Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
Potential Health Benefits of Mirabegron
- Treats symptoms of overactive bladder [1-25]
- Improves blood sugar levels [26-29]
- Improves heart health [30-34]
- Increases exercise capacity [35-37]
- Improves cognitive function [38]
What is Mirabegron?
Mirabegron is a medicine that is commonly prescribed for the treatment of overactive bladder, a condition that causes a frequent and sudden urge to pass urine. It comes as an extended-release tablet that you need to take by mouth. This means that the tablets slowly and evenly release mirabegron throughout the day.
How does Mirabegron Works?
Mirabegron belongs to a class of medications known as beta-3-adrenergic-receptor agonists. They work by stimulating the beta-3-adrenergic-receptor, resulting in decreased contraction of the smooth muscles of the bladder. As a result, the bladder stores more urine which decreases the symptoms of an overactive bladder.
Chemical Structure of Mirabegron
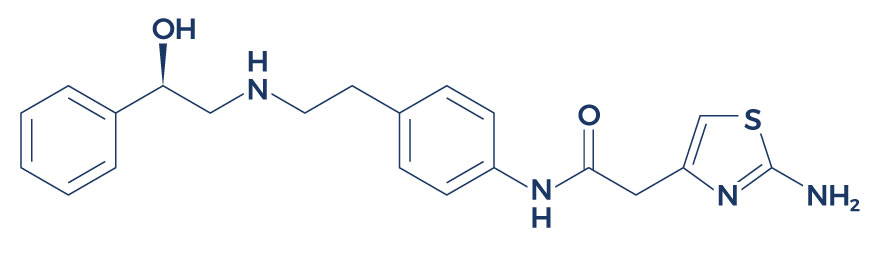
Research on Mirabegron
Treats Symptoms of Overactive Bladder
An overwhelming body of clinical evidence supports the primary benefits of mirabegron:
-
- Results from large phase 3 phase 3 trials showed that oral mirabegron administration for 12 weeks reduced urinary frequency, urgency, and incontinence, and improved the health-related quality of life of patients. [1]
- A review of phase II, III, and IV studies has shown mirabegron to be safe and efficacious in the treatment of symptoms of overactive bladder. [2]
- In Phase III clinical trials in patients with overactive bladder, the administration of mirabegron at various doses (25, 50, and 100 mg) resulted in significant reduction of symptoms. [3-4]
- In patients with overactive bladder, monotherapy with mirabegron decreased nocturia (uncontrolled urination at night), urgency episodes, and urgency urinary incontinence. [5]
- In patients with Parkinson’s disease, mirabegron demonstrated increased efficacy in treating the symptoms of overactive bladder. [6]
- A study reported that the administration of mirabegron in patients with overactive bladder at 25 and 50 mg/day was associated with clinically meaningful benefits. [7]
- In female patients with overactive bladder syndrome, mirabegron treatment for 12 weeks significantly improved the quality of life and sexual health of the subjects. [8]
- Data from 10 studies showed the safety and efficacy profiles of mirabegron in the treatment of overactive bladder for different age groups. [9-10]
- An analysis of multiple studies showed that mirabegron was more effective than placebo in the treatment of symptoms of overactive bladder. [11]
- A review of 64 studies showed that mirabegron 50 mg is significantly better than placebo at relieving symptoms of overactive bladder. [12]
- In men with overactive bladder (OAB) and benign prostatic hyperplasia (BPH), mirabegron has been shown to be effective in treating the symptoms of OAB and BPH with few adverse side effects when used alone or in combination with other medications. [13-20]
- The combination of solifenacin succinate 5 mg plus mirabegron 50 mg tablets over 12 months in patients with overactive bladder reduced urinary frequency, urinary urgency, and unintentional passing of urine. [21-22]
- Studies suggest that mirabegron appears to be a promising treatment for overactive bladder with a low occurrence of side effects. [23-25]
Improves Blood Sugar Levels

Evidence suggests that mirabegron can help improve blood sugar levels by affecting the body’s response to the hormone insulin:
-
- In obese, insulin-resistant humans, mirabegron treatment substantially improved multiple measures of blood sugar balance. [26]
- In healthy women of diverse ethnicities, treatment with 100 mg mirabegron resulted in improved insulin secretion and insulin sensitivity. [27]
- In high-fat diet-induced obese mice, mirabegron treatment improved blood sugar tolerance and insulin sensitivity. [28]
- In healthy young lean males, oral administration of mirabegron (200 mg) increased blood sugar uptake. [29]
Improves Heart Health

Mirabegron has also been shown to exert protective effect against heart failure:
-
- Studies suggest that beta-3-adrenergic-receptors such as mirabegron offer protective mechanism under conditions of increased stress to the heart muscle. [30-32]
- In mice, mirabegron reduced the incidence of heart enlargement. [33]
- In patients with reduced blood flow to the heart, mirabegron significantly increased blood circulation in the left ventricle of the heart. [34]
Increases Exercise Capacity

There are also studies supporting the beneficial effects of mirabegron on exercise capacity:
-
- A study reported that mirabegron can increase exercise capacity by indirectly participating in the regulation of carbohydrate metabolism, improving insulin sensitivity, and supporting cellular uptake of blood sugar. [35]
- In healthy lean men, mirabegron improved exercise capacity by increasing resting energy expenditure (REE). [36]
- A study found that mirabegron can increase exercise capacity by affecting blood sugar and lipid metabolism. [37]
Improves Cognitive Function

Mirabegron can also help improve memory in patients with debilitating injuries.
A study assessed the cognitive effects of mirabegron in older persons with spinal cord injury (SCI). [38] Twenty older persons aged 60 years and above with SCI who are taking medications for neurogenic lower urinary tract dysfunction were enrolled. All of the subjects were studied at baseline then switched to mirabegron treatment for 6 months. Researchers observed that the subjects had significant improvements in immediate Story A recall, and delayed story A and B recall, with no adverse effects on the heart and gut.
Associated Side Effects of Mirabegron
Mirabegron side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on mirabegron. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of mirabegron. Despite this, it was listed as a side effect associated with mirabegron even though these associated side effects are very uncommon.
Side effects associated with mirabegron may include the following:
- Back pain
- Changes in heart rhythm
- Constipation
- Dizziness
- Headache
- Increased heart rate
- Itchy welts
- Joint pain and swelling
- Palpitations
FAQ
What is mirabegron used for?
Mirabegron is primarily used to treat overactive bladder (OAB), which includes symptoms like urinary urgency, frequency, and incontinence. However, it may also affect blood pressure, so monitoring is recommended, especially in patients with a history of hypertension. If you have unused medicine at home, it’s important to check with your healthcare provider about the appropriate use and disposal of any remaining doses. Because mirabegron can increase blood pressure in some individuals, healthcare providers may evaluate baseline blood pressure before starting the medication and continue monitoring it during treatment. Additionally, unused medicine should be safely disposed of to prevent accidental ingestion or misuse.
Can mirabegron be crushed?
Mirabegron tablets should not be crushed or chewed. They should be swallowed whole to maintain their effectiveness and to prevent irritation of the digestive system, as altering the tablet may also affect how it influences blood pressure. Crushing or breaking the tablet could disrupt the process of amide hydrolysis, potentially leading to improper absorption. To ensure the drug works as intended, it is essential to avoid any modifications that could interfere with amide hydrolysis and the controlled release of the medication.
When to take Myrbetriq morning or night?
Myrbetriq (mirabegron) can be taken at any time of the day, but it is generally recommended to take it at the same time each day to help you remember. Whether in the morning or evening depends on personal preference. It is also used to treat neurogenic detrusor overactivity, a condition that causes involuntary bladder contractions. Some people may experience side effects, such as a skin rash, which should be monitored. If a skin rash develops or worsens, it is important to contact your healthcare provider.
What is the best time of day to take mirabegron?
The best time of day to take mirabegron is when it fits into your daily routine, typically in the morning or evening. The medication can be taken with or without food. If you experience any symptoms of a serious allergic reaction, such as swelling, rash, or difficulty breathing, seek medical help immediately. It’s important to note that mirabegron has been associated with potential risks during pregnancy, including increased fetal mortality. Always follow your healthcare provider’s instructions and stop taking the medication if a serious allergic reaction occurs. Additionally, consult your doctor regarding the risk of increased fetal mortality before taking mirabegron during pregnancy.
What is medicine mirabegron used for?
Mirabegron is used to treat symptoms of overactive bladder, such as frequent urination, sudden urges to urinate, and incontinence. The medication mirabegron helps relax the bladder muscle to increase storage capacity. Many patients report improvement in symptoms after starting mirabegron therapy. If mirabegron is not effective or causes side effects, a healthcare provider may suggest an alternate drug to manage these symptoms. Exploring an alternate drug may provide a suitable solution for patients seeking different treatment options for overactive bladder.
Is Myrbetriq bad for your kidneys?
Myrbetriq is generally not harmful to the kidneys when taken as prescribed. However, patients with severe kidney impairment, hepatic impairment, or high blood pressure may require dose adjustments or additional monitoring by their healthcare provider. It’s important to consult with a healthcare provider to determine the appropriate dosage for individuals with hepatic impairment to avoid potential complications.
Does mirabegron cause weight gain?
Weight gain is a potential side effect of mirabegron, though it does not occur in all patients. Some studies have shown a slight increase in weight for some individuals taking the medication, particularly those using it to manage symptoms such as urge urinary incontinence. Additionally, some patients have experienced increased serum ALT, which may contribute to changes in metabolism or weight. It is important for individuals using mirabegron to monitor for any potential changes and consult with their healthcare provider if they experience increased serum ALT or other side effects.
Are Myrbetriq and mirabegron the same thing?
Yes, Myrbetriq is the brand name for the medication mirabegron. Both names refer to the same active ingredient, which is commonly used to treat urge urinary incontinence. During the storage phase, the medication is stored in the bladder and can help manage symptoms like urgency and frequency. Once the medication reaches its therapeutic level, it continues to maintain its effect through the storage phase of treatment.
What are the most common side effects of mirabegron?
Common side effects of mirabegron include high blood pressure, headache, dry mouth, constipation, and urinary tract infection. While it is commonly prescribed to manage urge urinary incontinence, patients should be aware of these potential adverse effects. For more detailed information on side effects, refer to the patient information leaflet. If you are taking mirabegron for urge urinary incontinence and experience any severe side effects, consult your healthcare provider. Be sure to review the patient information leaflet for additional guidance on how to manage and report side effects.
What is the best overactive bladder medication with the least side effects?
The best overactive bladder medication with the least side effects can vary depending on individual response. Anticholinergic drugs (like oxybutynin) and beta-3 adrenergic agonists (like mirabegron) are commonly used, with mirabegron often cited as having a potentially more favorable side effect profile. However, both classes of drugs can carry a risk of urinary retention, especially in susceptible individuals. Individuals with liver disease may need closer monitoring when taking these medications, as liver function can affect drug metabolism. It’s important to monitor for signs of urinary retention when starting or adjusting these medications, particularly for those with liver disease.
Who should not take mirabegron?
Mirabegron should not be taken by individuals with severe uncontrolled high blood pressure, moderate to severe liver impairment, or severe kidney problems. It should also be avoided by those allergic to the drug or its components. Additionally, caution is advised in patients with bladder outlet obstruction, as the medication may worsen urinary retention by affecting the detrusor smooth muscle. People with a history of bladder outlet obstruction should consult their healthcare provider before using mirabegron, as it could impact the detrusor smooth muscle’s function and exacerbate symptoms.
How does mirabegron work?
Mirabegron works by stimulating beta-3 adrenergic receptors in the bladder, which helps relax the bladder muscle, increasing its capacity and reducing the need for frequent urination. It is formulated as extended release tablets to ensure a steady release of the medication throughout the day. Alternatively, an oral suspension formulation may be available for patients who have difficulty swallowing tablets. Taking extended release tablets or oral suspension like mirabegron consistently helps maintain effective symptom control over time.
What foods should you avoid while taking Myrbetriq?
There are no specific foods that need to be avoided while taking Myrbetriq. However, patients with neurogenic detrusor overactivity (NDO) should be cautious with grapefruit or grapefruit juice, as it can interact with some medications, although it has not been shown to directly affect mirabegron. Myrbetriq is also sometimes used in cases of neurogenic detrusor overactivity (NDO), and in such scenarios, it’s especially important to follow dietary and medication guidance from a healthcare provider. Additionally, for patients who require an oral suspension form of Myrbetriq, proper dosing instructions should be carefully followed to ensure effective treatment. If an oral suspension of mirabegron is prescribed, patients should be mindful of any dietary restrictions that could potentially interfere with its absorption or efficacy.
What is the therapeutic effect of mirabegron?
The therapeutic effect of mirabegron is the relaxation of the bladder muscle, which helps to reduce symptoms of overactive bladder, such as urinary urgency, frequency, and incontinence. In some cases, patients may experience lip edema as a side effect, which can cause swelling in the extremities. In the event of an overdose or adverse reaction, it is important to contact a poison control center for guidance. If you experience any severe side effects, such as lip edema or other symptoms, you should also reach out to a poison control center immediately for advice on how to manage the situation.
What is another name for mirabegron?
Mirabegron is also known by its brand name, Myrbetriq.
Why is Myrbetriq so expensive?
Myrbetriq can be expensive due to factors like its status as a branded drug, limited competition from generics, and research and development costs. Additionally, patients with subcutaneous tissue disorders may face higher healthcare costs due to the complexity of managing multiple conditions. The cost of Myrbetriq may also be influenced by steady state concentrations, which can vary between individuals based on factors such as metabolism and comorbid conditions. Pricing varies depending on the healthcare system and insurance coverage, which can also impact those with subcutaneous tissue disorders who require specialized treatments and are managing steady state concentrations of multiple medications.
What are the side effects of taking mirabegron?
The side effects of mirabegron can include increased blood pressure, headache, dry mouth, constipation, urinary tract infections, and dizziness. Most side effects are mild, but it’s important to contact a healthcare provider if any severe reactions occur. Additionally, mirabegron may affect renal function, including glomerular filtration, which is important to monitor in patients with kidney issues. It’s crucial to evaluate glomerular filtration rates to ensure that the medication is not causing any adverse effects on kidney function.
Is there a cheaper alternative to Myrbetriq?
Yes, generic versions of Myrbetriq are available, which are typically cheaper than the brand name. The generic form of mirabegron can offer a more affordable option for individuals experiencing trouble passing urine. This can be especially beneficial for those who need long-term treatment for overactive bladder symptoms, including trouble passing urine.
Which one is better, Myrbetriq or Gemtesa?
Both Myrbetriq (mirabegron) and Gemtesa (vibegron) are effective treatments for overactive bladder (OAB), but they work slightly differently. Myrbetriq tends to have a more significant impact on the bladder’s detrusor muscle, while Gemtesa may have fewer side effects for some individuals. The choice between the two depends on individual response and tolerability, especially when considering any underlying conditions such as reproductive system disorders. Both medications can be beneficial for those dealing with overactive bladder symptoms, but it is important to evaluate them in the context of any reproductive system disorders that may affect treatment outcomes.
How much does vibegron cost compared to mirabegron?
Vibegron (Gemtesa) is generally more expensive than mirabegron (Myrbetriq) because it is a newer medication. Prices vary depending on the pharmacy, insurance, and location, but Gemtesa tends to cost more out-of-pocket. If you miss a dose of vibegron, it is important to take it as soon as you remember unless it’s almost time for your next dose. In case of a missed dose, do not double up on the next dose to make up for the missed dose.
What drug is comparable to Gemtesa?
Toviaz (fesoterodine) and Ditropan (oxybutynin) are examples of drugs that are used for treating overactive bladder, including conditions like neurogenic detrusor overactivity, and can be considered alternatives to Gemtesa, though they work differently and may have different side effect profiles. These medications may also be used in patients with neurogenic detrusor overactivity to help manage symptoms such as urinary urgency and frequency.
What is stronger than mirabegron?
Medications like solifenacin (Vesicare) or oxybutynin (Ditropan) can be considered “stronger” in terms of their anticholinergic effects, though they may come with more side effects compared to mirabegron, which is a beta-3 adrenergic agonist. These medications are typically used in adults, but in some cases, may be prescribed cautiously to pediatric patients under strict supervision. Pediatric patients may have a different tolerance to the side effects of anticholinergic medications, making mirabegron a potentially safer alternative for certain individuals.
Does mirabegron increase metabolism?
Mirabegron has been shown to have mild thermogenic effects, which could slightly increase metabolism, though it is not primarily used as a weight loss drug. Some studies suggest it may increase energy expenditure slightly, making it a potential option for individuals with urinary disorders who may also have metabolic concerns. However, its primary use remains in the treatment of urinary disorders, specifically for overactive bladder symptoms.
What overactive bladder medication helps you lose weight?
Mirabegron (Myrbetriq) has been associated with slight weight loss in some patients, though weight loss is not its primary indication. This effect may be partly due to its influence on active tubular secretion, which can affect the metabolism. Gemtesa (vibegron) may have similar effects on weight due to its metabolism-boosting properties, possibly involving mechanisms like active tubular secretion.
Does Myrbetriq cause loss of appetite?
Loss of appetite is not a common side effect of Myrbetriq. However, some people may experience changes in appetite, gastrointestinal discomfort, or even bladder pain, but this is not typically a primary effect. In rare cases, individuals may also report bladder pain, though this is not commonly associated with the medication.
What is the yo yo effect weight loss?
The “yo-yo effect,” also known as weight cycling, refers to the pattern of losing weight, then regaining it, often repeatedly. This can occur with drastic diets or weight loss methods that are not sustainable long term, potentially leading to minimal oxidative metabolism. When weight loss is achieved too quickly, it can disrupt the body’s natural processes, including minimal oxidative metabolism, which may contribute to the difficulty of maintaining weight loss over time.
Why is mirabegron used in obesity?
Mirabegron has been studied for its potential to aid in weight loss due to its ability to increase thermogenesis and metabolism, particularly in individuals with obesity. However, it is not approved as a weight loss drug and is primarily prescribed for overactive bladder. While some studies suggest it may have an effect on body weight, concerns about its impact on pregnancy have been raised, including potential risks like decreased fetal weight. It is important to consult a healthcare provider before using mirabegron during pregnancy, as it could contribute to decreased fetal weight.
Is mirabegron safer than solifenacin?
Mirabegron tends to have a more favorable side effect profile compared to solifenacin, which is an anticholinergic drug. Solifenacin may cause dry mouth, constipation, and blurred vision, while mirabegron typically has fewer anticholinergic side effects. It is important to consult a health care professional to determine the best treatment for individual needs. A health care professional can help evaluate the risks and benefits of each medication based on the patient’s health status.
What overactive bladder medication has the least side effects?
Mirabegron (Myrbetriq) is often considered to have a more favorable side effect profile compared to other OAB medications, especially anticholinergics like oxybutynin or tolterodine, which may cause dry mouth and constipation. Unlike other medicines used for overactive bladder (OAB), mirabegron does not typically cause the same anticholinergic side effects. This makes it a preferable choice for many patients when compared to other medicines that may cause more bothersome symptoms.
Can mirabegron and solifenacin be used together?
Yes, mirabegron and solifenacin can be used together in certain cases, though combining these medications should be done cautiously under medical supervision due to the potential for increased side effects, especially urinary retention. It’s important to consider how these medications may interact with other medicines the patient may be taking. Always consult a healthcare provider to ensure that the combination of mirabegron, solifenacin, and other medicines is safe for your specific situation.
When is it best to take Myrbetriq?
Myrbetriq is typically taken once daily, with or without food. It is often recommended to take it at the same time each day for consistency, but it can be taken in the morning or evening based on personal preference. It’s important to monitor blood pressure regularly while taking Myrbetriq, especially for those with underlying heart conditions. Additionally, remember to monitor blood pressure throughout the treatment to ensure it remains within a healthy range.
What is the best time of day to take overactive bladder medication?
The best time to take overactive bladder medication depends on the specific medication and individual needs. For Myrbetriq, it is often taken in the morning or at a time when the patient is most likely to remember. However, clinically significant differences in effectiveness may be observed based on the timing of the dose. Some studies suggest that the time of day may affect how well the medication works, though these differences are typically subtle and may not always be clinically significant.
Does Myrbetriq cause sleep problems?
Myrbetriq may cause some individuals to experience sleep disturbances, though this is not a common side effect. If sleep problems occur, it is recommended to consult with a healthcare provider to assess any potential drug associated risk. It’s important to consider that the drug associated risk of sleep issues can vary from person to person, and a healthcare provider can help determine the best course of action.
How long does it take for Myrbetriq to wear off?
Myrbetriq has a half-life of approximately 50 hours, so its effects can last for a prolonged period. It may take a few days for the medication to fully wear off after discontinuation. If you experience any symptoms like a vaginal infection during this time, it’s important to contact your healthcare provider. In some rare cases, medications like Myrbetriq can contribute to changes in your body, which may increase the risk of a vaginal infection.
What happens if you crush mirabegron?
Crushing mirabegron should be avoided as it is designed to be taken as an extended-release tablet. Crushing the tablet can interfere with its proper release and may cause the medication to be released too quickly, potentially increasing the risk of side effects and requiring medical attention. If you accidentally crush or break the tablet, seek medical attention to ensure proper guidance on how to manage the situation.
Can you cut mirabegron in half?
It is not recommended to cut mirabegron tablets in half, as they are extended-release tablets. Cutting them could affect the controlled release of the medication, which may lead to an increased risk of side effects. If you accidentally cut or crush the tablet, seek immediate medical attention. If you experience any unusual symptoms, it is important to contact a healthcare provider for immediate medical attention.
What is the best way to take mirabegron?
Mirabegron should be taken orally once a day with or without food. It is important to swallow the tablet whole and avoid crushing or breaking it. If you experience a weak urine stream while taking mirabegron, consult your healthcare provider for further evaluation. In some cases, a weak urine stream may be a side effect that requires attention.
Can you break Myrbetriq?
Myrbetriq should not be broken, chewed, or crushed as it is an extended-release formulation. Doing so may lead to improper dosing, which could affect its renal elimination, and increase the risk of side effects. The medication relies on controlled release, and altering its form can disrupt its renal elimination process.
What is the best time of day to take mirabegron?
The best time to take mirabegron is usually in the morning or at a time that fits into your daily routine, especially for managing a bladder control condition caused by overactive bladder. Consistency is key to getting the best results and effectively managing the bladder control condition caused by frequent urination.
Does Myrbetriq stop frequent urination?
Myrbetriq is effective at reducing the symptoms of overactive bladder, including frequent urination, urgency, and incontinence, by relaxing the bladder muscles. The maximum dose of Myrbetriq is 50 mg once daily, which may be prescribed if the lower dose does not provide sufficient symptom relief. Taking the maximum dose can help achieve the desired effect while minimizing side effects.
What is the best overactive bladder medication?
The best overactive bladder medication depends on the individual’s response and tolerance, especially when considering multiple doses for effectiveness. Mirabegron (Myrbetriq) and Gemtesa (vibegron) are both newer options with favorable side effect profiles, but anticholinergic medications like oxybutynin are also effective, particularly when multiple doses are required throughout the day.
Is it better to take Myrbetriq at night or in the morning?
Myrbetriq can be taken either in the morning or at night, depending on your preference. However, taking it in the morning may help avoid potential sleep disturbances.
Can mirabegron cause frequent urination?
Mirabegron is designed to treat frequent urination caused by overactive bladder. It is not likely to cause frequent urination; in fact, it helps reduce the frequency of urination. However, as with any medication, it is important to note that mirabegron does not have a narrow therapeutic index, meaning the range between the minimum effective dose and the toxic dose is relatively wide compared to some other drugs. This makes it easier to adjust dosages if needed, but careful monitoring is still advised.
Can you take mirabegron and Amitriptyline together?
It is generally safe to take mirabegron and amitriptyline together, but caution should be exercised due to the potential for additive side effects like dry mouth or constipation. Additionally, if you drink alcohol while taking these medications, it may increase the risk of side effects. Always consult a healthcare provider before combining medications, especially if you drink alcohol.
Reference
Deeks ED. Mirabegron: A Review in Overactive Bladder Syndrome. Drugs. 2018 Jun;78(8):833-844. doi: 10.1007/s40265-018-0924-4. PMID: 29869204.
A Review in Overactive Bladder Syndrome
Overactive bladder syndrome is a complex, often misunderstood condition that significantly impacts quality of life and poses challenges for both patients and medical professionals, requiring accurate diagnosis, individualized treatment, and strong patient-physician collaboration for effective management.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC8549091/.Robinson D, Thiagamoorthy G, Cardozo L. A drug safety evaluation of mirabegron in the management of overactive bladder. Expert Opin Drug Saf. 2016 May;15(5):689-96. doi: 10.1517/14740338.2016.1165663. Epub 2016 Apr 21. PMID: 26980445.
A drug safety evaluation of mirabegron in the management of overactive bladder
Mirabegron is a β3 adrenoceptor agonist shown through extensive clinical trials to be a safe and effective treatment for overactive bladder, offering a well-tolerated alternative to antimuscarinic therapy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/26980445/.Chapple CR, Cardozo L, Nitti VW, Siddiqui E, Michel MC. Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability. Neurourol Urodyn. 2014 Jan;33(1):17-30. doi: 10.1002/nau.22505. Epub 2013 Oct 11. PMID: 24127366.
Mirabegron in overactive bladder: a review of efficacy, safety, and tolerability
Mirabegron, a β3-adrenoceptor agonist, has shown significant and sustained efficacy in improving overactive bladder (OAB) symptoms, including urgency, frequency, and incontinence, with a favorable safety and tolerability profile, especially at 50 and 100 mg doses, and is well tolerated even in older adults and those who discontinued antimuscarinic therapy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/24127366/Warren K, Burden H, Abrams P. Mirabegron in overactive bladder patients: efficacy review and update on drug safety. Ther Adv Drug Saf. 2016;7(5):204-216. doi:10.1177/2042098616659412.
Mirabegron in overactive bladder patients: efficacy review and update on drug safety
Mirabegron, the first approved β3 adrenoceptor agonist for overactive bladder, offers significant symptom improvement with fewer side effects like dry mouth compared to anticholinergics, making it a promising alternative, especially given concerns about anticholinergic-related dementia.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/27695622/.Shin JH, Choo MS. Effectiveness and persistence of mirabegron as a first-line treatment in patients with overactive bladder in real-life practice. Low Urin Tract Symptoms. 2019 May;11(3):151-157. doi: 10.1111/luts.12253. Epub 2019 Jan 8. PMID: 30623583.
Effectiveness and persistence of mirabegron as a first-line treatment in patients with overactive bladder in real-life practice
Mirabegron is an effective first-line treatment for overactive bladder, showing significant symptom improvement, especially in treatment-naïve patients, though its persistence rate declines to 39.4% at 12 months, with those having more severe symptoms often requiring add-on therapy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/30623583/.Cho SY, Jeong SJ, Lee S, Kim J, Lee SH, Choo MS, Oh SJ. Mirabegron for treatment of overactive bladder symptoms in patients with Parkinson’s disease: A double-blind, randomized placebo-controlled trial (Parkinson’s Disease Overactive bladder Mirabegron, PaDoMi Study). Neurourol Urodyn. 2021 Jan;40(1):286-294. doi: 10.1002/nau.24552. Epub 2021 Jan 3. PMID: 33389776.
Mirabegron for treatment of overactive bladder symptoms in patients with Parkinson’s disease: A double-blind, randomized placebo-controlled trial (Parkinson’s Disease Overactive bladder Mirabegron, PaDoMi Study)
Mirabegron was found to be effective and generally well-tolerated for treating overactive bladder symptoms in patients with Parkinsonism, showing significant improvement in symptoms with mostly mild adverse events.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/33389776/.Chapple CR, Siddiqui E. Mirabegron for the treatment of overactive bladder: a review of efficacy, safety and tolerability with a focus on male, elderly and antimuscarinic poor-responder populations, and patients with OAB in Asia. Expert Rev Clin Pharmacol. 2017 Feb;10(2):131-151. doi: 10.1080/17512433.2017.1275570. Epub 2017 Jan 16. PMID: 28001447.
Mirabegron for the treatment of overactive bladder: a review of efficacy, safety and tolerability with a focus on male, elderly and antimuscarinic poor-responder populations, and patients with OAB in Asia
You can read the abstract of this article atAthanasiou S, Pitsouni E, Grigoriadis T, Zacharakis D, Salvatore S, Serati M. Mirabegron in female patients with overactive bladder syndrome: What’s new? A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2020 Aug;251:73-82. doi: 10.1016/j.ejogrb.2020.05.018. Epub 2020 May 15. PMID: 32480182.
Mirabegron in female patients with overactive bladder syndrome: What’s new? A systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol
This review found that mirabegron is a safe and effective treatment for overactive bladder in women, significantly improving urgency, frequency, nocturia, urinary incontinence, quality of life, and sexual health, with fewer side effects than anticholinergics; however, more high-quality, long-term studies are needed.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/32480182/.Chapple CR, Cruz F, Cardozo L, Staskin D, Herschorn S, Choudhury N, Stoelzel M, Heesakkers J, Siddiqui E. Safety and Efficacy of Mirabegron: Analysis of a Large Integrated Clinical Trial Database of Patients with Overactive Bladder Receiving Mirabegron, Antimuscarinics, or Placebo. Eur Urol. 2020 Jan;77(1):119-128. doi: 10.1016/j.eururo.2019.09.024. Epub 2019 Oct 18. PMID: 31635815.
Safety and Efficacy of Mirabegron: Analysis of a Large Integrated Clinical Trial Database of Patients with Overactive Bladder Receiving Mirabegron, Antimuscarinics, or Placebo. Eur Urol
Mirabegron benefits include improved symptoms of overactive bladder—such as reduced incontinence, urgency, and nighttime urination—with a favorable safety profile across different ages and sexes, as confirmed by pooled data from 10 clinical trials.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/31635815/.Gras J. Mirabegron for the treatment of overactive bladder. Drugs Today (Barc). 2012 Jan;48(1):25-32. doi: 10.1358/dot.2012.48.1.1738056. PMID: 22384458.
Mirabegron for the treatment of overactive bladder
Mirabegron is a well-tolerated and effective alternative to antimuscarinics for treating overactive bladder, showing consistent benefits across diverse patient populations, with fewer side effects like dry mouth and constipation and sustained efficacy at approved doses.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/28001447/.Wu T, Duan X, Cao CX, Peng CD, Bu SY, Wang KJ. The role of mirabegron in overactive bladder: a systematic review and meta-analysis. Urol Int. 2014;93(3):326-37. doi: 10.1159/000361079. Epub 2014 Aug 7. PMID: 25115445.
The role of mirabegron in overactive bladder: a systematic review and meta-analysis
Mirabegron was found to be an effective and safe treatment for overactive bladder (OAB), showing improvements in the number of incontinence episodes and micturitions compared to placebo. It was also more effective than tolterodine for incontinence, with a lower rate of adverse reactions, making it a valuable pharmacologic option for managing OAB.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/25115445/.Kelleher C, Hakimi Z, Zur R, Siddiqui E, Maman K, Aballéa S, Nazir J, Chapple C. Efficacy and Tolerability of Mirabegron Compared with Antimuscarinic Monotherapy or Combination Therapies for Overactive Bladder: A Systematic Review and Network Meta-analysis. Eur Urol. 2018 Sep;74(3):324-333. doi: 10.1016/j.eururo.2018.03.020. Epub 2018 Apr 23. PMID: 29699858.
Efficacy and Tolerability of Mirabegron Compared with Antimuscarinic Monotherapy or Combination Therapies for Overactive Bladder: A Systematic Review and Network Meta-analysis
Mirabegron 50mg is an effective treatment for overactive bladder (OAB), offering similar efficacy to antimuscarinics with fewer side effects like dry mouth and constipation. While combination therapy with solifenacin and mirabegron may be slightly more effective, mirabegron alone provides significant symptom relief with better tolerability compared to other treatments.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29699858/.Mullen GR, Kaplan SA. Efficacy and Safety of Mirabegron in Men with Overactive Bladder Symptoms and Benign Prostatic Hyperplasia. Curr Urol Rep. 2021 Jan 7;22(1):5. doi: 10.1007/s11934-020-01017-7. PMID: 33411109.
Efficacy and Safety of Mirabegron in Men with Overactive Bladder Symptoms and Benign Prostatic Hyperplasia
Mirabegron is an effective and safe treatment for men with overactive bladder (OAB) and benign prostatic hyperplasia (BPH). Recent studies show that it successfully treats OAB symptoms with minimal side effects, whether used alone or in combination therapy.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/33411109/.Su S, Lin J, Liang L, Liu L, Chen Z, Gao Y. The efficacy and safety of mirabegron on overactive bladder induced by benign prostatic hyperplasia in men receiving tamsulosin therapy: A systematic review and meta-analysis. Medicine (Baltimore). 2020 Jan;99(4):e18802. doi: 10.1097/MD.0000000000018802. PMID: 31977871; PMCID: PMC7004736.
The efficacy and safety of mirabegron on overactive bladder induced by benign prostatic hyperplasia in men receiving tamsulosin therapy: A systematic review and meta-analysis
A meta-analysis of three randomized controlled trials involving 1,317 men with benign prostatic hyperplasia (BPH) treated with tamsulosin found that mirabegron effectively improved overactive bladder (OAB) symptoms, including reducing micturitions, urgency episodes, and total OAB symptom scores. Mirabegron was well tolerated, with a low occurrence of side effects, although it may increase post-void residual urine volume.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC7004736/.Bhide AA, Digesu GA, Fernando R, Khullar V. Use of mirabegron in treating overactive bladder. Int Urogynecol J. 2012 Oct;23(10):1345-8. doi: 10.1007/s00192-012-1724-0. Epub 2012 Mar 13. PMID: 22411211.
Use of mirabegron in treating overactive bladder
Mirabegron is a promising alternative to antimuscarinics for treating overactive bladder (OAB) by targeting β-3 adrenoreceptors to improve bladder relaxation and capacity. Clinical studies show it reduces micturitions and incontinence episodes, with mild to moderate side effects such as dry mouth and gastrointestinal issues. While it may slightly raise heart rate and blood pressure, ongoing research is needed to further assess its long-term efficacy, safety, and potential drug interactions.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/22411211/.Warren K, Burden H, Abrams P. Mirabegron in overactive bladder patients: efficacy review and update on drug safety. Ther Adv Drug Saf. 2016 Oct;7(5):204-216. doi: 10.1177/2042098616659412. Epub 2016 Jul 19. PMID: 27695622; PMCID: PMC5014049.
Mirabegron in overactive bladder patients: efficacy review and update on drug safety
Mirabegron, the first β3 adrenoceptor agonist approved for overactive bladder, offers significant improvements in key symptoms compared to a placebo. It has a lower incidence of dry mouth compared to anticholinergic medications, though side effects like constipation, hypertension, and tachycardia remain similar. Mirabegron can be safely combined with solifenacin and tamsulosin, and further studies comparing its efficacy with anticholinergics could enhance treatment strategies.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/27695622/.Bragg R, Hebel D, Vouri SM, Pitlick JM. Mirabegron: a Beta-3 agonist for overactive bladder. Consult Pharm. 2014 Dec;29(12):823-37. doi: 10.4140/TCP.n.2014.823. PMID: 25521658; PMCID: PMC4605389.
Mirabegron: a Beta-3 agonist for overactive bladder. Consult Pharm
Mirabegron has been shown to effectively reduce the number of micturitions and incontinence episodes in patients with overactive bladder (OAB), based on data from five randomized, placebo-controlled trials. Common side effects include hypertension, urinary tract infections, and headaches. Overall, mirabegron offers a promising alternative to antimuscarinics for OAB treatment, with further studies needed to assess its utility across different patient demographics.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/25521658/.Mirabegron for overactive bladder syndrome. Drug Ther Bull. 2013 Aug;51(8):90-2. doi: 10.1136/dtb.2013.8.0196. PMID: 23949821.
Mirabegron for overactive bladder syndrome
Mirabegron is an effective and well-tolerated treatment for overactive bladder (OAB), showing significant improvements in urgency urinary incontinence, total incontinence, and nocturia. Compared to other treatments like tolterodine, solifenacin, and oxybutynin, mirabegron demonstrates better results with fewer side effects, and when combined with anticholinergic medications, it offers an additional benefit without increasing adverse events.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/39343691/.Kakizaki H, Lee KS, Yamamoto O, Jong JJ, Katou D, Sumarsono B, Uno S, Yamaguchi O. Mirabegron Add-on Therapy to Tamsulosin for the Treatment of Overactive Bladder in Men with Lower Urinary Tract Symptoms: A Randomized, Placebo-controlled Study (MATCH). Eur Urol Focus. 2020 Jul 15;6(4):729-737. doi: 10.1016/j.euf.2019.10.019. Epub 2019 Nov 11. PMID: 31718957.
Mirabegron Add-on Therapy to Tamsulosin for the Treatment of Overactive Bladder in Men with Lower Urinary Tract Symptoms: A Randomized, Placebo-controlled Study (MATCH). Eur Urol Focus
Mirabegron add-on therapy to tamsulosin in men with lower urinary tract symptoms (LUTS) and overactive bladder (OAB) showed superior efficacy to placebo in improving micturition frequency, OAB symptoms, and patient-reported outcomes. The treatment was well tolerated, with no major safety concerns, though differences in urgency, incontinence, and nocturia were not significant.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/31718957/.Leone Roberti Maggiore U, Cardozo L, Ferrero S, Sileo F, Cola A, Del Deo F, Torella M, Colacurci N, Candiani M, Salvatore S. Mirabegron in the treatment of overactive bladder. Expert Opin Pharmacother. 2014 Apr;15(6):873-87. doi: 10.1517/14656566.2014.898752. Erratum in: Expert Opin Pharmacother. 2014 May;15(7):1059. Del Deo, Fabio [added]. PMID: 24646053.
Salvatore S. Mirabegron in the treatment of overactive bladder. Expert Opin Pharmacother
Mirabegron is a selective β3-adrenergic receptor agonist developed for treating overactive bladder (OAB) and offers an alternative to antimuscarinics. Phase II and III studies demonstrate its efficacy, safety, and tolerability in OAB treatment, with future research needed on CNS concentrations, combination therapies, and its use in pediatrics.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/24646053/.Gratzke, C., van Maanen, R., Chapple, C., Abrams, P., Herschorn, S., Robinson, D., Ridder, A., Stoelzel, M., Paireddy, A., Yoon, S. J., Al-Shukri, S., Rechberger, T., & Mueller, E. R. (2018). Long-term Safety and Efficacy of Mirabegron and Solifenacin in Combination Compared with Monotherapy in Patients with Overactive Bladder: A Randomised, Multicentre Phase 3 Study (SYNERGY II). European urology, 74(4), 501–509. https://doi.org/10.1016/j.eururo.2018.05.005.
Long-term Safety and Efficacy of Mirabegron and Solifenacin in Combination Compared with Monotherapy in Patients with Overactive Bladder: A Randomised, Multicentre Phase 3 Study (SYNERGY II)
No abstract availableYou can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/30025617/.Yamaguchi, O., Kakizaki, H., Homma, Y., Igawa, Y., Takeda, M., Nishizawa, O., Gotoh, M., Yoshida, M., Yokoyama, O., Seki, N., Okitsu, A., Hamada, T., Kobayashi, A., & Kuroishi, K. (2015). Safety and efficacy of mirabegron as ‘add-on’ therapy in patients with overactive bladder treated with solifenacin: a post-marketing, open-label study in Japan (MILAI study). BJU international, 116(4), 612–622. https://doi.org/10.1111/bju.13068.
Safety and efficacy of mirabegron as ‘add-on’ therapy in patients with overactive bladder treated with solifenacin: a post-marketing, open-label study in Japan (MILAI study)
Mirabegron add-on therapy with solifenacin for overactive bladder (OAB) was well tolerated and demonstrated significant improvements in OAB symptoms, including frequency, urgency, and incontinence. The combination therapy, with a possible dose increase of mirabegron to 50 mg, was effective in patients previously treated with solifenacin, showing favorable safety and efficacy outcomes.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/25639296/.Nozawa, Y., Kato, D., Tabuchi, H., & Kuroishi, K. (2018). Safety and Effectiveness of Mirabegron in Patients with Overactive Bladder in a Real-World Clinical Setting: A Japanese Post-Marketing Study. Lower urinary tract symptoms, 10(2), 122–130. https://doi.org/10.1111/luts.12148.
Safety and Effectiveness of Mirabegron in Patients with Overactive Bladder in a Real-World Clinical Setting: A Japanese Post-Marketing Study
No abstract available
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/31009978/.Cui, Y., Zong, H., Yang, C., Yan, H., & Zhang, Y. (2014). The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials. International urology and nephrology, 46(1), 275–284. https://doi.org/10.1007/s11255-013-0509-9.
The efficacy and safety of mirabegron in treating OAB: a systematic review and meta-analysis of phase III trials
Mirabegron is a safe and effective treatment for overactive bladder, significantly reducing incontinence and urinary frequency while being well tolerated, according to a meta-analysis of phase III trials involving over 5,700 patients.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/23896942/.Imran, M., Najmi, A. K., & Tabrez, S. (2013). Mirabegron for overactive bladder: a novel, first-in-class β3-agonist therapy. Urology journal, 10(3), 935–940.
Mirabegron for overactive bladder: a novel, first-in-class β3-agonist therapy
Mirabegron is a novel β3 receptor agonist approved for overactive bladder treatment, working by relaxing the detrusor muscle through nitric oxide and UDIF release, increasing bladder capacity without the common side effects of antimuscarinic drugs.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/24078498/.Finlin BS, Memetimin H, Zhu B, Confides AL, Vekaria HJ, El Khouli RH, Johnson ZR, Westgate PM, Chen J, Morris AJ, Sullivan PG, Dupont-Versteegden EE, Kern PA. The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans. J Clin Invest. 2020 May 1;130(5):2319-2331. doi: 10.1172/JCI134892. PMID: 31961829; PMCID: PMC7190997.
The β3-adrenergic receptor agonist mirabegron improves glucose homeostasis in obese humans
Mirabegron treatment in obese, insulin-resistant humans improved glucose tolerance, insulin sensitivity, and β cell function by stimulating beige fat formation in subcutaneous white adipose tissue, enhancing fat and muscle metabolism without directly acting on β cells or muscle.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/31961829/.O’Mara AE, Johnson JW, Linderman JD, et al. Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity. J Clin Invest. 2020;130(5):2209-2219. doi:10.1172/JCI131126.
Chronic mirabegron treatment increases human brown fat, HDL cholesterol, and insulin sensitivity
Chronic treatment with mirabegron increased brown fat activity, resting energy expenditure, insulin sensitivity, and beneficial metabolic biomarkers in healthy women, suggesting its potential as a therapy for obesity-related metabolic disease.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/31961826/.Hao L, Scott S, Abbasi M, Zu Y, Khan MSH, Yang Y, Wu D, Zhao L, Wang S. Beneficial Metabolic Effects of Mirabegron In Vitro and in High-Fat Diet-Induced Obese Mice. J Pharmacol Exp Ther. 2019 Jun;369(3):419-427. doi: 10.1124/jpet.118.255778. Epub 2019 Apr 2. PMID: 30940691; PMCID: PMC6530071.
Beneficial Metabolic Effects of Mirabegron In Vitro and in High-Fat Diet-Induced Obese Mice
Mirabegron, a β3-adrenergic receptor agonist, promotes brown fat activation and UCP1 expression, leading to reduced body weight, increased browning of white fat, and improved glucose tolerance and insulin sensitivity in high-fat diet-induced obese mice.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/30940691/.Cypess AM, Weiner LS, Roberts‐Toler C, Elia EF, Kessler SH, Kahn PA et al (2015). Activation of human brown adipose tissue by a β3‐adrenergic receptor agonist. Cell Metab 21: 33–38.
Activation of human brown adipose tissue by a β3‐adrenergic receptor agonist
Mirabegron, a β3-adrenergic receptor agonist approved for overactive bladder, was shown to activate brown adipose tissue (BAT) and increase resting metabolic rate in humans, suggesting its potential as a treatment for obesity and metabolic disease.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/25565203/.Michel MC, Harding SE, Bond RA (2011). Are there functional β3‐adrenoceptors in the human heart? Br J Pharmacol 162: 817–822.
Are there functional β3‐adrenoceptors in the human heart?
β₃-adrenoceptors are present in the human heart, but their exact role remains unclear and controversial; while they may affect ventricular function, current evidence suggests limited or no impact on atrial contraction, and more research is needed to confirm their specific cardiac effects, especially with future therapeutic use.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/20735409/.Germack R, Dickenson JM (2006). Induction of β3‐adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes. J Pharmacol Exp Ther 316: 392–402.
This study found that chronic exposure to noradrenaline down-regulates beta(1)- and beta(2)-adrenergic receptors while functionally up-regulating beta(3)-adrenergic receptors, which become coupled to G(i) protein in rat neonatal cardiomyocytes.
Induction of β3‐adrenergic receptor functional expression following chronic stimulation with noradrenaline in neonatal rat cardiomyocytes
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/16183708/.Moniotte S, Kobzik L, Feron O, Trochu JN, Gauthier C, Balligand JL (2001). Upregulation of β3‐adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 103: 1649–1655.
Upregulation of β3‐adrenoceptors and altered contractile response to inotropic amines in human failing myocardium
In failing human hearts, beta(3)-adrenoceptors are upregulated and continue to exert a mild negative inotropic effect, while beta(1)-adrenoceptors are downregulated, leading to a significant reduction in the heart’s contractile response and contributing to overall cardiac dysfunction.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/11273992/.Belge C, Hammond J, Dubois-Deruy E, Manoury B, Hamelet J, Beauloye C, Markl A, Pouleur AC, Bertrand L, Esfahani H, Jnaoui K, Götz KR, Nikolaev VO, Vanderper A, Herijgers P, Lobysheva I, Iaccarino G, Hilfiker-Kleiner D, Tavernier G, Langin D, Dessy C, Balligand JL. Enhanced expression of β3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase. Circulation. 2014 Jan 28;129(4):451-62. doi: 10.1161/CIRCULATIONAHA.113.004940. Epub 2013 Nov 4. PMID: 24190960.
Enhanced expression of β3-adrenoceptors in cardiac myocytes attenuates neurohormone-induced hypertrophic remodeling through nitric oxide synthase
Cardiac-specific overexpression of β3-adrenergic receptors (β3-AR) protects against neurohormonal-induced hypertrophy and fibrosis in mice through a nitric oxide synthase–mediated mechanism, suggesting a potential therapeutic role for β3-AR activation in preventing pathological cardiac remodeling.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/24190960/.Bundgaard H, Axelsson A, Hartvig Thomsen J, Sorgaard M, Kofoed KF, Hasselbalch R et al (2017). The first‐in‐man randomized trial of a β3 adrenoceptor agonist in chronic heart failure: the BEAT‐HF trial. Eur J Heart Fail 19: 566–575.
The first‐in‐man randomized trial of a β3 adrenoceptor agonist in chronic heart failure: the BEAT‐HF trial
Mirabegron, a β3 adrenergic receptor agonist, was tested in a trial involving patients with heart failure (HF) to evaluate its effect on left ventricular ejection fraction (LVEF). While the primary endpoint was not met, an exploratory analysis showed that mirabegron significantly increased LVEF in patients with baseline LVEF <40%, suggesting potential benefits in severe HF. The treatment was generally well tolerated, and further studies are needed to confirm these findings.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/27990717/.Leis K, Mazur E, Racinowski M, Świerczyński W, Baska A, Gałązka P. Effect of Mirabegron on the Body’s Exercise Capacity: A Review. Endocr Metab Immune Disord Drug Targets. 2020;20(9):1448-1455. doi: 10.2174/1871530320666200516164434. PMID: 32416711.
Effect of Mirabegron on the Body’s Exercise Capacity: A Review. Endocr Metab Immune Disord Drug Targets
Mirabegron, a β3-agonist approved for overactive bladder, activates adrenergic receptors to relax the detrusor muscle and also has lipolytic effects, reducing both brown and white adipose tissue. It decreases body weight, triglycerides, and improves insulin sensitivity, though caution is needed due to potential cardiovascular effects like elevated blood pressure and pulse. Additionally, recent studies explore its impact on exercise capacity and its potential use as a doping agent.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/32416711/.Nahon KJ, Janssen LGM, Sardjoe Mishre ASD, Bilsen MP, van der Eijk JA, Botani K, Overduin LA, Ruiz JR, Burakiewicz J, Dzyubachyk O, Webb AG, Kan HE, Berbée JFP, van Klinken JB, van Dijk KW, van Weeghel M, Vaz FM, Coskun T, Jazet IM, Kooijman S, Martinez-Tellez B, Boon MR, Rensen PCN. The effect of mirabegron on energy expenditure and brown adipose tissue in healthy lean South Asian and Europid men. Diabetes Obes Metab. 2020 Nov;22(11):2032-2044. doi: 10.1111/dom.14120. Epub 2020 Jul 29. PMID: 32558052; PMCID: PMC7771034.
The effect of mirabegron on energy expenditure and brown adipose tissue in healthy lean South Asian and Europid men. Diabetes Obes Metab
Mirabegron and cold exposure both induce beneficial metabolic effects in South Asians and Europids, increasing energy expenditure and lipid oxidation. While cold exposure raised serum lipid levels and decreased brown adipose tissue (BAT) fat fraction in both ethnicities, mirabegron primarily increased free fatty acids and enhanced lipid oxidation in Europids.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC7771034/.Bel JS, Tai TC, Khaper N, Lees SJ. Mirabegron: The most promising adipose tissue beiging agent. Physiol Rep. 2021 Mar;9(5):e14779. doi: 10.14814/phy2.14779. PMID: 33650753; PMCID: PMC7923552.
The most promising adipose tissue beiging agent
Mirabegron (Myrbetriq®), a β3-adrenergic receptor agonist, has shown potential in activating “beiging” of white adipose tissue (WAT), which may aid in treating metabolic disorders like obesity and type 2 diabetes. Recent studies suggest that mirabegron can improve adipokine levels, glucose metabolism, and lipid droplet size, showing similar benefits to intermittent cold exposure and exercise without the cardiovascular side effects. These findings position mirabegron as a promising and safe option for metabolic disorder treatment.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC7923552/.Trbovich M, Romo T, Polk M, Koek W, Kelly C, Stowe S, Kraus S, Kellogg D. The treatment of neurogenic lower urinary tract dysfunction in persons with spinal cord injury: An open label, pilot study of anticholinergic agent vs. mirabegron to evaluate cognitive impact and efficacy. Spinal Cord Ser Cases. 2021 Jun 10;7(1):50. doi: 10.1038/s41394-021-00413-6. PMID: 34112758; PMCID: PMC8192499.
An open label, pilot study of anticholinergic agent vs. mirabegron to evaluate cognitive impact and efficacy
Mirabegron benefits older individuals with spinal cord injury (SCI) by improving cognitive function, including memory and executive function, when replacing oral anticholinergic medications for neurogenic lower urinary tract dysfunction (NLUTD). In a pilot study, participants showed significant improvements in memory recall and executive function, as well as enhanced bladder control, with no adverse effects on bowel or cardiovascular health.You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/34112758/.
Other Peptides
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








