Health Library
Human Chorionic Gonadotropin (HCG)
Author: Dr. George Shanlikian, M.D. | Last Updated: May 23rd, 2025
- Home
- >
- Health Library
- >
- Human Chorionic Gonadotropin (HCG)
Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Health Benefits of Human Chorionic Gonadotropin
- Key Takeaways
- What is Human Chorionic Gonadotropin?
- How Human Chorionic Gonadotropin Works
- Chemical Structure of Human Chorionic Gonadotropin
- Research on Human Chorionic Gonadotropin
- Associated Side Effects of Human Chorionic Gonadotropin
- Human Chorionic Gonadotropin Injection
- HCG Blood Test
- HCG Drops
- Human Chorionic Gonadotropin Test
- When is HCG Detectable?
- How to take HCG?
- HCG Function in Males
- What does HCG do?
- Where is HCG produced?
- FAQ
- Reference
Table of Contents
- Potential Health Benefits of Human Chorionic Gonadotropin
- Key Takeaways
- What is Human Chorionic Gonadotropin?
- How Human Chorionic Gonadotropin Works
- Chemical Structure of Human Chorionic Gonadotropin
- Research on Human Chorionic Gonadotropin
- Associated Side Effects of Human Chorionic Gonadotropin
- Human Chorionic Gonadotropin Injection
- HCG Blood Test
- HCG Drops
- Human Chorionic Gonadotropin Test
- When is HCG Detectable?
- How to take HCG?
- HCG Function in Males
- What does HCG do?
- Where is HCG produced?
- FAQ
- Reference
Overall Health Benefits of Human Chorionic Gonadotropin (HCG)
Human Chorionic Gonadotropin (HCG) benefits include stimulating testosterone production and sperm development in men, supporting ovulation and fertility in women, and aiding in the treatment of hormonal imbalances. It is also used in medical therapies for conditions like hypogonadism and certain fertility disorders.
- Increases sex drive by enhancing testosterone production [1-4]
- Treats and prevents testicular atrophy (shrinking of the testicles) [5-12]
- Increases sperm production [13-26]
- Treats male infertility [27-31]
- Lowers the risk of heart disease [32-35]
- Boosts immunity [36-39]
- Increases the chances of pregnancy [40-41]
- Improves brain health [42-43]
- Prevents breast cancer [44-48]
- Increases muscle mass [49-50]
Key Takeaways
- Hormone Regulation: Human Chorionic Gonadotropin (HCG) mimics luteinizing hormone (LH), stimulating testosterone production in men and supporting ovulation in women.
- Fertility Treatment: HCG is commonly used to treat male and female infertility, aiding in sperm production and egg maturation.
- Medical Uses: It is prescribed for hypogonadism, delayed puberty, and certain hormonal disorders, helping restore normal hormone levels.
- Performance Enhancement: Some athletes and bodybuilders use HCG to maintain testosterone levels while on anabolic steroids, though this is not an FDA-approved use.
- Pregnancy Indicator: HCG is naturally produced during pregnancy and serves as the basis for pregnancy tests, detecting its presence in urine or blood.
What is Human Chorionic Gonadotropin (HCG)?
Human chorionic gonadotropin or HCG is a hormone found in a woman’s blood and urine during pregnancy. This hormone plays a significant role during pregnancy by stimulating the corpus luteum (a part of the ovary) to produce another hormone known as progesterone. HCG is usually administered to help increase the chances of pregnancy in women.
In men, HCG administration boosts the levels of the hormone testosterone and stimulates sperm production. It’s used in conjunction with testosterone to reduce some of the side effects of testosterone replacement therapy (TRT), mainly preventing testicular atrophy (shrinking of the testicles). HCG is also used to treat male infertility associated with TRT.
How Human Chorionic Gonadotropin Works
Human chorionic gonadotropin (HCG) works by stimulating the corpus luteum to produce progesterone, a hormone essential for maintaining the uterine lining and supporting early pregnancy. This increase in progesterone enhances the chances of pregnancy by creating a favorable environment for embryo implantation. In men, HCG mimics luteinizing hormone (LH), stimulating the testes to produce more testosterone and increasing sperm production, which can help address hormonal imbalances and improve fertility.
Chemical Structure of Human Chorionic Gonadotropin
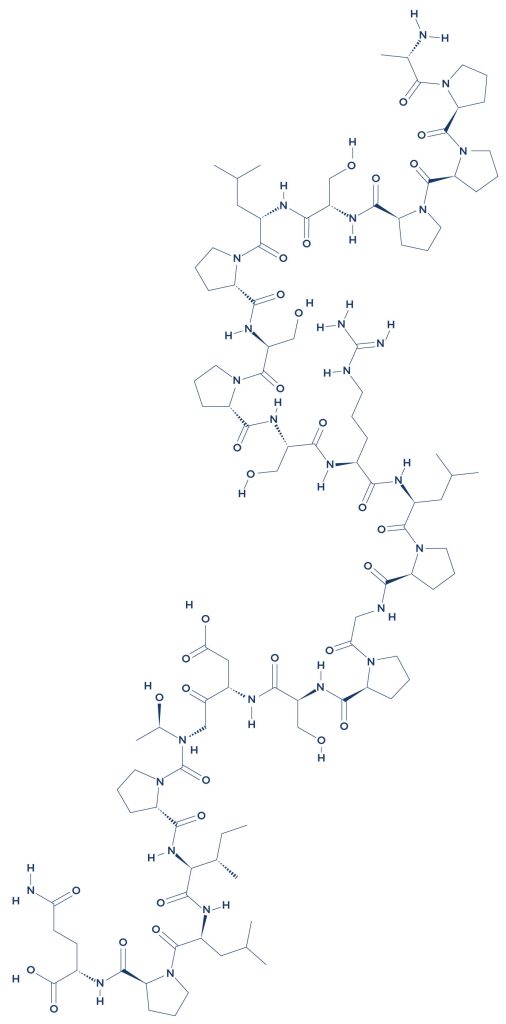
Research on Human Chorionic Gonadotropin
A. Increases Sex Drive by Enhancing Testosterone Production

Human Chorionic Gonadotropin (HCG) increases sex drive by enhancing testosterone production, making it a valuable treatment for men with low testosterone levels or hypogonadism. By mimicking luteinizing hormone (LH), HCG stimulates the testes to produce more testosterone, which plays a crucial role in libido, energy levels, and overall sexual function. This boost in hormone levels can help improve mood, muscle strength, and reproductive health. However, while HCG is effective in restoring testosterone levels, its use should be carefully monitored to prevent potential side effects such as fluid retention, gynecomastia, or hormonal imbalances.
- In men with erectile dysfunction and lack of sexual desire, HCG administration resulted in improved sexual behavior. [1]
- In horses with a lack of libido, HCG administration increased sex drive by boosting testosterone levels. [2]
- In men with testosterone deficiency, HCG treatment alone or with clomiphene citrate (CC) successfully increased their testosterone levels while preserving their fertility. [3]
- In bulls, HCG administration increased blood testosterone levels which in turn improved sexual behavior. [4]
B. Treats and Prevents Testicular Atrophy (Shrinking of the Testicles)

Human Chorionic Gonadotropin (HCG) helps treat and prevent testicular atrophy by stimulating the production of testosterone and maintaining testicular function. In men with hypogonadism or those using anabolic steroids, HCG mimics luteinizing hormone (LH), signaling the testes to continue producing testosterone and sperm, preventing shrinkage. This is particularly beneficial for individuals undergoing testosterone replacement therapy (TRT), where natural testosterone production is suppressed. By supporting testicular size and function, HCG can help preserve fertility and hormonal balance, making it a crucial treatment option for those experiencing testicular atrophy.
- In men with sex hormone deficiency, HCG therapy stimulated testis growth and sperm production. [5]
- In adolescent boys with testicular atrophy caused by delayed puberty, HCG was effective in treating the condition. [6]
- In men with testis atrophy, HCG therapy successfully treated the condition and led to sperm production. [7]
- A study showed that HCG could prevent testosterone replacement therapy-induced testicular atrophy. [8]
- In boys aged 1-12 years old, HCG administration resulted in greater improvements in testicular volume (TV) and testicular atrophy index (TAI). [9]
- In male patients who were treated for hypogonadism, HCG monotherapy improved intratesticular testosterone while preventing testicular atrophy. [10]
- In boys with undescended testicles, HCG therapy resulted in the descent of the testes to the scrotum. [11]
- In men with androgen deficiency symptoms, HCG treatment was found to reduce the prevalence of testicular atrophy. [12]
C. Increases Sperm Production
Human Chorionic Gonadotropin (HCG) increases sperm production by stimulating the testes to produce testosterone, a key hormone necessary for spermatogenesis. By mimicking luteinizing hormone (LH), HCG signals the Leydig cells in the testes to release testosterone, which in turn supports the development and maturation of sperm. This makes HCG a valuable treatment for men with hypogonadism or infertility caused by low testosterone levels. When used as part of fertility therapy, HCG can significantly improve sperm count and motility, increasing the chances of conception. However, proper medical supervision is essential to ensure optimal dosage and minimize potential side effects.
Several studies suggest that HCG can help improve male fertility by boosting sperm production:
- In patients with testosterone deficiency, long-term administration of HCG resulted in increased sperm density and motility. [13]
- In men with normal testes, HCG administration drastically elevated the rate of sperm secretion. [14]
- In patients with testosterone deficiency, injection with HCG maintained normal sperm production. [15]
- In men with testosterone deficiency, HCG treatment alone was found to be effective in stimulating complete sperm production regardless of the testicular volume. [16]
- A study showed that HCG treatment was enough for both the initiation and maintenance of sperm production in most patients. [17]
- In patients with testosterone deficiency, HCG treatment once a week resulted in increased sperm count. [18]
- In pre-pubertal mice, HCG injection stimulated sperm production even in immature testicles. [19]
- In pubertal boys with a history of orchiopexy (surgery to correct undescended testicles), weekly HCG administration restored sperm production. [20]
- In adult men with low sexual function, HCG treatment led to the normalization of sperm production. [21]
- In men who underwent varicocelectomy (removal of enlarged veins in the scrotum), the administration of HCG increased sperm production. [22]
- In men with secondary testosterone deficiency, HCG administration triggered testosterone and sperm production. [23]
- In men undergoing testosterone replacement therapy, HCG treatment successfully preserved fertility during the therapy by boosting sperm production. [24]
- In healthy men with suppressed production of sex hormones, low dose-administration of HCG maintained normal testosterone and sperm production. [25]
- In adolescent males with sex hormone deficiency, HCG administration with follicle-stimulating hormone therapy stimulated puberty, improved sexual function, and increased sperm production. [26]
D. Treats Male Infertility
Human Chorionic Gonadotropin (HCG) is an effective treatment for male infertility, particularly in men with low testosterone levels or hypogonadotropic hypogonadism. HCG mimics luteinizing hormone (LH), stimulating the testes to produce testosterone and sperm, which can improve fertility outcomes. It is often used in combination with follicle-stimulating hormone (FSH) therapy to enhance sperm production in men with low sperm counts. Additionally, HCG can help restore testicular size and function in individuals who have experienced testicular shrinkage due to prolonged anabolic steroid use or other hormonal imbalances. Regular medical monitoring is essential to ensure proper dosing and minimize potential side effects such as acne, fluid retention, or mood swings.
- In men with testosterone deficiency, HCG stimulated testosterone and sperm production without affecting their fertility. [27]
- In men with active hypogonadism (sex hormone deficiency), HCG treatment was effective in restoring testosterone levels either alone or in combination with Clomiphene citrate. [28]
- A study showed that HCG therapy is an effective option for treating male infertility caused by testosterone replacement therapy. [29]
- In late-onset hypogonadism (LOH) patients, HCG administration preserved fertility and increased testosterone levels. [30]
- In men with testosterone-related infertility, HCG therapy was shown to significantly elevate total testosterone levels while maintaining sperm production. [31]
E. Lowers the Risk of Heart Disease
Human Chorionic Gonadotropin (HCG) may help lower the risk of heart disease by supporting hormonal balance, particularly in men with low testosterone levels. Adequate testosterone is essential for maintaining healthy cholesterol levels, reducing visceral fat, and improving overall metabolic function, all of which contribute to cardiovascular health. By stimulating natural testosterone production, HCG can aid in regulating blood pressure, reducing inflammation, and enhancing blood vessel function. However, while HCG may have potential cardiovascular benefits, its use should be carefully monitored to avoid complications such as fluid retention or hormone imbalances that could negatively impact heart health.
- In obese women, daily treatment with HCG via nasal spray with dietary restriction and vitamin supplementation significantly lowered cardiovascular risk factors. [32]
- In anesthetized pigs, intracoronary infusion of HCG improved heart function and increased blood flow. [33]
- In pregnant women in their first trimester, a low concentration of HCG was associated with a high possibility of coronary heart disease. [34]
- In animals, the combination of erythropoietin (EPO) and HCG treatment promoted cardiac remodeling after a heart attack. [35]
F. Boosts Immunity
Human Chorionic Gonadotropin (HCG) plays a role in boosting immunity by modulating the immune system and promoting a balanced inflammatory response. During pregnancy, HCG helps protect the developing fetus by regulating immune tolerance, preventing the mother’s body from rejecting the embryo. Additionally, HCG has been studied for its potential immunomodulatory effects in autoimmune conditions and certain medical treatments. By influencing cytokine production and supporting hormonal balance, HCG may contribute to a stronger immune response, though further research is needed to fully understand its long-term effects on immune health.
- A study showed that the increased production of HCG during pregnancy enhanced immunity against L. tropica infection. [36]
- A study suggested that HCG may play a role in the modulation of immune system cells during pregnancy. [37]
- In non-obese mice, HCG treatment showed immunomodulatory effects that prevented autoimmune diabetes. [38]
- In a murine model, the immune-modulating effects of HCG promoted fetal survival. [39]
G. Increases the Chances of Pregnancy
Human Chorionic Gonadotropin (HCG) increases the chances of pregnancy by stimulating ovulation in women struggling with infertility. It mimics luteinizing hormone (LH), triggering the release of a mature egg from the ovaries, which is essential for conception. In assisted reproductive treatments, such as in vitro fertilization (IVF) or intrauterine insemination (IUI), HCG is often administered to ensure timely ovulation and improve fertilization success rates. Additionally, in some cases, it helps support the early stages of pregnancy by promoting progesterone production, which is crucial for maintaining a healthy uterine lining for embryo implantation.
- In infertile women, HCG treatment resulted in a high pregnancy rate. [40]
- The administration of a low-dose HCG alone in infertile women resulted in a higher pregnancy rate without any side effects. [41]
H. Improves Brain Health
Human Chorionic Gonadotropin (HCG) may contribute to improved brain health by supporting hormone balance, which plays a crucial role in cognitive function and mental well-being. By stimulating testosterone production and regulating estrogen levels, HCG can help maintain neurotransmitter activity, potentially enhancing memory, focus, and mood stability. Some research suggests that balanced hormone levels, particularly testosterone, may reduce the risk of neurodegenerative diseases such as Alzheimer’s and Parkinson’s. Additionally, HCG’s role in reducing inflammation and oxidative stress may further support brain health, though more studies are needed to fully understand its neuroprotective effects.
- In rats, HCG reduced plaque formation in the brain. [42]
- In mice, HCG prevented the progression of Alzheimer’s disease. [43]
I. Prevents Breast Cancer
Human Chorionic Gonadotropin (HCG) has been studied for its potential role in preventing breast cancer by regulating hormone levels and promoting cellular differentiation in breast tissue. Research suggests that HCG may exert protective effects by reducing estrogen-driven cell proliferation, which is a key factor in the development of hormone-sensitive breast cancers. Additionally, HCG influences gene expression in breast cells, promoting a more differentiated and less cancer-prone state. While further studies are needed to fully understand its protective mechanisms, HCG’s hormonal regulatory properties make it a promising area of research in breast cancer prevention.
- Research suggests that HCG may play a role in reducing the risk of breast cancer. [44]
- One study found that HCG can lower breast cancer risk by 50% in post-partum women. [45]
- In animal models, HCG treatment demonstrated protective effects by preventing both the initiation and progression of breast cancer in female rats. [46]
- Another study indicated that mimicking pregnancy through HCG treatment could be a promising approach for breast cancer prevention. [47]
- Increased HCG levels during the first trimester of pregnancy may contribute to long-term protection against breast cancer. [48]
J. Increases Muscle Mass
Human Chorionic Gonadotropin (HCG) plays a role in increasing muscle mass by stimulating the production of testosterone, a key hormone for muscle growth and strength. By mimicking luteinizing hormone (LH), HCG signals the testes to produce more testosterone, which enhances protein synthesis, supports muscle recovery, and promotes lean muscle development. This makes it a potential treatment for conditions like hypogonadism, where low testosterone levels lead to muscle weakness and reduced physical performance.
- In men with age-related testosterone deficiency, HCG injections increased muscle mass. [49]
- In older men, HCG significantly increased muscle mass by boosting testosterone levels. [50]
Associated Side Effects of Human Chorionic Gonadotropin
Human chorionic gonadotropin side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on human chorionic gonadotropin. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of human chorionic gonadotropin. Despite this, it was listed as a side effect associated with human chorionic gonadotropin even though these associated side effects are very uncommon.
Side effects associated with human chorionic gonadotropin may include the following:
- Diarrhea
- Feeling short of breath
- Little or no urination
- Pelvic pain
- Rapid weight gain
- Severe nausea
- Severe stomach pain
- Swelling around the waist
- Vomiting
Human Chorionic Gonadotropin Injection
Human chorionic gonadotropin (HCG) injection is a medical treatment used primarily to support fertility and hormonal balance in both men and women. In women, HCG injections help trigger ovulation by mimicking luteinizing hormone (LH), which is essential for egg maturation and release. This makes it a key component of assisted reproductive treatments, such as in vitro fertilization (IVF) and ovulation induction for women struggling with infertility.
In men, HCG injections are used to stimulate the production of testosterone and sperm, making it a common treatment for conditions like hypogonadism and low sperm count. By acting similarly to LH, HCG signals the testes to produce more testosterone, helping to restore hormonal balance and improve reproductive function. It is also sometimes used in young boys with delayed puberty to encourage natural testosterone production and normal development.
While HCG injections are generally well tolerated, they can have side effects, including headaches, mood changes, swelling, and, in some cases, an increased risk of ovarian hyperstimulation syndrome (OHSS) in women undergoing fertility treatments. Proper dosage and medical supervision are crucial to minimizing risks and ensuring effective treatment. HCG is typically administered as a subcutaneous or intramuscular injection, with dosage and frequency depending on the condition being treated.
HCG Blood Test
The HCG blood test is a diagnostic tool used to measure the levels of human chorionic gonadotropin (HCG) in the bloodstream. HCG is a hormone produced during pregnancy by the placenta, making this test one of the most reliable methods for early pregnancy detection. It can also help assess pregnancy progression by tracking hormone levels over time, with rapidly increasing HCG levels indicating a healthy pregnancy.
Beyond pregnancy, the HCG blood test is also used in diagnosing certain medical conditions, such as ectopic pregnancies, gestational trophoblastic disease, and some cancers, including testicular and ovarian tumors. In men, elevated HCG levels may signal testicular cancer, prompting further evaluation. Additionally, doctors may use this test to monitor the effectiveness of fertility treatments or hormone-related therapies.
The test is performed through a simple blood draw, and results are typically available within a few hours to a few days, depending on the laboratory. Unlike home pregnancy tests that detect HCG in urine, blood tests can measure both qualitative (presence of HCG) and quantitative (exact levels of HCG) values, providing more precise information. While generally accurate, results may be affected by factors like medications or medical conditions, requiring careful interpretation by a healthcare professional.
HCG Drops
HCG drops are a form of Human Chorionic Gonadotropin (HCG) supplementation, often marketed for weight loss and hormone regulation. These drops are typically taken sublingually (under the tongue) and are claimed to help reduce appetite, boost metabolism, and promote fat burning when combined with a low-calorie diet. However, many over-the-counter HCG drops do not contain real HCG but instead rely on homeopathic formulations, which lack scientific evidence for effectiveness.
While some individuals report weight loss with HCG drops, most experts believe this is primarily due to the extremely low-calorie diet often recommended alongside them rather than the drops themselves. Medical HCG injections, prescribed for fertility and hormone-related conditions, differ significantly from these over-the-counter products. The FDA has also warned against the use of non-prescription HCG drops, stating that they are ineffective for weight loss and may lead to nutritional deficiencies.
For those considering HCG drops, it is essential to consult a healthcare provider before use, especially if they are being used for weight management. A well-balanced diet and regular exercise remain the safest and most effective ways to achieve sustainable weight loss. Additionally, individuals looking to regulate hormone levels should seek medically supervised treatments rather than relying on unregulated HCG supplements.
Human Chorionic Gonadotropin Test
The human chorionic gonadotropin (HCG) test is a medical test used to detect the presence of HCG, a hormone produced during pregnancy. It is commonly performed through a blood or urine sample to confirm pregnancy, as HCG levels rise significantly in the early weeks. This test is also used to estimate gestational age and monitor pregnancy progression.
Beyond pregnancy, the HCG test can help diagnose certain medical conditions, including trophoblastic disease, ectopic pregnancy, and some types of cancers, such as testicular and ovarian cancer. In men, elevated HCG levels may indicate testicular tumors, making the test a valuable diagnostic tool for reproductive health concerns.
The test is typically quick and simple, with urine tests often available over-the-counter in the form of pregnancy test kits, while blood tests provide more precise measurements. In cases of abnormal HCG levels, further medical evaluation is necessary to determine the underlying cause and ensure appropriate treatment.
When is HCG Detectable?
HCG (Human Chorionic Gonadotropin) becomes detectable in the body shortly after implantation occurs, typically around 6 to 12 days after ovulation. During early pregnancy, HCG levels rise rapidly, doubling approximately every 48 to 72 hours. The exact timing of detection depends on the sensitivity of the test being used and individual variations in implantation timing.
In blood tests, HCG can be detected as early as 8 to 10 days post-ovulation, making it one of the earliest indicators of pregnancy. Blood tests measure the exact concentration of HCG, allowing for early and accurate detection. In contrast, urine pregnancy tests usually detect HCG a few days later, around 12 to 14 days post-ovulation, since the hormone must reach a high enough concentration to be detectable in urine.
For the most accurate results, it is recommended to wait until the first day of a missed period before taking a home pregnancy test. Testing too early may result in a false negative due to low HCG levels. If pregnancy is suspected but an early test is negative, repeating the test after a few days can help confirm the results as HCG levels continue to rise.
How to take HCG?
HCG (Human Chorionic Gonadotropin) is typically administered as an injection either subcutaneously (under the skin) or intramuscularly (into the muscle), depending on the prescribed dosage and treatment plan. It is important to follow the instructions given by a healthcare provider to ensure proper administration. The injection site is usually the abdomen, thigh, or buttocks, and rotating the injection site can help prevent irritation or discomfort.
The dosage and frequency of HCG injections vary based on the condition being treated. For fertility treatments, women may receive a single injection to trigger ovulation, while men treating hypogonadism or low testosterone may take multiple doses per week over a longer period. If using HCG for weight loss or athletic purposes, which is not an FDA-approved use, it should be done under medical supervision to avoid side effects.
Proper storage and handling of HCG are crucial for maintaining its effectiveness. The powder form must be mixed with a sterile diluent before injection and should be refrigerated after reconstitution. It is important to use a clean syringe and needle for each injection to prevent infections, and any unused solution should be discarded after the recommended period. Always consult a doctor before starting HCG treatment to ensure safe and effective use.
HCG Function in Males
Human Chorionic Gonadotropin (HCG) plays a crucial role in stimulating testosterone production in males by mimicking luteinizing hormone (LH). In the testes, LH signals the Leydig cells to produce testosterone, which is essential for male reproductive health, muscle growth, and overall vitality. This function makes HCG particularly useful in treating conditions like hypogonadism, where the body produces insufficient testosterone, leading to symptoms such as fatigue, reduced libido, and loss of muscle mass.
HCG is also widely used in fertility treatments for men, as it helps stimulate sperm production by increasing intratesticular testosterone levels. This is especially beneficial for men with low sperm counts (oligospermia) or those experiencing infertility due to hormonal imbalances. In some cases, HCG is prescribed alongside other fertility medications, such as follicle-stimulating hormone (FSH), to improve sperm quantity and quality, enhancing the chances of conception.
Beyond medical treatments, HCG is sometimes used in post-cycle therapy (PCT) by athletes and bodybuilders who take anabolic steroids. Since steroid use can suppress natural testosterone production, HCG helps reactivate the body’s ability to produce testosterone, preventing severe hormonal imbalances and testicular shrinkage. However, improper or excessive use of HCG for this purpose can lead to adverse effects, including hormone fluctuations and desensitization of the testes, making medical supervision essential.
What does HCG do?
Human Chorionic Gonadotropin (HCG) is a hormone that plays a crucial role in regulating reproductive functions in both men and women. In women, HCG supports pregnancy by maintaining progesterone production in the early stages, ensuring the uterine lining remains stable for embryo implantation. It is also used in fertility treatments to trigger ovulation and enhance the chances of conception.
In men, HCG stimulates the testes to produce testosterone by mimicking luteinizing hormone (LH). This makes it a common treatment for hypogonadism, a condition where the body produces insufficient testosterone, leading to symptoms like fatigue, muscle loss, and reduced libido. Additionally, HCG can help stimulate sperm production in men experiencing infertility due to low sperm count.
Beyond fertility, HCG has been used in certain medical therapies to address hormonal imbalances. Some bodybuilders and athletes use it to restore natural testosterone production after anabolic steroid use, though this is not an FDA-approved application. While HCG has several benefits, it should only be used under medical supervision to avoid potential side effects such as hormone fluctuations, fluid retention, and testicular atrophy.
16. Where is HCG produced?
Human Chorionic Gonadotropin (HCG) is primarily produced in the placenta during pregnancy. Specifically, it is secreted by the syncytiotrophoblast cells, which are responsible for supporting the early development of the embryo. HCG plays a crucial role in maintaining the corpus luteum, which in turn produces progesterone to sustain the pregnancy.
Outside of pregnancy, small amounts of HCG can also be produced by the pituitary gland in both men and women. This pituitary-derived HCG is structurally similar to luteinizing hormone (LH) and plays a minor role in regulating reproductive functions. In some cases, elevated HCG levels in non-pregnant individuals may indicate certain medical conditions, including hormone-secreting tumors.
Additionally, HCG can be found in certain cancerous tumors, such as those of the testes, ovaries, and trophoblastic diseases like choriocarcinoma. Because of this, HCG testing is sometimes used as a tumor marker in diagnosing and monitoring specific cancers. However, its primary and most well-known source remains the placenta during pregnancy.
FAQ
What does human chorionic gonadotropin do?
HCG stimulates the production of progesterone during pregnancy to support fetal development and can also promote testosterone production in men. This pregnancy hormone plays a crucial role in maintaining early pregnancy and can be detected through a urine test.
What does hCG do in males?
The HCG diet involves taking beta hCG to aid in weight loss, but in males, HCG mimics luteinizing hormone (LH) to stimulate testosterone production and support sperm development in the testes.
What is a normal hCG level in a woman?
In non-pregnant women, HCG levels are typically less than 5 mIU/mL, while in early pregnancy, they can range from 5 to over 100,000 mIU/mL depending on the stage. In some cases, a false negative result may occur if HCG levels are too low to be detected early in pregnancy.
What is hCG for baby boys?
HCG helps stimulate the development of male reproductive organs in the fetus and may be used to treat undescended testicles in infants. It can also help maintain testosterone production in certain cases. However, some dangerous HCG diet products are marketed for weight loss, despite potential health risks.
What hCG level indicates pregnancy?
An HCG level above 25 mIU/mL is generally considered indicative of pregnancy, but some people also explore HCG weight loss products for their potential effects on metabolism. Diagnostic tests play a crucial role in confirming HCG levels and assessing related health conditions.
What does hCG do to males?
HCG increases testosterone production, supports fertility, and may help maintain testicular size in men using anabolic steroids or testosterone replacement therapy.
What does gonadotropin hormone do in pregnancy?
Gonadotropin hormones, particularly HCG, maintain the corpus luteum, ensuring progesterone production to sustain early pregnancy and help the body lose weight in certain medical protocols, such as a low calorie diet.
What exactly does hCG do?
HCG supports pregnancy by stimulating progesterone production and, in men, promotes testosterone synthesis and sperm production. HCG levels rise quickly and are commonly detected through a urine pregnancy test result.
What does an hCG test tell you?
An HCG test can confirm pregnancy, detect certain cancers, assess fertility or hormonal disorders, and may occasionally produce a false positive result. Some weight loss clinics also use HCG as part of their treatment programs.
What happens if hCG is positive?
A positive HCG test typically indicates normal pregnancy, but it can also suggest other conditions, such as certain tumors or medical disorders. In some cases, a blood sample is required to confirm and monitor HCG levels accurately.
What does hCG do to your body?
HCG, including hyperglycosylated hCG, regulates hormones, supports pregnancy, influences testosterone levels in men, and can aid in fertility treatments. It is also sometimes considered a weight loss aid.
What does hCG do to a man?
HCG helps men by stimulating natural testosterone production, increasing sperm count, and preventing testicular shrinkage.
Does hCG mean you are pregnant?
HCG is a strong indicator of pregnancy, but false positives can occur due to medical conditions or hormone treatments.
What are the benefits of hCG in bodybuilding?
Bodybuilders use HCG to maintain testosterone levels, prevent testicular shrinkage, and aid recovery after steroid use.
What does human chorionic gonadotropin injection do?
HCG injections stimulate ovulation in women and increase testosterone production in men for fertility or hormone therapy.
What does HCG do to males?
HCG promotes natural testosterone production, supports sperm production, helps maintain testicular function, and is sometimes used as a screening test for hormonal imbalances. Bariatric physicians may also use HCG as part of hormone management protocols.
Where to inject hCG for men?
HCG injections are typically administered subcutaneously (into fat) or intramuscularly (into muscle), usually in the abdomen, thigh, or buttocks. According to Henry’s Clinical Diagnosis, proper administration technique is crucial for effectiveness and safety.
Does hCG increase testicle size?
Yes, HCG can help restore or maintain testicular size, particularly in men experiencing shrinkage due to testosterone therapy or steroid use. It is also sometimes used to assess hormonal balance in relation to the last menstrual period in certain medical evaluations.
What does an hCG blood test tell you?
An HCG blood test can confirm pregnancy, detect hormone-related disorders, or indicate certain cancers. It is often used to assess early pregnancy by measuring HCG levels after the last menstrual period, including cases like molar pregnancies.
How soon can blood hCG detect pregnancy?
HCG products can be detected in blood through laboratory methods as early as 6–8 days after ovulation or implantation.
How many weeks is a 15,000 hCG level?
An HCG level of 15,000 mIU/mL typically corresponds to around 5–6 weeks of pregnancy. Many people explore HCG products during early pregnancy for various reasons. Understanding HCG levels and the role of HCG products can be important for monitoring pregnancy progress.
What happens when your hCG drops?
A drop in HCG during early pregnancy may indicate a miscarriage or an ectopic pregnancy. However, false results can occur due to laboratory errors or testing too early. It’s important to confirm any abnormal HCG levels with follow-up tests to avoid misinterpreting false results.
Why do bodybuilders take hCG?
Bodybuilders use HCG to prevent testosterone suppression, maintain fertility, and avoid testicular shrinkage during or after steroid cycles. The FDA warns that misuse of HCG may lead to serious health risks. Additionally, the FDA warns against using HCG for weight loss or performance enhancement due to potential side effects.
How long after hCG drops will I get my period?
After a miscarriage or stopping HCG treatment, a period usually returns within 4–6 weeks. Clinical pharmacology helps explain how hormonal changes influence this timeline. Understanding clinical pharmacology is essential for managing post-treatment recovery effectively.
What is replacing hCG?
Some alternatives to HCG include Kisspeptin, clomiphene citrate, and gonadotropin-releasing hormone (GnRH) therapies. Most tests suggest these therapies can be effective in stimulating hormone production. Additionally, most tests indicate that individualized treatment plans may optimize outcomes.
What does chorionic gonadotropin test for?
It tests for pregnancy, hormone disorders, and certain cancers, including trophoblastic and testicular tumors. There is substantial evidence supporting its accuracy in detecting these conditions. Additionally, substantial evidence indicates its usefulness in monitoring treatment efficacy.
What hCG level is pregnant?
An HCG level above 25 mIU/mL typically indicates pregnancy. In some cases, hormonal changes during pregnancy may contribute to an irregular heartbeat. It’s essential to monitor any symptoms, such as an irregular heartbeat, during this time for proper medical evaluation.
What is your hCG when not pregnant?
In non-pregnant individuals, HCG levels are usually less than 5 mIU/mL. The free beta subunit of HCG is typically undetectable in these cases. Measuring the free beta subunit can help identify abnormal HCG production.
When should an hCG test be done?
An HCG test should be done after a missed period to confirm pregnancy or when diagnosing fertility or hormonal conditions. It may also help reduce hunger in some weight management programs. Additionally, certain treatments involving HCG can reduce hunger while addressing hormonal imbalances.
How long does it take for hCG to show up in urine?
HCG can be detected in urine 10–14 days after conception, typically measured in milli international units. The concentration of HCG in milli international units increases as pregnancy progresses.
How early can hCG be detected?
HCG can be detected in blood at varying levels as early as 6–8 days after ovulation and in urine at varying levels around 10–14 days post-conception.
How early in pregnancy does hCG start?
HCG production starts right after implantation, usually around 6–7 days after fertilization.
How long does it take for hCG to test positive?
HCG tests turn positive within 10–14 days after conception in urine and 6–8 days in blood.
Do you inject hCG in muscle or fat?
HCG can be injected either intramuscularly (into the muscle) or subcutaneously (into the fat, usually the abdomen or thigh).
How many times a week should I take hCG?
Dosage frequency depends on the purpose; for testosterone maintenance, 2–3 times per week is common, while fertility treatments may require different schedules.
What is the best time of day to take hCG?
There is no strict best time, but many people take it in the morning for consistency.
How do you take hCG with testosterone?
HCG is often used alongside testosterone replacement therapy to maintain natural testosterone production and fertility.
What is the function of hCG in males?
HCG stimulates testosterone production and supports sperm development in the testes.
What does hCG in men indicate?
HCG stimulates testosterone production and supports sperm development in the testes.
What does hCG in men indicate?
HCG presence in men may indicate testosterone therapy, fertility treatment, or, in some cases, testicular tumors.
When does hCG kick in for men?
Effects of HCG in men, such as increased testosterone, can be noticeable within a few days to weeks.
What does hCG do with testosterone?
HCG helps maintain natural testosterone production and prevents testicular shrinkage in men using testosterone therapy.
What do bodybuilders use hCG for?
Bodybuilders use HCG to prevent testosterone suppression, maintain fertility, and restore hormone balance after steroid cycles.
What is the main function of hCG?
The main function of HCG is to support pregnancy by maintaining progesterone levels and, in men, to stimulate testosterone production.
When and where is hCG produced?
HCG is produced mainly in the placenta during pregnancy, but small amounts can be made by the pituitary gland and some tumors.
Where is hCG secretion from?
HCG is primarily secreted by syncytiotrophoblast cells in the placenta during pregnancy.
Is hCG produced by the pituitary gland?
Yes, small amounts of HCG can be produced by the pituitary gland in both men and women.
Is hCG secreted by the ovary?
No, HCG is not directly secreted by the ovary but stimulates the ovaries to produce hormones like progesterone during pregnancy. Follow up care is important to monitor hormone levels and ensure proper ovarian function. Regular follow up care during pregnancy helps track hormonal changes and overall reproductive health.
Reference
Buvat J, Lemaire A, Buvat-Herbaut M. Human chorionic gonadotropin treatment of nonorganic erectile failure and lack of sexual desire: a double-blind study. Urology. 1987 Sep;30(3):216-9. doi: 10.1016/0090-4295(87)90237-8. PMID: 3307093.
Human chorionic gonadotropin treatment of nonorganic erectile failure and lack of sexual desire: a double-blind study
A double-blind study treating 45 cases of erectile failure and lack of sexual desire found that HCG was more effective than a placebo (47% vs. 12%, p < 0.05) in improving sexual parameters, with its benefits appearing independent of plasma testosterone levels.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/3307093/.Parlevliet JM, Bevers MM, van de Broek J, Colenbrander B. Effect of GnRH and hCG administration on plasma LH and testosterone concentrations in normal stallions, aged stallions and stallions with lack of libido. Vet Q. 2001 Apr;23(2):84-7. doi: 10.1080/01652176.2001.9695088. PMID: 11361105.
Effect of GnRH and hCG administration on plasma LH and testosterone concentrations in normal stallions, aged stallions and stallions with lack of libido
The study evaluated the effects of GnRH and hCG on LH and testosterone levels in control, aged, and low-libido stallions during the breeding season. GnRH increased LH in all groups, with the highest response in aged stallions, while hCG increased testosterone, though the response was minimal in aged stallions.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/11361105/.Habous M, Giona S, Tealab A, Aziz M, Williamson B, Nassar M, Abdelrahman Z, Remeah A, Abdelkader M, Binsaleh S, Muir G. Clomiphene citrate and human chorionic gonadotropin are both effective in restoring testosterone in hypogonadism: a short-course randomized study. BJU Int. 2018 Nov;122(5):889-897. doi: 10.1111/bju.14401. Epub 2018 Jun 14. PMID: 29772111.
Clomiphene citrate and human chorionic gonadotropin are both effective in restoring testosterone in hypogonadism: a short-course randomized study
Clomiphene citrate (CC), human chorionic gonadotropin (hCG), and their combination effectively increased testosterone levels in men with hypogonadism over three months, with no significant difference between groups. CC alone is a simple, cost-effective treatment option for hypogonadism while preserving fertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29772111/.Sundby A, Torjesen PA. Plasma levels of testosterone in bulls. Response to repeated HCG injections. Acta Endocrinol (Copenh). 1978 Aug;88(4):787-92. doi: 10.1530/acta.0.0880787. PMID: 581119.
Plasma levels of testosterone in bulls. Response to repeated HCG injections
HCG administration in bulls initially increased plasma testosterone levels, but repeated injections shortened the response due to antibody formation against HCG. Continuous HCG stimulation is required to maintain elevated testosterone, and antibody development correlates with decreased testosterone production.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/581119/.Yang L, Zhang SX, Dong Q, Xiong ZB, Li X. Application of hormonal treatment in hypogonadotropic hypogonadism: more than ten years experience. Int Urol Nephrol. 2012 Apr;44(2):393-9. doi: 10.1007/s11255-011-0065-0. Epub 2011 Oct 12. PMID: 21989858.
Application of hormonal treatment in hypogonadotropic hypogonadism: more than ten years experience
Hormone therapy for hypogonadotropic hypogonadism (HH) is most effective with hCG or hCG/hMG combination therapy, which significantly increases testicular volume and promotes spermatogenesis. Testosterone therapy, however, does not stimulate testicular growth or sperm production.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/21989858/.Soliman AT, Nasr I, Thabet A, Rizk MM, El Matary W. Human chorionic gonadotropin therapy in adolescent boys with constitutional delayed puberty vs those with beta-thalassemia major. Metabolism. 2005 Jan;54(1):15-23. doi: 10.1016/j.metabol.2004.07.006. PMID: 15562375.
Human chorionic gonadotropin therapy in adolescent boys with constitutional delayed puberty vs those with beta-thalassemia major
hCG therapy effectively stimulated puberty in 58% of thalassemic adolescents with delayed puberty, while 42% with higher iron overload showed testicular dysfunction and inadequate testosterone response. Proper iron chelation may help prevent testicular damage, with hCG suitable for those with mild dysfunction and testosterone replacement for those with severe impairment.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/15562375/.Nilsson S, Hellberg D. Recovery of spermatozoa after rFSH/hCG treatment, and subsequent ICSI/IVF, in a male with testicular atrophy due to severe congenital hypogonadotrophic hypogonadism. Arch Androl. 2006 Mar-Apr;52(2):135-8. doi: 10.1080/01485010500315891. PMID: 16443591.
Recovery of spermatozoa after rFSH/hCG treatment, and subsequent ICSI/IVF, in a male with testicular atrophy due to severe congenital hypogonadotrophic hypogonadism
A 47-year-old man with congenital idiopathic hypogonadotropic hypogonadism and absent testes was treated with rFSH/hCG, leading to the development of almond-sized testes and the production of mobile spermatozoa. Although intracytoplasmic sperm injection (ICSI) was performed, resulting embryo transfers did not achieve a clinical pregnancy, marking the first reported case of successful testicular growth from atrophy using this treatment.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/16443591/.Lee JA, Ramasamy R. Indications for the use of human chorionic gonadotropic hormone for the management of infertility in hypogonadal men. Transl Androl Urol. 2018;7(Suppl 3):S348-S352. doi:10.21037/tau.2018.04.11.
Indications for the use of human chorionic gonadotropic hormone for the management of infertility in hypogonadal men
This review discusses the use of human chorionic gonadotropin (hCG) therapy to maintain or restore fertility in hypogonadal men, particularly those undergoing testosterone replacement therapy (TRT), which can cause infertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/30159241/.Niedzielski J, Kucharski P, Slowikowska-Hilczer J. The volume of unilaterally undescended testis after hCG therapy compared to orchidopexy and combined methods. Andrology. 2018 Sep;6(5):742-747. doi: 10.1111/andr.12507. Epub 2018 Jun 4. PMID: 29869442.
The volume of unilaterally undescended testis after hCG therapy compared to orchidopexy and combined methods
The study compared the effects of hCG therapy, surgical treatment, and combined therapy on testicular volume (TV) in boys with unilateral canalicular undescended testis (UDT). While all treatments increased TV and reduced the testicular atrophy index (TAI) over time, patient age and treatment type did not significantly affect outcomes, and hCG therapy did not impair testicular development.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29869442/.Madhusoodanan, Vinayak et al. “Human Chorionic Gonadotropin monotherapy for the treatment of hypogonadal symptoms in men with total testosterone > 300 ng/dL.” International braz j urol : official journal of the Brazilian Society of Urology vol. 45,5 (2019): 1008-1012. doi:10.1590/S1677-5538.IBJU.2019.0132.
Human Chorionic Gonadotropin monotherapy for the treatment of hypogonadal symptoms in men with total testosterone > 300 ng/dL
This study evaluated hCG monotherapy for men with hypogonadal symptoms and testosterone levels above 300 ng/dL. Results showed a 49.9% increase in mean testosterone levels and symptom improvement in 50% of patients, suggesting that hCG is a safe and effective treatment option.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC6844348/.Kucharski P, Niedzielski J. Neoadjuvant human Chorionic Gonadotropin (hCG) therapy may improve the position of undescended testis: a preliminary report. Cent European J Urol. 2013;66(2):224-228. doi:10.5173/ceju.2013.02.art29.
Neoadjuvant human Chorionic Gonadotropin (hCG) therapy may improve the position of undescended testis: a preliminary report
Neoadjuvant hCG therapy for cryptorchidism resulted in 44.5% of undescended testes (UDT) descending to the scrotum, avoiding surgery, and improved the testicular position in an additional 37.3% of cases before orchiopexy, with an overall effectiveness of 81.8%. The initial testicle position did not significantly impact treatment success or future gonad atrophy.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC3936151/.Shiraishi K, Ohmi C, Matsuyama H. Patient-reported outcomes and biochemical alterations during hormonal therapy in men with hypogonadotropic hypogonadism who have finished infertility treatment. Endocr J. 2021 Feb 28;68(2):221-229. doi: 10.1507/endocrj.EJ20-0365. Epub 2020 Oct 3. PMID: 33012744.
Patient-reported outcomes and biochemical alterations during hormonal therapy in men with hypogonadotropic hypogonadism who have finished infertility treatment
This study compared testosterone replacement therapy (TRT) and human chorionic gonadotropin (hCG) in men with male hypogonadotropic hypogonadism (MHH) after infertility treatment. Both treatments improved quality of life and erectile function, but hCG showed better mental and general health outcomes, while TRT caused testicular atrophy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/33012744/.Okuyama A, Nakamura M, Namiki M, Aono T, Matsumoto K, Utsunomiya M, Yoshioka T, Itoh H, Itatani H, Mizutani S, et al. Testicular responsiveness to long-term administration of hCG and hMG in patients with hypogonadotrophic hypogonadism. Horm Res. 1986;23(1):21-30. doi: 10.1159/000180284. PMID: 3079723.
Testicular responsiveness to long-term administration of hCG and hMG in patients with hypogonadotrophic hypogonadism
Long-term intramuscular administration of hCG and hMG in males with hypogonadotrophic hypogonadism increased testosterone and 17 beta-estradiol levels, with faster and higher hormonal responses in hypophysectomized patients. While some patients showed improved sperm density and motility, only 3 hypophysectomized patients achieved fertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/3079723/.Weinstein RL, Kelch RP, Jenner MR, Kaplan SL, Grumbach MM. Secretion of unconjugated androgens and estrogens by the normal and abnormal human testis before and after human chorionic gonadotropin. J Clin Invest. 1974 Jan;53(1):1-6. doi: 10.1172/JCI107526. PMID: 4271572; PMCID: PMC301431.
Secretion of unconjugated androgens and estrogens by the normal and abnormal human testis before and after human chorionic gonadotropin
The study compared androgen and estrogen secretion in normal men and those with unilateral testicular atrophy, finding that HCG stimulation significantly increased hormone levels in normal testes but not in atrophic ones. It also concluded that the human testis secretes a small amount of estrone (E1), and peripheral hormone measurements may not reflect reduced hormone production in atrophic testes.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/4271572/.Depenbusch M, von Eckardstein S, Simoni M, Nieschlag E. Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone. Eur J Endocrinol. 2002 Nov;147(5):617-24. doi: 10.1530/eje.0.1470617. PMID: 12444893.
Maintenance of spermatogenesis in hypogonadotropic hypogonadal men with human chorionic gonadotropin alone
FSH is essential for maintaining normal sperm production in men with hypogonadotropic hypogonadism. While hCG alone can sustain spermatogenesis qualitatively after induction, sperm counts gradually decline without FSH.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/12444893/.Vicari E, Mongioì A, Calogero AE, Moncada ML, Sidoti G, Polosa P, D’Agata R. Therapy with human chorionic gonadotrophin alone induces spermatogenesis in men with isolated hypogonadotrophic hypogonadism–long-term follow-up. Int J Androl. 1992 Aug;15(4):320-9. doi: 10.1111/j.1365-2605.1992.tb01131.x. PMID: 1516981.
Therapy with human chorionic gonadotrophin alone induces spermatogenesis in men with isolated hypogonadotrophic hypogonadism–long-term follow-up
Long-term hCG treatment in male patients with isolated hypogonadotrophic hypogonadism (IHH) increased testicular volume, normalized plasma testosterone levels, and induced spermatogenesis in 70% of patients. While hCG alone was effective, some patients showed further improvements in testicular volume, sperm output, and pregnancy outcomes with the addition of hMG.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/1516981/.Ley SB, Leonard JM. Male hypogonadotropic hypogonadism: factors influencing response to human chorionic gonadotropin and human menopausal gonadotropin, including prior exogenous androgens. J Clin Endocrinol Metab. 1985 Oct;61(4):746-52. doi: 10.1210/jcem-61-4-746. PMID: 3928676.
Male hypogonadotropic hypogonadism: factors influencing response to human chorionic gonadotropin and human menopausal gonadotropin, including prior exogenous androgens
Gonadotropin therapy with hCG and hMG effectively stimulates testosterone production, increases testicular volume, and induces spermatogenesis in men with hypogonadotropic hypogonadism, regardless of prior testosterone treatment. Most patients developed sperm in their ejaculate, with some achieving pregnancies, and hCG alone was sufficient to maintain sperm production in certain cases.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/3928676/.Yasukawa A, Nakahara M, Kume T, Muromoto T, Mizutani H. [Combined administration of human chorionic gonadotropin and human menopausal gonadotropin in idiopathic male infertility]. Hinyokika Kiyo. 1984 Feb;30(2):279-84. Japanese. PMID: 6430049.
[Combined administration of human chorionic gonadotropin and human menopausal gonadotropin in idiopathic male infertility]
A study on 44 cases of idiopathic male infertility examined the effects of hCG/hMG therapy over 12 weeks, showing increased sperm count in 26% of group I and 24% of group II, with four pregnancies in group I but no significant changes in testicular volume or sex hormone levels.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/6430049/.Zhou B, Hutson JM, Watts LM, Hasthorpe S, Naim-Ul-Islam. Human chorionic gonadotrophin (hCG) stimulates spermatogenesis in immature mice in vivo. J Pediatr Surg. 2002 Dec;37(12):1751-3. doi: 10.1053/jpsu.2002.36713. PMID: 12483648.
Human chorionic gonadotrophin (hCG) stimulates spermatogenesis in immature mice in vivo
This study shows that human chorionic gonadotropin (hCG) accelerates the transformation of primary spermatocytes to round spermatids in immature mouse testes. These findings suggest that hCG treatment could potentially induce premature spermatogenesis in prepubertal cryptorchid boys.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/12483648/.Okuyama A, Namiki M, Aono T, Matsumoto K, Utsunomiya M, Itoh H, Yoshioka T, Itatani H, Sonoda T. Improvement of spermatogenesis by hCG and hMG treatment in pubertal boys with history of orchiopexy at early childhood. Arch Androl. 1984;12 Suppl:29-33. PMID: 6152576.
Improvement of spermatogenesis by hCG and hMG treatment in pubertal boys with history of orchiopexy at early childhood
Long-term hCG and hMG treatment during puberty in boys with a history of bilateral cryptorchidism and orchiopexy showed limited improvement in spermatogenesis, with some patients developing oligozoospermia while others remained azoospermic. The findings suggest that gonadotropin therapy during puberty may partially restore sperm production but does not guarantee normal fertility outcomes.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/6152576/.Kobori Y, Suzuki K, Iwahata T, Shin T, Sato R, Nishio K, Yagi H, Arai G, Soh S, Okada H. Hormonal therapy (hCG and rhFSH) for infertile men with adult-onset idiopathic hypogonadotropic hypogonadism. Syst Biol Reprod Med. 2015 Apr;61(2):110-2. doi: 10.3109/19396368.2014.994789. Epub 2014 Dec 18. PMID: 25518839.
Hormonal therapy (hCG and rhFSH) for infertile men with adult-onset idiopathic hypogonadotropic hypogonadism
This study evaluated the efficacy and safety of combined hCG and rhFSH therapy in men with adult-onset idiopathic male hypogonadotropic hypogonadism (IMHH), restoring spermatogenesis in five of seven patients, with one achieving spontaneous pregnancy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/25518839/.Babak J, Behruz F, Mohammadreza Y, Morteza FK. The Effect of Human Chorionic Gonadotropin Therapy on Semen Parameters and Pregnancy Rate after Varicocelectomy. Curr Urol. 2018 Feb;11(2):92-96. doi: 10.1159/000447200. Epub 2017 Dec 30. PMID: 29593468; PMCID: PMC5836152.
The Effect of Human Chorionic Gonadotropin Therapy on Semen Parameters and Pregnancy Rate after Varicocelectomy
The study investigated the effect of hCG on spermatogenesis in infertile patients with varicocele undergoing varicocelectomy. Results showed that combining hCG with varicocelectomy significantly increased pregnancy rates compared to varicocelectomy alone, though further research is needed to confirm these findings.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC5836152/.Fink J, Schoenfeld BJ, Hackney AC, Maekawa T, Horie S. Human chorionic gonadotropin treatment: a viable option for management of secondary hypogonadism and male infertility. Expert Rev Endocrinol Metab. 2021 Jan;16(1):1-8. doi: 10.1080/17446651.2021.1863783. Epub 2020 Dec 21. PMID: 33345656.
Human chorionic gonadotropin treatment: a viable option for management of secondary hypogonadism and male infertility
Human chorionic gonadotropin (HCG) is an effective treatment for male infertility and secondary hypogonadism due to its ability to stimulate testosterone and sperm production without the fertility risks associated with testosterone replacement therapy. This review summarizes research from 1977-2020, comparing the benefits and drawbacks of HCG versus testosterone replacement for treating low testosterone.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/33345656/Hsieh TC, Pastuszak AW, Hwang K, Lipshultz LI. Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy. J Urol. 2013 Feb;189(2):647-50. doi: 10.1016/j.juro.2012.09.043. Epub 2012 Dec 20. PMID: 23260550.
Concomitant intramuscular human chorionic gonadotropin preserves spermatogenesis in men undergoing testosterone replacement therapy
Co-administration of low-dose human chorionic gonadotropin (hCG) with testosterone replacement therapy (TRT) in hypogonadal men helps maintain intratesticular testosterone and preserves semen parameters, preventing azoospermia. This combined therapy may support fertility in men undergoing TRT.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/23260550/.Coviello AD, Matsumoto AM, Bremner WJ, Herbst KL, Amory JK, Anawalt BD, Sutton PR, Wright WW, Brown TR, Yan X, Zirkin BR, Jarow JP. Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression. J Clin Endocrinol Metab. 2005 May;90(5):2595-602. doi: 10.1210/jc.2004-0802. Epub 2005 Feb 15. PMID: 15713727.
Low-dose human chorionic gonadotropin maintains intratesticular testosterone in normal men with testosterone-induced gonadotropin suppression
This study examined the relationship between human chorionic gonadotropin (hCG) and intratesticular testosterone (ITT) in healthy men, showing that low doses of hCG can maintain ITT within the normal range despite gonadotropin suppression. The findings suggest a linear increase in ITT with rising hCG doses, providing a foundation for determining the ITT threshold needed for normal spermatogenesis.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/15713727/.Barrio R, de Luis D, Alonso M, Lamas A, Moreno JC. Induction of puberty with human chorionic gonadotropin and follicle-stimulating hormone in adolescent males with hypogonadotropic hypogonadism. Fertil Steril. 1999 Feb;71(2):244-8. doi: 10.1016/s0015-0282(98)00450-6. PMID: 9988392.
Induction of puberty with human chorionic gonadotropin and follicle-stimulating hormone in adolescent males with hypogonadotropic hypogonadism
Long-term combined hCG and FSH therapy effectively induces puberty, increases testicular volume, and stimulates spermatogenesis in adolescent males with isolated or panhypopituitarism-associated hypogonadotropic hypogonadism. All patients achieved normal sexual maturation and testosterone levels.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/9988392/Fink J, Schoenfeld BJ, Hackney AC, Maekawa T, Horie S. Human chorionic gonadotropin treatment: a viable option for management of secondary hypogonadism and male infertility. Expert Rev Endocrinol Metab. 2021 Jan;16(1):1-8. doi: 10.1080/17446651.2021.1863783. Epub 2020 Dec 21. PMID: 33345656.
Human chorionic gonadotropin treatment: a viable option for management of secondary hypogonadism and male infertility
HCG therapy is an effective treatment for low testosterone and secondary hypogonadism, as it stimulates testosterone and sperm production without the fertility risks associated with testosterone replacement therapy. This review summarizes research from 1977-2020, comparing the benefits and drawbacks of HCG versus testosterone replacement therapy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/33345656/.Habous M, Giona S, Tealab A, Aziz M, Williamson B, Nassar M, Abdelrahman Z, Remeah A, Abdelkader M, Binsaleh S, Muir G. Clomiphene citrate and human chorionic gonadotropin are both effective in restoring testosterone in hypogonadism: a short-course randomized study. BJU Int. 2018 Nov;122(5):889-897. doi: 10.1111/bju.14401. Epub 2018 Jun 14. PMID: 29772111.
Clomiphene citrate and human chorionic gonadotropin are both effective in restoring testosterone in hypogonadism: a short-course randomized study
Clomiphene citrate (CC), human chorionic gonadotropin (hCG), and their combination effectively increased testosterone levels in men with hypogonadism over 3 months, with no significant difference between treatments. CC alone is a simple, cost-effective option for treating hypogonadism while preserving fertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29772111/.Crosnoe LE, Grober E, Ohl D, Kim ED. Exogenous testosterone: a preventable cause of male infertility. Transl Androl Urol. 2013 Jun;2(2):106-13. doi: 10.3978/j.issn.2223-4683.2013.06.01. PMID: 26813847; PMCID: PMC4708215.
Exogenous testosterone: a preventable cause of male infertility. Transl Androl Urol
Testosterone replacement therapy suppresses spermatogenesis by reducing intratesticular testosterone levels. Treatments like clomiphene citrate or hCG can preserve fertility, with most men regaining normal sperm production within a year after stopping testosterone therapy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/26813847/.Sukegawa G, Tsuji Y. [Risk of Male Infertility Due to Testosterone Replacement Therapy for Late-Onset Hypogonadism (LOH)]. Hinyokika Kiyo. 2020 Nov;66(11):407-409. Japanese. doi: 10.14989/ActaUrolJap_66_11_407. PMID: 33271659.
[Risk of Male Infertility Due to Testosterone Replacement Therapy for Late-Onset Hypogonadism (LOH)]
Testosterone replacement therapy for late-onset hypogonadism (LOH) can suppress the hypothalamic-pituitary-gonadal axis, leading to spermatogenic failure and infertility. Clinicians should avoid testosterone in men wishing to maintain fertility and consider alternatives like clomiphene or hCG.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/33271659/.Ramasamy R, Armstrong JM, Lipshultz LI. Preserving fertility in the hypogonadal patient: an update. Asian J Androl. 2015 Mar-Apr;17(2):197-200. doi: 10.4103/1008-682X.142772. PMID: 25337850; PMCID: PMC4378070.
Preserving fertility in the hypogonadal patient: an update. Asian J Androl
An increasing number of young men are experiencing hypogonadism due to testosterone supplementation and anabolic steroid use, leading to infertility risks like azoospermia. Treatments such as human chorionic gonadotropin (hCG) and selective estrogen receptor modulators can help preserve fertility and maintain testosterone production.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/25337850/.Mikirova NA, Casciari JJ, Hunninghake RE, Beezley MM. Effect of weight reduction on cardiovascular risk factors and CD34-positive cells in circulation. Int J Med Sci. 2011;8(6):445-452. doi:10.7150/ijms.8.445.
Effect of weight reduction on cardiovascular risk factors and CD34-positive cells in circulation
A low-calorie diet combined with vitamins, minerals, probiotics, and hCG supplementation reduced body fat, improved lipid profiles, and increased circulating CD34-positive progenitor cells. These findings suggest that improving body composition may positively impact cardiovascular risk factors and stem/progenitor cell numbers.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC3156990/.Grossini E, Surico D, Mary DA, Molinari C, Surico N, Vacca G. In anesthetized pigs human chorionic gonadotropin increases myocardial perfusion and function through a β-adrenergic-related pathway and nitric oxide. J Appl Physiol (1985). 2013 Aug 15;115(4):422-35. doi: 10.1152/japplphysiol.00425.2013. Epub 2013 Jun 20. PMID: 23788572.
In anesthetized pigs human chorionic gonadotropin increases myocardial perfusion and function through a β-adrenergic-related pathway and nitric oxide
This study found that human chorionic gonadotropin (hCG) increases cardiac function and coronary blood flow in anesthetized pigs through the activation of β-adrenoceptors and nitric oxide (NO) release. The effects are mediated by cAMP/PKA signaling and other intracellular pathways in coronary endothelial cells.
You can read the abstract of this article at|
https://pubmed.ncbi.nlm.nih.gov/23788572/.Alanen J, Korpimaki T, Kouru H, Sairanen M, Leskinen M, Gissler M, Ryynanen M, Nevalainen J. First trimester combined screening biochemistry in detection of congenital heart defects. J Matern Fetal Neonatal Med. 2019 Oct;32(19):3272-3277. doi: 10.1080/14767058.2018.1462324. Epub 2018 Apr 22. PMID: 29683008.
First trimester combined screening biochemistry in detection of congenital heart defects
The study evaluated first-trimester biochemical markers (PAPP-A and fβ-hCG) and nuchal translucency (NT) for detecting severe congenital heart defects (CHDs). Results showed that low PAPP-A and fβ-hCG levels improved CHD detection, particularly for ventricular septal defects (VSD) and hypoplastic left heart syndrome (HLHS), even when NT measurements were normal.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29683008/.Erythropoietin (EPO)Kanashiro-Takeuchi RM, Takeuchi LM, Hatzistergos K, Quevedo H, Selem SM, Treuer AV, Premer C, Balkan W, Margitich I, Song Y, Hu Q, Hare JM. Effects of combination of proliferative agents and erythropoietin on left ventricular remodeling post-myocardial infarction. Clin Transl Sci. 2011 Jun;4(3):168-74. doi: 10.1111/j.1752-8062.2011.00278.x. PMID: 21707946; PMCID: PMC3408228.
Effects of combination of proliferative agents and erythropoietin on left ventricular remodeling post-myocardial infarction
Combining erythropoietin (EPO) with human chorionic gonadotrophin (hCG) improves cardiac function and reduces apoptosis after myocardial infarction (MI). While EPO enhances neovascularization, the combination of hCG and EPO also promotes c-kit+ cell proliferation, suggesting a synergistic effect on heart repair.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/21707946/.Abu Alshamat E, Al-Okla S, Soukkarieh CH, Kweider M. Human chorionic gonadotrophin (hCG) enhances immunity against L. tropica by stimulating human macrophage functions. Parasite Immunol. 2012 Oct;34(10):449-54. doi: 10.1111/j.1365-3024.2012.01368.x. PMID: 22540351.
Human chorionic gonadotrophin (hCG) enhances immunity against L. tropica by stimulating human macrophage functions
This study found that human chorionic gonadotropin (hCG) enhances immune responses against Leishmania tropica by increasing nitric oxide, IL-6, and IL-12p40 production in macrophages, reducing infection in a dose-dependent manner, with the strongest effect at 250 U/mL.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/22540351/.Kayisli UA, Selam B, Guzeloglu-Kayisli O, Demir R, Arici A. Human chorionic gonadotropin contributes to maternal immunotolerance and endometrial apoptosis by regulating Fas-Fas ligand system. J Immunol. 2003 Sep 1;171(5):2305-13. doi: 10.4049/jimmunol.171.5.2305. PMID: 12928375.
Human chorionic gonadotropin contributes to maternal immunotolerance and endometrial apoptosis by regulating Fas-Fas ligand system
hCG may play a key role in promoting maternal immune tolerance during early pregnancy by increasing apoptosis in endometrial cells and stimulating FasL expression. This process may help facilitate trophoblast invasion and support successful implantation.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/12928375/.Khil, LY., Jun, HS., Kwon, H. et al. Human chorionic gonadotropin is an immune modulator and can prevent autoimmune diabetes in NOD mice. Diabetologia 50, 2147–2155 (2007). https://doi.org/10.1007/s00125-007-0769-y.
Human chorionic gonadotropin is an immune modulator and can prevent autoimmune diabetes in NOD mice
The study found that treating young NOD mice with human chorionic gonadotropin (hCG) prevented the development of type 1 diabetes by modulating the immune system. hCG reduced harmful T cell activity, suppressed pro-inflammatory cytokines, and increased regulatory T cells, which are essential for preventing autoimmune responses.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/17676307/.Dauven D, Ehrentraut S, Langwisch S, Zenclussen AC, Schumacher A. Immune Modulatory Effects of Human Chorionic Gonadotropin on Dendritic Cells Supporting Fetal Survival in Murine Pregnancy. Front Endocrinol (Lausanne). 2016;7:146. Published 2016 Nov 15. doi:10.3389/fendo.2016.00146.
Immune Modulatory Effects of Human Chorionic Gonadotropin on Dendritic Cells Supporting Fetal Survival in Murine Pregnancy
Human chorionic gonadotropin (hCG) helps maintain dendritic cells (DCs) in a tolerogenic state, increasing regulatory T cells (Tregs) and promoting fetal survival. In a murine model, hCG-treated DCs transferred before conception improved pregnancy outcomes by enhancing Treg cell numbers and anti-inflammatory cytokines.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/27895621/.Zimmermann R, Soor B, Braendle W, Lehmann F, Weise HC, Bettendorf G. Gonadotropin therapy of female infertility. Analysis of results in 416 cases. Gynecol Obstet Invest. 1982;14(1):1-18. doi: 10.1159/000299438. PMID: 6811376.
Gonadotropin therapy of female infertility
Gonadotropin therapy is an effective treatment for anovulatory infertility in women with hypothalamic-pituitary failure (WHO group I), showing higher pregnancy (72.2%) and ‘take-home’ baby rates (57.1%) compared to those with hypothalamic-pituitary dysfunction (WHO group II), where success rates are much lower. Most pregnancies occurred within the first four cycles, and ovarian hyperstimulation syndrome was rare but more common in group II.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/2494828/.estrogenic ovulatory dysfunctionLee KL, Couchman GM, Walmer DK. Successful pregnancies in patients with estrogenic anovulation after low-dose human chorionic gonadotropin therapy alone following hMG for controlled ovarian hyperstimulation. J Assist Reprod Genet. 2005 Jan;22(1):37-40. doi: 10.1007/s10815-005-0819-7. PMID: 15807221; PMCID: PMC3455389.
Successful pregnancies in patients with estrogenic anovulation after low-dose human chorionic gonadotropin therapy alone following hMG for controlled ovarian hyperstimulation
Low-dose hCG after FSH-priming successfully supported folliculogenesis and resulted in pregnancy in three women with estrogenic ovulatory dysfunction and high risk for severe OHSS. This approach allowed follicular growth while reducing smaller follicles, minimizing the risk of OHSS and leading to successful pregnancies, including singleton, twin, and triplet deliveries.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/15807221/.Nikmahzar E, Jahanshahi M, Elyasi L, Saeidi M, Babakordi F, Bahlakeh G. Human chorionic gonadotropin attenuates amyloid-β plaques induced by streptozotocin in the rat brain by affecting cytochrome c-ir neuron density. Iran J Basic Med Sci. 2019;22(2):166-172. doi:10.22038/ijbms.2018.31412.7569.
Human chorionic gonadotropin attenuates amyloid-β plaques induced by streptozotocin in the rat brain by affecting cytochrome c-ir neuron density
The study investigated the effects of hCG on amyloid β plaques and cytochrome c-immunoreactive neurons in an Alzheimer’s disease rat model induced by streptozotocin. Results showed that hCG treatment significantly reduced the density of amyloid β plaques and cytochrome c-immunoreactive neurons in the hippocampus, prefrontal cortex, and cerebellum, suggesting its potential to mitigate Alzheimer’s-related brain damage.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/30834082/.A. M. Barron, G. Verdile, K. Taddei, K. A. Bates, R. N. Martins, Effect of Chronic hCG Administration on Alzheimer’s-Related Cognition and Aβ Accumulation in PS1KI Mice, Endocrinology, Volume 151, Issue 11, 1 November 2010, Pages 5380–5388, https://doi.org/10.1210/en.2009-1168.
Effect of Chronic hCG Administration on Alzheimer’s-Related Cognition and Aβ Accumulation in PS1KI Mice, Endocrinology, Volume 151, Issue 11, 1 November 2010
This study suggests that gonadotropin hormones, independent of estrogen, may influence Alzheimer’s-related behavior and cognition. In ovariectomized mice, gonadotropin administration caused hyperactivity, anxiety, and working memory dysfunction but had minimal impact on toxic Aβ42 levels.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/20844010/.0Bernstein L, Hanisch R, Sullivan-Halley J, Ross RK. Treatment with human chorionic gonadotropin and risk of breast cancer. Cancer Epidemiol Biomarkers Prev. 1995 Jul-Aug;4(5):437-40. PMID: 7549796.
Treatment with human chorionic gonadotropin and risk of breast cancer
This study suggests that human chorionic gonadotropin (hCG) administration may reduce breast cancer risk, particularly in women with a body mass index (BMI) below 27.5 kg/m², supporting previous findings from animal models. The risk reduction was more significant in nulliparous women, though the overall results are not definitive.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/7549796/.Rao CV. Protective Effects of Human Chorionic Gonadotropin Against Breast Cancer: How Can We Use This Information to Prevent/Treat the Disease? Reprod Sci. 2017 Aug;24(8):1102-1110. doi: 10.1177/1933719116676396. Epub 2016 Nov 15. PMID: 28715966.
Protective Effects of Human Chorionic Gonadotropin Against Breast Cancer: How Can We Use This Information to Prevent/Treat the Disease? Reprod Sci
Human chorionic gonadotropin (hCG) may reduce breast cancer risk by promoting cell differentiation, apoptosis, and blocking cancer-related pathways. Higher hCG levels during pregnancy are linked to lower breast cancer incidence, suggesting potential clinical use for breast cancer prevention and treatment.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/28715966/.Russo IH, Koszalka M, Russo J. Human chorionic gonadotropin and rat mammary cancer prevention. J Natl Cancer Inst. 1990 Aug 1;82(15):1286-9. doi: 10.1093/jnci/82.15.1286. PMID: 2115592.
Human chorionic gonadotropin and rat mammary cancer prevention. J Natl Cancer Inst
This study shows that human chorionic gonadotropin (hCG) significantly reduces the incidence of mammary cancer in Sprague-Dawley rats exposed to the carcinogen DMBA, both when administered before and after carcinogen exposure. The protective effect of hCG was dose-dependent, with higher doses leading to a greater reduction in tumor development.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/2115592/.Schüler-Toprak S, Treeck O, Ortmann O. Human Chorionic Gonadotropin and Breast Cancer. Int J Mol Sci. 2017 Jul 21;18(7):1587. doi: 10.3390/ijms18071587. PMID: 28754015; PMCID: PMC5536074.
Human Chorionic Gonadotropin and Breast Cancer
HCG plays a dual role in breast cancer: placental HCG offers protective effects by promoting cellular differentiation and inhibiting growth, while ectopic β-HCG expression is linked to poor prognosis and tumor promotion. This suggests HCG treatment may help prevent breast cancer, while targeting β-HCG could be a therapeutic approach.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC5536074/.Toniolo P, Grankvist K, Wulff M, et al. Human chorionic gonadotropin in pregnancy and maternal risk of breast cancer. Cancer Res. 2010;70(17):6779-6786. doi:10.1158/0008-5472.CAN-09-4622.
Human chorionic gonadotropin in pregnancy and maternal risk of breast cancer
Higher levels of hCG during the first trimester of a first full-term pregnancy are associated with a reduced maternal breast cancer risk, especially for pregnancies before age 25 and diagnoses after age 40. However, hCG may increase the risk of breast cancer diagnosed before age 40 or within 10 years after pregnancy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/20713523/.Peter Y. Liu, Susan M. Wishart, David J. Handelsman, A Double-Blind, Placebo-Controlled, Randomized Clinical Trial of Recombinant Human Chorionic Gonadotropin on Muscle Strength and Physical Function and Activity in Older Men with Partial Age-Related Androgen Deficiency, The Journal of Clinical Endocrinology & Metabolism, Volume 87, Issue 7, 1 July 2002, Pages 3125–3135, https://doi.org/10.1210/jcem.87.7.8630.
A Double-Blind, Placebo-Controlled, Randomized Clinical Trial of Recombinant Human Chorionic Gonadotropin on Muscle Strength and Physical Function and Activity in Older Men with Partial Age-Related Androgen Deficiency
Three months of twice-weekly r-hCG treatment in older men with partial androgen deficiency increased testosterone, estradiol, body weight, and lean body mass while reducing fat mass, but it did not improve muscle strength, physical activity, or functional performance. The treatment was generally well-tolerated with minor side effects, including nipple tenderness in some participants.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/12107212/.Handelsman DJ. Clinical review: The rationale for banning human chorionic gonadotropin and estrogen blockers in sport. J Clin Endocrinol Metab. 2006 May;91(5):1646-53. doi: 10.1210/jc.2005-2569. Epub 2006 Feb 14. PMID: 16478815.
The rationale for banning human chorionic gonadotropin and estrogen blockers in sport
The study supports banning hCG and estrogen blockers in male athletes due to their significant increase in blood testosterone, which enhances muscle growth and strength. However, since these substances do not meaningfully raise testosterone levels in women, the authors argue that their prohibition should apply only to men, as testing in women may also risk invading privacy by detecting unrecognized pregnancies.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/16478815/.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








