers, with haloperidol ranking highest. Olanzapine, risperidone, and quetiapine also had lower discontinuation rates than lithium and other agents. These findings suggest that antipsychotics should be prioritized in clinical guidelines for treating manic episodes.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/21851976/.Tseng PT, Chen YW, Chung W, et al. Significant Effect of Valproate Augmentation Therapy in Patients With Schizophrenia: A Meta-analysis Study. Medicine (Baltimore). 2016;95(4):e2475.
Significant Effect of Valproate Augmentation Therapy in Patients With Schizophrenia: A Meta-analysis Study
A meta-analysis of 11 clinical trials (889 patients) evaluated the efficacy of valproate augmentation in schizophrenia and schizoaffective disorder. Patients receiving antipsychotics with valproate showed significantly greater improvement in total psychopathology than those on antipsychotics alone, particularly in open trials and short-term treatment. However, randomized controlled trials did not confirm this benefit, and long-term effects remain unclear. There was no significant difference in dropout rates. These findings suggest valproate augmentation may enhance treatment response in schizophrenia, though further research is needed on its long-term efficacy and safety.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC5291556/.Suzuki T, Uchida H, Takeuchi H, et al. Augmentation of atypical antipsychotics with valproic acid. An open-label study for most difficult patients with schizophrenia. Hum Psychopharmacol. 2009;24(8):628-38.
Augmentation of atypical antipsychotics with valproic acid. An open-label study for most difficult patients with schizophrenia
An open-label study of 28 severe schizophrenia inpatients assessed valproate augmentation of atypical antipsychotics. Despite long illness duration and high baseline severity, 9 patients responded, 12 partially responded, and 7 did not. Significant but limited improvements were observed, with risperidone and olanzapine as the main antipsychotics. Valproate was generally well tolerated, though some experienced thrombocytopenia, anemia, and sedation-related falls. While promising, these findings require validation against clozapine-based treatments or intensive monotherapy.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/19946935/.Sajatovic M, Coconcea N, Ignacio RV, et al. Adjunct extended-release valproate semisodium in late life schizophrenia. Int J Geriatr Psychiatry. 2008;23(2):142-7.
Adjunct extended-release valproate semisodium in late life schizophrenia
A 12-week open-label study evaluated adjunctive extended-release valproate semisodium in 20 older adults with schizophrenia. Significant improvements were observed in psychosis (PANSS, p<0.01), global functioning (GAS, p<0.01), and depression (GDS, p<0.05), with a mean dose of 587.5 mg/day. The medication was generally well tolerated, with sedation as the main side effect, which was dose-dependent. Weight change was not significant. While findings suggest efficacy and tolerability, larger controlled trials are needed.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/17582828/.Yoshimura R, Shinkai K, Ueda N, Nakamura J. Valproic acid improves psychotic agitation without influencing plasma risperidone levels in schizophrenic patients. Pharmacopsychiatry. 2007;40(1):9-13.
Valproic acid improves psychotic agitation without influencing plasma risperidone levels in schizophrenic patients
The addition of valproic acid to risperidone in schizophrenic patients effectively reduced excitement and hostility symptoms without altering risperidone metabolism or causing significant side effects. This combination also decreased dopaminergic activity, suggesting its efficacy and tolerability for managing impulsiveness and agitation in schizophrenia.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/17327954/.Schaff MR, Fawcett J, Zajecka JM. Divalproex sodium in the treatment of refractory affective disorders. J Clin Psychiatry. 1993;54(10):380-4.
Divalproex sodium in the treatment of refractory affective disorders
Divalproex sodium was effective in 75% of 63 patients with refractory affective disorders, including bipolar I, bipolar II, and schizoaffective disorder. It showed significant benefits in those who had previously failed lithium (84% response) or carbamazepine (69% response), particularly in rapid-cycling patients (81% response). While well tolerated overall, 14% discontinued due to side effects. These findings support divalproex as a valuable treatment for mania and rapid mood cycling, including in some cases of covert cycling misdiagnosed as unipolar depression.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8262880/.Bogan AM, Brown ES, Suppes T. Efficacy of divalproex therapy for schizoaffective disorder. J Clin Psychopharmacol. 2000;20(5):520-2.
Efficacy of divalproex therapy for schizoaffective disorder
A retrospective study of 20 patients with schizoaffective disorder, bipolar type, found that adjunctive divalproex therapy led to significant clinical improvement in 75% of cases without any worsening or discontinuation due to side effects. With an average dose of 1,150 mg and a mean peak serum level of 61 µg/mL, divalproex was well tolerated and showed promise as a treatment for schizoaffective disorder, similar to its efficacy in bipolar disorder. Further controlled trials are needed to confirm these findings.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/11001235/.Puzyński S, Kłosiewicz L. Valproic acid amide in the treatment of affective and schizoaffective disorders. J Affect Disord. 1984;6(1):115-21.
Valproic acid amide in the treatment of affective and schizoaffective disorders
A 26- to 51-month study of valproic acid amide treatment in 15 patients with affective and schizoaffective disorders showed a reduction in the frequency, duration, and severity of affective episodes, particularly mania. Some patients experienced milder, shorter episodes instead of prolonged relapses. Hospital admissions also decreased in all patients, suggesting sustained therapeutic benefits.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/6142911/#:~:text=During%2026%2D51%20months%20of,of%20affective%20episodes%2C%20especially%20mania..Forsythe I., Butler R., Berg I., McGuire R. (1991) Cognitive impairment in new cases of epilepsy randomly assigned to carbamazepine, phenytoin and sodium valproate. Dev Med Child Neurol 33: 524–534.
Cognitive impairment in new cases of epilepsy randomly assigned to carbamazepine, phenytoin and sodium valproate
A study of 64 children with epilepsy randomly assigned to carbamazepine, phenytoin, or sodium valproate found that while carbamazepine in moderate doses negatively impacted memory, phenytoin and sodium valproate did not affect cognitive function over a year of follow-up.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/1864478/.Coenen A.M.L., Konings G.M.L.G., Aldenkamp A.P., Reiner W.O., van Luijtelaar E.L.J.M. (1995) Effects of chronic use of carbamazepine and valproate on cognitive processes. J Epilepsy 8: 250–254.
Effects of chronic use of carbamazepine and valproate on cognitive processes
A study comparing the cognitive effects of chronic carbamazepine (CBZ) and valproate (VPA) use in young epilepsy patients found similar overall cognitive profiles, though VPA users performed better on attention and memory tasks, while CBZ users had faster motor responses. Both drugs caused only mild cognitive impairments compared to older antiepileptic drugs like phenytoin and phenobarbital.
You can read the abstract of this article at https://www.sciencedirect.com/science/article/abs/pii/089669749500041B.Craig I, Tallis R. Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study. Epilepsia. 1994;35(2):381-90.
Impact of valproate and phenytoin on cognitive function in elderly patients: results of a single-blind randomized comparative study
A study of 38 elderly patients with late-onset seizures found that monotherapy with phenytoin (PHT) or valproate (VPA) had minimal impact on cognitive function, with some measures even showing improvement. Contrary to previous findings, there was little difference between the two drugs in cognitive effects, though noncognitive side effects were common. The results suggest that AED selection in elderly patients should prioritize other adverse effects rather than cognitive concerns.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/8156961/.Stores G, Williams PL, Styles E, Zaiwalla Z. Psychological effects of sodium valproate and carbamazepine in epilepsy. Arch Dis Child. 1992;67(11):1330-7.
Psychological effects of sodium valproate and carbamazepine in epilepsy
A study of 63 schoolchildren with newly diagnosed epilepsy found that treatment with sodium valproate or carbamazepine was effective at modest doses without significant long-term psychological effects. While some temporary cognitive impairments were observed early in treatment, these may have been due to uncontrolled seizures. After 12 months, cognitive improvements were similar between children with epilepsy and matched controls, though some psychological abnormalities were present before treatment, suggesting early effects of the epileptic process itself.
You can read the abstract of this article at https://pmc.ncbi.nlm.nih.gov/articles/PMC1793785/.De araujo filho GM, Pascalicchio TF, Lin K, Sousa PS, Yacubian EM. Neuropsychiatric profiles of patients with juvenile myoclonic epilepsy treated with valproate or topiramate. Epilepsy Behav. 2006;8(3):606-9.
Neuropsychiatric profiles of patients with juvenile myoclonic epilepsy treated with valproate or topiramate
A cross-sectional study comparing 42 patients with juvenile myoclonic epilepsy (JME) treated with valproate (VPA) or topiramate (TPM) found that TPM was associated with worse neuropsychological performance, particularly in attention, short-term memory, processing speed, and verbal fluency—functions linked to frontal lobe dysfunction in JME. Anxiety disorders were more common in patients with poor seizure control and a history of over 20 generalized tonic-clonic seizures.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16504593/.Schifitto G, Peterson DR, Zhong J, et al. Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report. Neurology. 2006;66(6):919-21.
Valproic acid adjunctive therapy for HIV-associated cognitive impairment: a first report
A pilot 10-week placebo-controlled study of valproic acid (VPA) 250 mg twice daily in 22 HIV-infected individuals found that VPA was safe and well tolerated, with trends toward improved neuropsychological performance and brain metabolism, particularly in those with cognitive impairment.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/16510768/.Wu P, Hong S, Zhong M, Guo Y, Chen H, Jiang L. Effect of Sodium Valproate on Cognitive Function and Hippocampus of Rats After Convulsive Status Epilepticus. Med Sci Monit. 2016;22:5197–5205. Published 2016 Dec 29. doi:10.12659/MSM.898859.
Effect of Sodium Valproate on Cognitive Function and Hippocampus of Rats After Convulsive Status Epilepticus
A study on a rat model of convulsive status epilepticus (CSE) found that sodium valproate (VPA) improved spatial cognitive dysfunction in older (P35) rats but impaired memory in both younger (P15) and normal P35 rats. VPA enhanced long-term potentiation (LTP) and inhibited CaMKII and p-CaMKII expression, suggesting its cognitive effects are linked to LTP modulation and CaMKII signaling.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC5218388/.Dash PK, Orsi SA, Zhang M, et al. Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats. PLoS ONE. 2010;5(6):e11383.
Valproate administered after traumatic brain injury provides neuroprotection and improves cognitive function in rats
A study using a rodent model of traumatic brain injury (TBI) found that post-injury administration of valproate (400 mg/kg) improved blood-brain barrier integrity, reduced neural damage, and enhanced motor and cognitive function. These effects were linked to increased histone acetylation and reduced GSK-3 activity, suggesting valproate’s neuroprotective potential as an acute treatment for TBI.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC2894851/.Zhang C, Yuan X, Hu Z, et al. Valproic Acid Protects Primary Dopamine Neurons from MPP+-Induced Neurotoxicity: Involvement of GSK3β Phosphorylation by Akt and ERK through the Mitochondrial Intrinsic Apoptotic Pathway. Biomed Res Int. 2017;2017:8124501. doi:10.1155/2017/8124501.
Valproic Acid Protects Primary Dopamine Neurons from MPP+-Induced Neurotoxicity: Involvement of GSK3β Phosphorylation by Akt and ERK through the Mitochondrial Intrinsic Apoptotic Pathway
Valproic acid (VPA) exhibits neuroprotective effects in a Parkinson’s disease (PD) model by reversing mitochondrial apoptosis, activating ERK and Akt pathways, and inhibiting GSK3β activation through phosphorylation. These effects were blocked by PI3K and MAPK pathway inhibitors, highlighting VPA’s potential as a therapeutic candidate for PD by targeting the mitochondrial apoptotic pathway.
You can read the abstract of this article at https://pmc.ncbi.nlm.nih.gov/articles/PMC5380829/.Zhang XZ, Li XJ, Zhang HY. Valproic acid as a promising agent to combat Alzheimer’s disease. Brain Res Bull. 2010;81(1):3-6.
Valproic acid as a promising agent to combat Alzheimer’s disease
Valproic acid (VPA), a widely used mood stabilizer and antiepileptic drug, shows neuroprotective potential in Alzheimer’s disease (AD) by promoting neurogenesis through multiple signaling pathways. Given the lack of effective AD treatments, VPA emerges as a promising therapeutic candidate.
You can read the abstract of this article at https://pubmed.ncbi.nlm.nih.gov/19748552/.Zaky A, Mahmoud M, Awad D, El Sabaa BM, Kandeel KM, Bassiouny AR. Valproic acid potentiates curcumin-mediated neuroprotection in lipopolysaccharide induced rats. Front Cell Neurosci. 2014;8:337. Published 2014 Oct 21. doi:10.3389/fncel.2014.00337.
Valproic acid potentiates curcumin-mediated neuroprotection in lipopolysaccharide induced rats
A study investigating the combined neuroprotective effects of curcumin (Cur) and valproic acid (VPA) in a rat model of lipopolysaccharide-induced neuroinflammation found that co-treatment significantly reduced markers of neuroinflammation, including COX-2, BACE1, APP, and iNOS. The combination also restored the expression of let-7 miRNA family members, which were suppressed in the inflamed group, suggesting a potential role in neuroprotection. These findings highlight the synergistic effects of Cur and VPA in enhancing brain recovery and suggest let-7 miRNAs as potential therapeutic targets for neuroinflammation-related disorders.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4204527/.Suda S, Katsura K, Kanamaru T, Saito M, Katayama Y. Valproic acid attenuates ischemia-reperfusion injury in the rat brain through inhibition of oxidative stress and inflammation. Eur J Pharmacol. 2013;707(1-3):26-31.
Valproic acid attenuates ischemia-reperfusion injury in the rat brain through inhibition of oxidative stress and inflammation
Valproic acid (VPA) demonstrates neuroprotective effects in an experimental ischemic stroke model by reducing infarct volume, improving neurological outcomes, and decreasing inflammation and oxidative stress when administered within 90 minutes of ischemia onset.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0014299913002100?via%3Dihub.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








