Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Health Benefits of Thymosin Beta 4
- Key Takeaways
- What is Thymosin Beta 4?
- How Thymosin Beta 4 Works
- Chemical Structure
- Research on Thymosin Beta 4
- Thymosin Beta 4 Side Effects
- Does Thymosin Beta 4 Cause Cancer?
- Thymosin Beta 4 Bodybuilding
- What does Thymosin Beta 4 do to the Body?
- Thymosin Beta 4 Hair Growth
- Thymosin Beta 4 Injection
- Thymosin Alpha 1 vs Thymosin Beta 4
- Thymosin Beta 4 Capsules
- FAQ
- Reference
Table of Contents
- Potential Health Benefits of Thymosin Beta 4
- Key Takeaways
- What is Thymosin Beta 4?
- How Thymosin Beta 4 Works
- Chemical Structure
- Research on Thymosin Beta 4
- Thymosin Beta 4 Side Effects
- Does Thymosin Beta 4 Cause Cancer?
- Thymosin Beta 4 Bodybuilding
- What does Thymosin Beta 4 do to the Body?
- Thymosin Beta 4 Hair Growth
- Thymosin Beta 4 Injection
- Thymosin Alpha 1 vs Thymosin Beta 4
- Thymosin Beta 4 Capsules
- FAQ
- Reference
Potential Health Benefits of Thymosin Beta 4
Thymosin Beta 4 benefits include promoting wound healing, reducing inflammation, enhancing tissue regeneration, supporting hair growth, and improving recovery from injuries by facilitating cellular repair and stem cell migration.
- Boosts the immune system [1-12]
- Improves heart health [13-20]
- Improves liver health [21-26]
- Accelerates wound healing [27-35]
- Improves eye health [36-40]
- Promotes nerve regeneration [41-44]
- Improves brain health [45-50]
- Improves lung health [51]
- Normalizes blood sugar levels [52-53]
- Improves blood pressure [54]
- Promotes hair growth [55-63]
Key Takeaways
- Promotes Healing and Tissue Repair: Thymosin Beta 4 accelerates the healing of wounds and injuries by enhancing cellular regeneration and reducing inflammation.
- Supports Hair Growth: It has been shown to potentially promote hair growth by improving blood circulation to hair follicles and reducing scalp inflammation.
- Regenerative Properties: Thymosin Beta 4 aids in the regeneration of various tissues, including muscles, skin, and nerves, making it beneficial for recovery after trauma or surgery.
- Performance Enhancement: Banned by WADA, Thymosin Beta 4 is considered performance-enhancing due to its ability to accelerate recovery and improve athletic performance.
- Potential Side Effects: While generally safe in controlled settings, Thymosin Beta 4 can cause mild side effects like irritation at injection sites, and its long-term safety is not well established.
What is Thymosin Beta 4?
Thymosin is a hormone secreted by the thymus gland. This powerful hormone serves a vital role in the training and development of a special type of white blood cell known as T-lymphocytes or T cells. In addition, thymosin also assists in the development of antibodies necessary for a strong immune system. The predominant form of thymosin known as thymosin beta 4 is an actin, a multi-functional protein that helps muscles and cells move. Thymosin beta 4 also plays a crucial role in tissue regeneration and protection.
How Thymosin Beta 4 Works
Thymosin beta 4 has the ability to increase the number of immune system cells and decrease inflammatory substances. By binding to actin, it promotes the migration and mobilization of stem/progenitor cells, which are special cells that help form new blood vessels (angiogenesis) and stimulate tissue regeneration. Thymosin beta 4 also decreases scar formation by reducing the number of myofibroblasts (cells responsible for wound healing and tissue repair) in wounds.
Chemical Structure
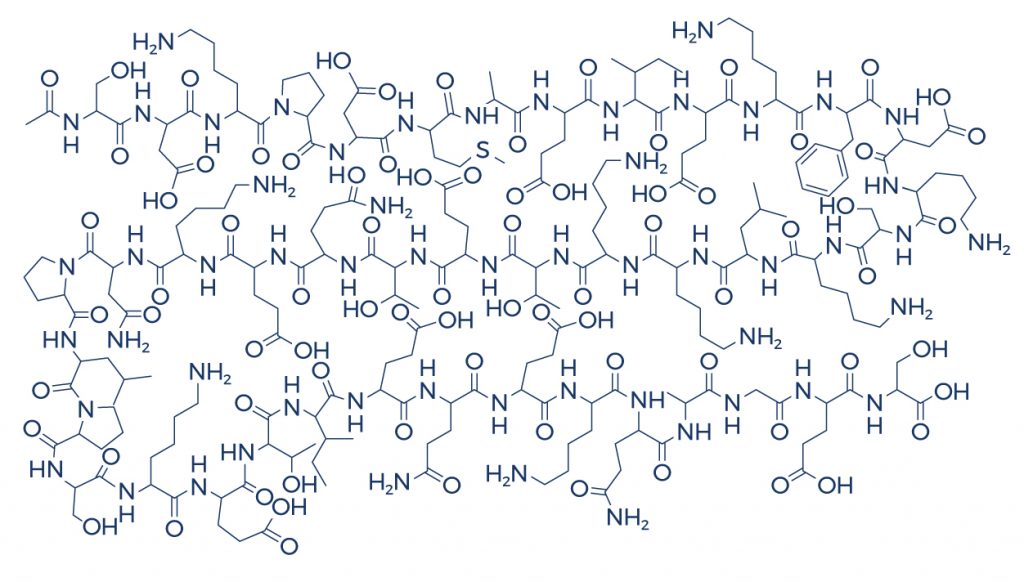
Research on Thymosin Beta 4
A. Boosts the Immune System

Thymosin increases the levels of white blood cells and antibodies, thus strengthening the immune system and protecting against a wide array of diseases. Its predominant form thymosin beta 4 has been shown to heighten the immune response through the following important mechanisms:
- In human gingival fibroblasts (tissues of the gums), thymosin beta 4 suppressed the section of the proinflammatory cytokine interleukin 8 (IL8) and protected against programmed cell death (apoptosis) induced by tumor necrosis factor-α. [1]
- In the human colon, thymosin beta 4 modulated the function of lymphocytes. [2]
- In a murine model, thymosin beta 4 potently inhibited white blood cell infiltration and significantly decreased the levels of pro-inflammatory cytokines. [3]
- Thymosin beta 4 facilitates the recognition of a variety of molecular targets, thus making it easier to detect harmful microorganisms. [4]
- Thymosin beta 4 is involved in the production of hemocytes, which are cells that play a crucial role in the immune system of invertebrates. [5]
- Thymosin beta 4 appears to have antiviral properties. [6]
- Thymosin beta 4 enhances the ability of antibodies to bind with antigens (toxins or other foreign substances) to easily destroy foreign invaders and remove them from the body. [7]
- Thymosin beta 4 enhances natural killer cells’ cytotoxicity. [8]
- Thymosin beta 4 is involved in stabilin-2-mediated engulfment of cells that trigger apoptosis. [9]
- In patients with uremia (build-up of waste products in the kidneys), thymosin beta 4 appears to have a restorative effect on depressed lymphocyte response. [10]
- Phase I and Phase II clinical trials with thymosin in the treatment of primary immunodeficiency diseases, autoimmune diseases, and cancer have shown that this hormone exerts its therapeutic benefits by maintaining immune balance and preventing thymus gland malfunction. [11]
- A cell study found that thymosin beta 4 has antibacterial effects against Pseudomonas aeruginosa. [12]
B. Improves Heart Health

The human heart can repair itself at a very slow pace. However, muscle and tissue damage caused by a heart attack cannot be repaired. Interestingly, numerous studies show that thymosin beta 4 could be sufficiently used to improve heart health and regenerate heart damage:
- In mammals, thymosin beta 4 administration prevented the death of heart muscle cells, stimulated vessel growth, and activated heart stem cells to initiate regeneration. [13]
- In patients with acute ST-segment elevation myocardial infarction (STEMI), transplantation of heart stem cells pre-treated with thymosin beta 4 improved exercise capacity and left ventricular function. [14]
- In patients with ischemic heart disease, a condition that affects the blood supply to the heart, thymosin beta 4 administration promoted the migration and survival of heart muscle cells. [15]
- In rats, thymosin beta 4 reduced the levels of harmful free radicals in heart muscle cells. [16]
- In mice, thymosin beta 4 treatment improved left ventricular function after myocardial infarction. [17]
- In mice, thymosin beta 4 reduced inflammatory cell infiltration and promoted cardiac wound healing. [18]
- In pigs, thymosin beta 4 appears to reduce rejection after heart transplantation. [19]
- A cell study found that thymosin beta 4 promoted cardiac cell migration, survival, and cardiac repair. [20]
C. Improves Liver Health
- Thymosin beta 4 suppresses the activation of hepatic stellate cells (HSCs), which are the main matrix-producing cells in the process of liver fibrosis (scarring). [21]
- Thymosin beta 4 has anti-inflammatory and anti-scarring properties which help prevent liver scarring and damage. [22-23]
- Thymosin beta 4 stimulates liver regeneration through the promotion of liver cell migration, blood vessel formation, cell survival, and stem cell maturation. [24]
- In patients with chronic hepatitis B combined with non-alcoholic fatty liver disease (NAFLD), thymosin beta 4 inhibited oxidative stress and proinflammatory factors. [25]
- Thymosin beta 4 inhibits the production of substances that cause liver scarring. [26]
D. Accelerates Wound Healing
Emerging evidence suggests that thymosin beta 4 is involved in a wide range of cellular responses that help accelerate wound healing:
- In both normal and aged rodents, thymosin beta 4 increased angiogenesis (development of new blood vessels) and cell migration in damaged tissues. [27]
- In a rat full-thickness wound model, thymosin beta 4 administration increased the formation of granulation tissues, collagen deposition, and angiogenesis. [28]
- In diabetic mice, thymosin beta 4 improved angiogenesis by activating the VEGF/AKT pathway. [29]
- In mice, thymosin beta 4 administration improved wound healing markers such as wound closure, granulation, and vascularization. [30]
- In mice with bone fractures, the group treated with thymosin beta 4 (6 mg/kg) had nearly 47% less old cortical bone and had a 31% increase in new trabecular bone area/total callus area fraction compared with the group treated with saline. [31]
- In patients with corneal wounds, the administration of thymosin beta 4 eyedrops to the affected eye 2 drops 4 times a day resulted in faster wound healing. [32-33]
- In patients and animal models with ulcers and other types of wounds, thymosin beta 4 accelerated the rate of repair. [34-35]
E. Improves Eye Health

Studies show that thymosin beta 4 may help maintain eye health by protecting against injury and accelerating the eye’s regenerative process:
- In cultured human corneal epithelial cells, thymosin beta 4 treatment significantly decreased the levels of proinflammatory substances and accelerated the wound healing process after injury. [36-37]
- In diabetic patients, treatment with 2 drops 4 times daily of thymosin beta 4 for 4 weeks appears to heal diabetic corneal defects. [38]
- In animals with severe traumatic corneal wound disorders, thymosin beta 4administration promoted rapid corneal wound healing and decreased the infiltration of inflammatory substances. [39]
- In patients with moderate to severe dry eye, the administration of thymosin beta 4 ophthalmic solution significantly relieved signs and symptoms. [40]
F. Promotes Nerve Regeneration
Thymosin beta 4 is widely distributed in the nervous system, suggesting that it has a role in nerve protection and regeneration. Numerous studies show that thymosin beta 4 is beneficial in several types of nerve injuries:
- In mice with brain inflammation, thymosin beta 4 improved functional recovery by reducing inflammatory infiltrates and stimulating oligodendrogenesis (formation of the protective covering of nerve cells or neurons). [41]
- In rats with spinal cord injury, thymosin beta 4 treatment is associated with a significant decrease in proinflammatory cytokines and spinal lesions. [42]
- In animal models, thymosin beta 4 played a crucial role in stem cell maturation and regeneration, and repair of injuries. [43]
- In mice with multiple sclerosis, treatment with thymosin beta 4 for 30 days significantly increased the number of newly generated oligodendrocytes (cells that produce the protective covering of axons, which allow the transmission of nerve signals). [44]
G. Improves Brain Health
With thymosin beta 4’s role in tissue regeneration and protection, it seems logical that this powerful protein may also be beneficial in supporting brain health. Numerous studies found that thymosin beta 4 may actually help protect against stroke and other brain injuries:
- In mice, thymosin beta 4 stimulated the regeneration and formation of new blood vessels. [45]
- In rats with ischemic stroke (caused by a blood clot), treatment with thymosin beta 4 significantly improved neurological functional outcomes. [46-47]
- In a rat model of ischemic stroke, thymosin beta 4 administration improves neurological functional outcomes by stimulating oligodendrogenesis. [48]
- In patients with stroke and other neurological diseases, thymosin beta 4 treatment appears to remodel or rebuild the nervous system by stimulating self-healing processes in the brain, spinal cord, and nerves. [49]
- In animal models of traumatic brain injury (TBI,) early (6 hours post-injury) treatment with thymosin beta 4 at doses of 6 and 30 mg/kg reduces brain lesions and cell loss and improves functional recovery. [50]
H. Improves Lung Health
The anti-scarring properties of thymosin beta 4 may also play a role in maintaining lung health. In mice, thymosin beta 4 administration helps counter bleomycin-induced lung damage by suppressing inflammation and preventing lung scarring. [51]
I. Normalizes Blood Sugar Levels
Not only does thymosin beta 4 protect against nerve injury caused by diabetes, but it also helps stabilize blood sugar levels. [52] In diabetic mice, thymosin beta 4 ameliorates high blood sugar levels (hyperglycemia) and improves insulin resistance. [53]
J. Improves Blood Pressure
The ability of thymosin to train and develop T cells may also have a positive effect on blood pressure not just on immune function. In mammals, studies show that administration of thymosin induces the growth of the T cell population and thus reduces the autoimmune state, thereby reducing blood pressure. [54]
K. Promotes Hair Growth
The regenerative properties of thymosin beta 4 allow it to stimulate hair growth. This beneficial effect is backed by a number of studies:
- In rats, thymosin beta 4 accelerated hair growth by stimulating the active phase of the hair follicle cycle and promoting the migration of stem cells to the base of the follicle. [55]
- In mice, thymosin beta 4 influenced the growth of blood vessels around the hair follicle by regulating the levels of vascular endothelial growth factor (VEGF). [56]
- In various rat and mouse models, thymosin beta 4 promoted hair growth by affecting follicle stem cell growth and migration. [57-62]
- In patients with alopecia areata, an autoimmune disorder that causes hair loss, thymosin beta 4 treatment for 3 months reduced the severity of the disease and induced significant hair growth. [63]
Thymosin Beta 4 Side Effects
Thymosin beta 4 peptide side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on thymosin beta 4. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of thymosin beta 4. Despite this, it was listed as a side effect associated with thymosin beta 4 even though these associated side effects are very uncommon.
Side effects associated with thymosin beta 4 may include the following:
- Dizziness
- Headache
- Lethargy
- Nausea
- Tiredness
Does Thymosin Beta 4 Cause Cancer?
Current research has not definitively established a direct causal relationship between Thymosin Beta 4 and cancer development. Studies in controlled settings indicate that TB4 does not inherently induce cancer in healthy cells. However, its ability to accelerate tissue repair and promote cellular growth may raise concerns in individuals with pre-existing cancer, as it could potentially support the proliferation of cancerous cells or enhance tumor vascularization.
In conclusion, while there is no strong evidence to suggest that Thymosin Beta 4 causes cancer, its use in individuals with an active or high risk of cancer should be approached cautiously. Further research is needed to fully understand its safety profile, particularly in the context of long-term use and its effects on cancerous and precancerous conditions. Healthcare providers should evaluate its benefits and risks on a case-by-case basis.
Thymosin Beta 4 Bodybuilding
Thymosin Beta 4 (TB4) has gained attention in the bodybuilding community due to its potential to enhance recovery and repair processes. Bodybuilders often face intense physical strain and are prone to injuries, micro-tears in muscles, and inflammation. TB4’s ability to promote cellular repair, reduce inflammation, and improve tissue regeneration makes it an appealing supplement for those seeking quicker recovery and reduced downtime between workouts. While not directly responsible for muscle growth, its supportive role in recovery allows athletes to train harder and more consistently.
Another benefit of TB4 in bodybuilding is its reported effect on flexibility and mobility. By reducing scar tissue formation and promoting angiogenesis (the growth of new blood vessels), it may improve joint health and overall muscular function. These effects can enhance workout performance, reduce the risk of chronic injuries, and contribute to long-term physical resilience. This makes TB4 particularly useful for athletes looking to extend their training careers and achieve peak performance over time.
Despite its benefits, TB4 remains controversial and is banned in professional sports by organizations like WADA due to its classification as a performance-enhancing substance. Moreover, its safety profile is not fully established, and long-term effects are not well-documented. Bodybuilders considering TB4 should proceed with caution, ensuring they source it responsibly and understand the legal and health implications. Consulting a healthcare provider before use is strongly recommended to minimize risks and optimize outcomes.
What does Thymosin Beta 4 do to the Body?
Thymosin Beta 4 is a naturally occurring peptide that plays a crucial role in tissue regeneration and repair. It is abundant in various cells and tissues throughout the body and is especially significant in processes such as wound healing, reducing inflammation, and promoting cellular migration. Thymosin Beta 4 achieves this by regulating actin, a protein essential for cell structure and movement, enabling cells to migrate to areas of injury more effectively.
In addition to its regenerative properties, Thymosin Beta 4 supports angiogenesis, the formation of new blood vessels, which is critical for delivering oxygen and nutrients to damaged tissues. It also exhibits anti-inflammatory effects by modulating the activity of immune cells and reducing the production of pro-inflammatory molecules. These functions make it a potential therapeutic candidate for conditions involving chronic inflammation or impaired healing.
Beyond its physical repair capabilities, Thymosin Beta 4 has shown potential neuroprotective effects, supporting recovery in neurodegenerative diseases and injuries. It is also being studied for its role in hair growth, where it improves circulation to hair follicles and reduces inflammation. While promising, its full therapeutic potential and long-term effects require further research to fully understand its impact on human health.
Thymosin Beta 4 Hair Growth
Thymosin Beta 4 (TB4) is a naturally occurring peptide in the human body, known for its regenerative and healing properties. Its role in hair growth is linked to its ability to stimulate stem cell migration and differentiation, processes that are vital for follicle regeneration. TB4 also promotes angiogenesis, or the formation of new blood vessels, which improves blood flow to hair follicles, ensuring they receive the necessary nutrients and oxygen for healthy growth.
In addition to enhancing blood circulation, TB4 reduces inflammation in the scalp, creating an environment conducive to hair follicle repair and rejuvenation. By modulating inflammation, it may help prevent follicle miniaturization, a common cause of hair thinning and baldness. This anti-inflammatory effect is particularly beneficial for conditions like alopecia areata or other inflammatory scalp disorders that can impede hair growth.
Though TB4 shows promise for stimulating hair regrowth and improving follicle health, most evidence is derived from preclinical studies or anecdotal reports. Its effectiveness and safety for long-term use in humans remain under investigation, and more clinical trials are needed to establish its role in treating hair loss conditions. Despite its potential, users should consult a healthcare professional before considering TB4 for hair growth.
Thymosin Beta 4 Injection
Thymosin Beta 4 (TB4) injections are widely studied for their regenerative and healing properties. This peptide naturally occurs in the human body and plays a crucial role in tissue repair, cell migration, and reducing inflammation. Administered via injection, TB4 is often explored in clinical and experimental settings for conditions involving tissue damage, such as injuries, surgical recovery, and even cardiovascular and neurodegenerative diseases. Its ability to promote angiogenesis (the formation of new blood vessels) and facilitate the migration of stem cells to injured areas has made it a promising candidate for advanced therapeutic approaches.
Despite its potential, TB4 injections remain unapproved for widespread medical use and are banned in sports by the World Anti-Doping Agency (WADA). This is due to their classification as a performance-enhancing substance, as TB4 accelerates recovery and improves physical resilience. While anecdotal reports suggest benefits like reduced recovery time and enhanced healing, comprehensive clinical trials are necessary to establish its efficacy and safety for general use. Concerns about its potential impact on uncontrolled cell proliferation also require further investigation.
The safety profile of TB4 injections appears generally favorable, with minimal reported side effects like mild irritation at the injection site or transient fatigue. However, the long-term effects of its use are not yet fully understood, raising concerns about its application without medical supervision. As research advances, TB4 continues to show promise in regenerative medicine, but ethical considerations and rigorous testing will be essential before it can transition from experimental therapy to mainstream medical treatment.
Thymosin Alpha 1 vs Thymosin Beta 4
Thymosin Alpha 1 (Tα1) and Thymosin Beta 4 (Tβ4) are peptides derived from thymosin, with distinct biological functions. Tα1 primarily focuses on immune system modulation, enhancing T-cell function, and boosting the body’s ability to fight infections and diseases, including cancer. In contrast, Tβ4 is primarily involved in tissue repair and regeneration, aiding wound healing, reducing inflammation, and promoting angiogenesis.
Tα1 has been clinically utilized for immune-related disorders, such as chronic infections, autoimmune conditions, and as an adjunctive treatment in cancer therapies. It supports immune resilience and regulation, making it valuable for combating diseases that compromise immunity. Tβ4, on the other hand, is widely studied for its regenerative properties, benefiting conditions like muscle injuries, surgical recovery, and even hair growth. While both peptides support recovery, their mechanisms and clinical applications differ significantly.
Tα1 works by modulating immune responses, enhancing the production and activity of T-cells, and regulating inflammation. Tβ4 operates at the cellular level, influencing actin dynamics to promote cell migration, repair, and regeneration. Both peptides have shown promising safety profiles in research, but their uses are highly specialized. Tα1 is suitable for systemic immune modulation, while Tβ4 excels in localized tissue repair and recovery.
Thymosin Beta 4 Capsules
Thymosin Beta 4 capsules are an oral form of the peptide, developed for their potential to enhance tissue repair and reduce inflammation. Unlike the injectable form, which is more commonly studied, capsules provide a non-invasive alternative that may appeal to those uncomfortable with needles. These capsules are often marketed for their ability to promote wound healing, improve recovery from injuries, and support overall cellular repair processes.
The use of Thymosin Beta 4 in capsule form remains controversial, as its bioavailability through oral ingestion is not well-established. Peptides like Thymosin Beta 4 are typically broken down in the digestive system, which may reduce their effectiveness when taken orally. Despite these concerns, proponents claim that encapsulated formulations can still deliver therapeutic benefits, though scientific evidence supporting these claims is limited.
As with other forms of Thymosin Beta 4, capsules are not approved for widespread medical use and may carry potential risks. Long-term safety data are lacking, and individuals considering these supplements should approach them with caution, consulting healthcare professionals for guidance. Additionally, consumers should ensure they purchase capsules from reputable sources to avoid counterfeit or low-quality products.
FAQ
What is Thymosin Beta 4 used for?
Thymosin Beta 4 is primarily used for its regenerative properties, including promoting wound healing, reducing inflammation, and supporting tissue repair. As an anti-inflammatory agent generated, it has been shown to enhance the migration of mesenchymal stem cells, which play a key role in tissue regeneration. Thymosin Beta 4 is also studied for its potential in treating cardiovascular and neurodegenerative diseases, where mesenchymal stem cells may be involved in repairing damaged tissues, acting as an anti-inflammatory agent generated to promote healing. This anti-inflammatory agent generated by Thymosin Beta 4 has sparked interest in its broader therapeutic applications.
Is Thymosin Beta 4 performance-enhancing?
Yes, it is considered performance-enhancing as it promotes faster healing, reduces inflammation, and may improve physical recovery, which can benefit athletes. Thymosin Beta 4 is one of the developmentally essential secreted peptides, playing a key role in tissue regeneration. As a developmentally essential secreted peptide, it facilitates cellular repair and regeneration, contributing to improved recovery times. The use of developmentally essential secreted peptides like Thymosin Beta 4 can provide significant advantages in athletic performance and recovery.
Is TB 500 the same as Thymosin Beta 4?
TB 500 is a synthetic derivative of Thymosin Beta 4, known for its potential to improve cardiac function and promote tissue regeneration. While they share similar effects, TB 500 is a fragment of the full Thymosin Beta 4 molecule, optimized for specific uses like muscle and tissue repair, which can also support cardiac function recovery. In addition to muscle healing, TB 500’s ability to enhance cellular regeneration may have a positive impact on overall cardiac function.
Is Thymosin Beta 4 safe?
Thymosin Beta 4 appears to have a good safety profile in research, particularly in its effects on endothelial cells, but comprehensive studies on long-term use and potential risks are lacking. Its ability to promote endothelial cells’ regeneration makes it promising for tissue repair, including in the adult heart, though its safety for human use is not fully established. Further research is needed to better understand the impact of Thymosin Beta 4 on endothelial cells and its overall long-term effects, especially in tissues like the adult heart. Understanding its potential in the adult heart will help evaluate its role in regenerative therapies.
What are the effects of Thymosin Beta 4?
Thymosin Beta 4 promotes wound healing, reduces inflammation, increases cell migration, and enhances tissue regeneration, which can have a positive impact on post ischemic cardiac function. Additionally, it is associated with improved blood vessel formation, further benefiting post ischemic cardiac function by supporting vascular health. Research involving terminal deoxynucleotidyl transferase suggests that Thymosin Beta 4 may influence DNA repair mechanisms, aiding in cellular regeneration. Thymosin Beta 4’s regenerative properties, in combination with terminal deoxynucleotidyl transferase activity, contribute to better recovery and tissue repair, potentially improving overall post ischemic cardiac function.
Is Thymosin Beta 4 safe to take?
Safety depends on the context of use, especially in the realm of anti-aging regenerative therapies, as highlighted in studies by Goldstein et al. While it is generally considered safe in experimental settings, its use outside controlled environments or for unapproved purposes poses risks, including unknown long-term effects. For those exploring anti-aging regenerative therapies, it is crucial to understand the potential risks and benefits before considering its use, a point emphasized in research by Goldstein al. As with all treatments, particularly in the field of anti-aging regenerative therapies, consulting a healthcare professional is essential to ensure safety, as noted in findings by Goldstein al.
Does Thymosin Beta 4 cause cancer?
There is no strong evidence that Thymosin Beta 4, a multi-functional regenerative peptide, directly causes cancer. However, due to its role in cell migration and proliferation, this multi-functional regenerative peptide raises theoretical concerns about its potential to affect tumor growth, as noted by Goldstein al. Despite its benefits as a multi-functional regenerative peptide, further research is needed to fully understand its long-term effects on cancer risk, with studies like those conducted by Goldstein al highlighting the importance of continued investigation. Goldstein al’s research emphasizes the need for further exploration to assess the true impact of Thymosin Beta 4 on cancer development.
What are the side effects of Thymosin Beta 4?
Reported side effects are minimal but can include localized irritation at injection sites, fatigue, dizziness, and nausea. Long-term side effects, particularly concerning the role of epicardium-derived progenitor cells, are not well-documented. Some studies suggest that Thymosin Beta 4 may interact with epicardium-derived progenitor cells, influencing tissue repair and regeneration processes, potentially benefiting heart regeneration. The peptide’s role in heart regeneration is still being explored, with research focusing on how it may enhance the repair of cardiac tissues. However, the exact impact on epicardium-derived progenitor cells and potential long-term risks related to heart regeneration remains unclear.
What does Thymosin Beta 4 do?
It supports cellular repair, reduces inflammation, and accelerates wound healing by promoting the migration and differentiation of cells, including stem cells, through mechanisms such as adult epicardial progenitor mobilization, as discussed in Nature Medicine. Additionally, its effects on tissue regeneration are thought to involve pathways related to adult epicardial progenitor mobilization, further enhancing its therapeutic potential, as highlighted in Nature Medicine research. By facilitating processes like stem cell activity and adult epicardial progenitor mobilization, it demonstrates significant promise in promoting recovery and healing, aligning with findings in Nature Medicine.
What are the benefits of Thymosin Beta 4?
Benefits include faster tissue repair across various cell types, reduced inflammation, improved healing of injuries, enhanced angiogenesis (blood vessel formation) in critical cell types, and potential neuroprotective effects that support different cell types in the nervous system, promoting early myocyte survival. The ability to enhance early myocyte survival contributes to better cardiac tissue repair and may improve outcomes in heart conditions. Additionally, early myocyte survival plays a key role in the regeneration of damaged tissues, supporting the recovery process in various injury contexts.
What does thymosin do to the body?
Thymosin peptides like Beta 4 and Alpha 1, which are small acidic peptides, regulate immune function, support tissue repair, and modulate inflammation. These small acidic peptides play a crucial role in enhancing the body’s healing processes, especially after a heart attack, by promoting tissue regeneration and reducing inflammation. The unique properties of these small acidic peptides make them valuable in promoting tissue regeneration and immune system regulation, offering potential benefits for recovery following a heart attack.
What does peptide Thymosin Beta 4 do?
Thymosin Beta 4 enhances tissue regeneration, regulates the cell cycle, reduces scar tissue formation, and supports cellular healing processes in various organs and tissues. By influencing the cell cycle, it promotes efficient tissue repair and regeneration, including the regulation of b cells, which are critical in immune responses. Its effects on the cell cycle and b cells contribute to accelerated healing and reduced scarring, further supporting its potential therapeutic benefits.
What are the side effects of thymosin injections?
Side effects of thymosin injections, as studied in cell biology, can include redness or irritation at the injection site, mild flu-like symptoms, or transient fatigue. Cell biology research suggests that these effects are generally mild and manageable. Serious side effects related to thymosin injections are rare, as supported by ongoing cell biology investigations. The basic properties of thymosin injections indicate they are typically well-tolerated by most individuals. Understanding the basic properties of thymosin can help researchers and healthcare providers assess its safety and effectiveness. Overall, the basic properties of thymosin injections contribute to their potential for therapeutic use with minimal adverse effects.
Is TB 500 good for muscle growth?
TB 500 may promote muscle growth indirectly by enhancing repair and recovery processes, reducing inflammation, and improving flexibility. Additionally, it is believed to play a role in cardiac regeneration, supporting the repair of damaged heart tissues. Through partial purification processes, TB 500 can be optimized for greater effectiveness in tissue regeneration. By aiding in cardiac regeneration and overall tissue repair, TB 500 offers potential benefits beyond muscle recovery. Its ability to reduce inflammation also contributes to improved flexibility and may indirectly support cardiac regeneration in certain conditions, with partial purification potentially enhancing its therapeutic potential.
What is TB4 good for?
Thymosin β4 is beneficial for wound healing, reducing inflammation, and improving tissue regeneration. The molecular weight of thymosin β4 plays a role in its ability to target specific tissues and facilitate these processes. Additionally, thymosin β4 may play a role in supporting hair growth by enhancing blood circulation and reducing scalp inflammation. The regenerative properties of thymosin β4, influenced by its molecular weight, make it a promising candidate for promoting recovery and overall tissue health.
What are the side effects of Thymosin Beta 4?
Side effects are usually mild and can include injection site discomfort, dizziness, and nausea. Despite its multiple functions in tissue repair and regeneration, including its role as an anti-inflammatory agent, long-term safety data is limited. As a peptide with multiple functions, including acting as an anti-inflammatory agent, it’s important to monitor any adverse reactions during use, especially in the absence of extensive long-term studies. The anti-inflammatory agent properties of Thymosin Beta 4 may offer benefits in reducing inflammation, but these effects need further investigation.
What is the benefit of Thymosin Beta 4?
Its primary benefit is enhancing healing and tissue regeneration, which can be useful for injuries, surgical recovery, and chronic conditions in the context of molecular biology. The application of Thymosin Beta 4 is a key focus in molecular biology due to its ability to regulate plasma cells and cellular repair processes. Research in molecular biology continues to explore how Thymosin Beta 4 influences plasma cells, tissue regeneration, and its potential therapeutic uses. Additionally, scientists are investigating how Thymosin Beta 4 interacts with plasma cells to enhance immune responses and tissue repair.
Does Thymosin Beta 4 build muscle?
Yes, though mild, such as injection site irritation, fatigue, and nausea. Comprehensive long-term effects on adult organs remain unclear, particularly with intravenous injections. While Thymosin Beta 4 shows potential for tissue regeneration, its impact on adult organs over extended use, especially with intravenous injections, is not fully understood. More research in biological chemistry is needed to assess its safety and effectiveness on adult organs, particularly when administered through intravenous injections, especially in the long term. Understanding its biological chemistry and how it interacts with various systems in the body is crucial for determining potential risks. Further studies on the biological chemistry of Thymosin Beta 4 are necessary to ensure its safe application in medical treatments.
Does Thymosin Beta 4 have side effects?
Yes, though mild, such as injection site irritation, fatigue, and nausea. Comprehensive long-term effects on adult organs remain unclear, particularly with intravenous injections. While Thymosin Beta 4 shows potential for tissue regeneration, its impact on adult organs over extended use, especially with intravenous injections, is not fully understood. More research is needed to assess its safety and effectiveness on adult organs, particularly when administered through intravenous injections, especially in the long term.
What is the role of Thymosin Beta 4 in hair growth?
Thymosin Beta 4 is thought to promote hair growth by improving blood circulation to hair follicles, reducing inflammation, and supporting stem cell migration, which also aids in dermal healing. This process is linked to the concept of the embryonic state new directions, where the body’s natural regenerative capabilities are enhanced to promote tissue restoration. Additionally, its regenerative properties support dermal healing by accelerating tissue repair and reducing scarring, opening up embryonic state new directions for new therapeutic approaches. This makes Thymosin Beta 4 a potential aid not only for hair restoration but also for overall dermal healing and recovery, further advancing the embryonic state new directions in regenerative medicine.
Does BPC 157 help hair growth?
BPC 157 is believed to support hair growth by improving vascularization and reducing inflammation, though evidence is mainly anecdotal or from preclinical studies. The recovery process is thought to be enhanced by better blood flow to hair follicles, which may aid in the regeneration of hair. As part of the recovery process, reducing inflammation around the scalp can also contribute to healthier hair growth over time. Additionally, thymosin β has been shown to play a role in stimulating tissue repair and enhancing cellular regeneration, which may further support hair growth. The use of thymosin β alongside other peptides could potentially optimize recovery and improve the overall health of the scalp. Thymosin β helps facilitate the repair and regeneration of tissues, which is essential for maintaining healthy hair follicles and promoting hair regrowth.
What does Thymosin Beta 4 do to the body?
It enhances cellular repair processes, reduces inflammation, and accelerates healing in various tissues, which may help limit microbial growth. By supporting tissue regeneration, thymosin β can also reduce the likelihood of microbial growth in areas of injury. Additionally, its role in promoting healing may indirectly prevent microbial growth by facilitating faster recovery and immune response, thanks to thymosin β’s regenerative properties. The action of thymosin β in reducing inflammation further contributes to an environment less favorable for microbial proliferation.
Does TB 500 help with hair growth?
TB 500 may promote hair growth by improving blood flow and reducing inflammation around hair follicles. Additionally, thymosin β 500 has shown potential in aiding the recovery of corneal injury by enhancing tissue regeneration and reducing inflammation in the eye. This regenerative effect may extend to other areas of the body, including the healing of corneal injury, where accelerated repair could be beneficial for restoring vision. The use of thymosin β in promoting recovery highlights its broad potential in tissue regeneration across various bodily functions.
What is thymosin injection used for?
Thymic lymphocytopoietic factor, found in thymosin β injections, is used for immune system modulation, wound healing, and tissue repair in experimental or off-label contexts. By influencing the thymic lymphocytopoietic factor, thymosin β injections help enhance immune function and support tissue regeneration. Research into thymosin β and thymic lymphocytopoietic factor continues to explore its potential therapeutic uses in various medical conditions.
What is Thymosin Alpha 1 good for?
Thymosin Alpha 1 enhances immune system function and is used to treat infections, autoimmune conditions, and as an adjuvant in cancer therapy. Additionally, it plays a role in regulating monomeric actin, which is crucial for maintaining cell structure and movement. By influencing monomeric actin, Thymosin Alpha 1 can help modulate immune responses and promote early differentiation of immune cells. Moreover, its impact on monomeric actin supports the immune system’s ability to fight infections, control inflammation, and facilitate early differentiation of immune cells during immune responses. This further enhances its ability to support cellular repair and immune system function.
How long should you take Thymosin Alpha 1?
Treatment duration depends on the condition being treated, typically ranging from a few weeks to several months under medical supervision. In cases involving bone marrow regeneration or disorders, the treatment may be prolonged to support the recovery of bone marrow cells and improve capillary density. For individuals undergoing therapies that target bone marrow health and capillary density, close monitoring is essential to ensure optimal recovery and prevent complications. Additionally, maintaining capillary density during treatment can aid in the healing process and enhance nutrient delivery to affected areas.
Who shouldn't take Thymosin Alpha 1?
People with a history of hypersensitivity to thymosin, pregnant or breastfeeding women, and individuals with specific immune disorders should avoid systemic injection of Thymosin Beta 4 unless advised by a healthcare provider. Additionally, those who are considering a systemic injection should consult a healthcare professional to ensure it is appropriate for their condition. The use of systemic injection in individuals with immune system concerns requires careful monitoring and professional oversight.
Reference
Reti R, Kwon E, Qiu P, Wheater M, Sosne G. Thymosin beta4 is cytoprotective in human gingival fibroblasts. European journal of oral sciences. 2008; 116(5):424-30. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18821984.
Thymosin beta4 is cytoprotective in human gingival fibroblasts
Thymosin beta4 (Tbeta(4)) is a non-toxic protein with known wound-healing, anti-inflammatory, and tissue-repair properties. In an in vitro model of human gingival fibroblasts, Tbeta(4) significantly reduced the secretion of interleukin-8 (IL-8) in response to tumor necrosis factor-alpha (TNF-alpha), suggesting its role in suppressing inflammation. Tbeta(4) also protected gingival fibroblasts from cytotoxic effects of chlorhexidine and carbamide peroxide, as well as from apoptosis induced by TNF-alpha. Despite not being effective against bacterial lipopolysaccharides, Tbeta(4) shows potential as an oral healthcare aid due to its antimicrobial, anti-inflammatory, and cytoprotective properties.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1111/j.1600-0722.2008.00569.x.
Elitsur Y, Mutchnick MG, Sakr WA, Luk GD. Thymosin alpha 1 and thymosin beta 4 modulate human colonic lamina propria lymphocyte function. Immunopharmacology. ; 20(2):89-96. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2266003.
Thymosin alpha 1 and thymosin beta 4 modulate human colonic lamina propria lymphocyte function
Thymosin alpha 1 and thymosin beta 4, derived from thymosin fraction 5, can influence immune functions in humans and animals. In a study on human colonic lamina propria lymphocytes (LPL), both peptides suppressed thymidine incorporation, indicating inhibition of LPL proliferation. However, they did not affect thymidine incorporation in phorbol ester- and calcium ionophore-stimulated LPL, nor did they alter ornithine decarboxylase (ODC) activity in Con A-stimulated LPL. These findings suggest that the peptides’ inhibition of LPL proliferation likely involves protein kinase C rather than calcium fluxes or the ODC pathway, and may play a role in modulating the human mucosal immune system.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/0162310990900113?via%3Dihub
Sosne G, Chan CC, Thai K, et al. Thymosin beta 4 promotes corneal wound healing and modulates inflammatory mediators in vivo. Exp Eye Res. 2002;74:293–9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11950239.
Bubb MR. Thymosin beta 4 interactions. Vitamins and hormones. 2003; 66:297-316. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/12852258.
Thymosin beta 4 interactions
Thymosin beta 4 is a 5-kDa protein with various functions, including acting as an actin monomer sequestering protein, an anti-inflammatory agent, and an inhibitor of bone marrow stem cell proliferation. While its effects on the actin cytoskeleton are well understood, the protein’s largely unfolded structure allows it to recognize multiple ligands, which may explain its broad range of functions. This flexible structure may enable thymosin beta 4 to integrate the actin cytoskeleton with key immune and cell growth-signaling pathways, contributing to its diverse biological activities.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/12852258/.
Li J, Zhang Y, Liu Y. A thymosin beta-4 is involved in production of hemocytes and immune defense of Hong Kong oyster, Crassostreahongkongensis. Developmental and comparative immunology. 2016; 57:1-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26695126.
A thymosin beta-4 is involved in production of hemocytes and immune defense of Hong Kong oyster, Crassostreahongkongensis
Thymosin beta-4 (Tβ4) was identified in the oyster species Crassostrea hongkongensis (ChTβ4) and shown to play a crucial role in immune defense and wound healing. The ChTβ4 protein, consisting of 41 amino acids, is highly conserved with an actin-binding motif and is widely expressed, especially in hemocytes. In vivo studies revealed that injecting recombinant ChTβ4 increased hemocyte counts and facilitated bacterial clearance, while its knockdown decreased hemocyte levels. ChTβ4 also reduced reactive oxygen species (ROS) in hemocytes and boosted antioxidant enzyme expression, suggesting its key involvement in immune responses and pathogen defense in oysters.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0145305X15300902?via%3Dihub
Retrieved from https://www.sciencedirect.com/science/article/pii/S0145305X15000713.
β-Thymosins participate in antiviral immunity of red swamp crayfish (Procambarus clarkii)
In the red swamp crayfish (Procambarus clarkii), nine β-thymosin isoforms (PcThy-1 to PcThy-8) were identified, each containing varying numbers of thymosin β actin-binding motifs. Among them, PcThy-4, with four motifs, showed significant involvement in immune responses. It was widely distributed across tissues and upregulated after white spot syndrome virus (WSSV) infection. PcThy-4 inhibited WSSV replication, enhanced crayfish survival, and promoted hemocyte phagocytosis of the virus. These findings highlight the critical role of β-thymosins, particularly PcThy-4, in the antiviral immune defense of crayfish.
You can read the full article at https://www.sciencedirect.com/science/article/pii/S0145305X15000713.
Retrieved from http://onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.2012.06659.x/abstract.
Antibodies in research of thymosin β4: investigation of cross-reactivity and influence of fixatives
Antibodies against thymosin β4 are widely used in research methods like immunohistochemistry, ELISA, and Western blot, but their specificity and compatibility with fixation techniques are not fully characterized. This is critical as β-thymosins share highly homologous sequences and often coexist in tissues, increasing the risk of cross-reactivity. Additionally, fixatives like formaldehyde may chemically modify thymosin β4, altering antibody recognition. Therefore, thorough validation of these antibodies is essential to ensure specificity and reliability in experimental applications.
You can read the abstract of the article at http://onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.2012.06659.x/abstract.
Lee HR, Yoon SY, Kang HB. Thymosin beta 4 enhances NK cell cytotoxicity mediated by ICAM-1. Immunology letters. 2009; 123(1):72-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19369144.
Thymosin beta 4 enhances NK cell cytotoxicity mediated by ICAM-1
Thymosin beta 4 (Tβ4), known for its role as a G-actin-sequestering protein with diverse biological activities, has been identified as a key activator of natural killer (NK) cell cytotoxicity. This study demonstrates that synthetic Tβ4 enhances NK cell function by increasing cytotoxicity via intercellular adhesion molecule-1 (ICAM-1) and promoting the secretion of cytolytic granules to target cells.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S016524780900056X?via%3Dihub.
Lee SJ, So IS, Park SY, Kim IS. Thymosin beta4 is involved in stabilin-2-mediated apoptotic cell engulfment. FEBS letters. 2008; 582(15):2161-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18519035.
Thymosin beta4 is involved in stabilin-2-mediated apoptotic cell engulfment
Stabilin-2, a receptor for phosphatidylserine on apoptotic cells, interacts with thymosin beta 4 (Tβ4) through its cytoplasmic domain. This interaction, localized at the phagocytic cup, is crucial for stabilin-2-mediated clearance of apoptotic cells. Knockdown of Tβ4 reduces stabilin-2’s phagocytic activity, while Tβ4 overexpression enhances it, with amino acids 2504-2514 of stabilin-2 identified as the interaction site. These findings highlight Tβ4 as a downstream molecule essential for stabilin-2-driven cell corpse clearance.
You can read the full article at https://febs.onlinelibrary.wiley.com/doi/10.1016/j.febslet.2008.03.058.
Abiko T, Sekino H. Synthesis of a thymosin beta 4-like peptide, deacetyl-thymosin beta Xen4, and its restorative effect on depressed lymphocyte blastogenic response to phytohemagglutinin (PHA) in uremic patients. Chemical & pharmaceutical bulletin. 1989; 37(9):2467-71. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2605693.
Synthesis of a thymosin beta 4-like peptide, deacetyl-thymosin beta Xen4, and its restorative effect on depressed lymphocyte blastogenic response to phytohemagglutinin (PHA) in uremic patients
An analog of thymosin beta Xen4, deacetyl-thymosin beta Xen4, was synthesized and shown to restore the impaired blastogenic response of T-lymphocytes from uremic patients. The synthesis involved assembling six peptide fragments, deprotection with trifluoromethanesulfonic acid, and reduction of methionine sulfoxide with dithiothreitol, demonstrating its potential immunomodulatory effects.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/2605693/.
Low TL, Thurman GB, Chincarini C. Current status of thymosin research: evidence for the existence of a family of thymic factors that control T-cell maturation. Annals of the New York Academy of Sciences. 1979; 332:33-48. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/394636.
Current status of thymosin research: evidence for the existence of a family of thymic factors that control T-cell maturation
Thymosin fraction 5 contains several hormonal-like peptides that regulate T-cell differentiation and immune function. Key peptides such as thymosin beta 3 and beta 4 induce TdT-positive precursor T-cells, thymosin alpha 1 promotes functional helper cells, and thymosin alpha 7 facilitates suppressor T-cell formation. These peptides act at different stages of T-cell maturation, highlighting the thymus’s critical role in immune balance. Clinical studies suggest thymosin’s therapeutic potential in immunodeficiency, autoimmune diseases, and cancer, emphasizing that T-cell maturation involves a complex interplay of thymic-specific factors rather than a single regulatory peptide.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/394636/.
Carion, T. W., Ebrahim, A. S., Alluri, S., Ebrahim, T., Parker, T., Burns, J., Sosne, G., & Berger, E. A. (2020). Antimicrobial Effects of Thymosin Beta-4 and Ciprofloxacin Adjunctive Therapy in Pseudomonas aeruginosa Induced Keratitis. International journal of molecular sciences, 21(18), 6840. https://doi.org/10.3390/ijms21186840.
Antimicrobial Effects of Thymosin Beta-4 and Ciprofloxacin Adjunctive Therapy in Pseudomonas aeruginosa Induced Keratitis
Thymosin beta 4 (Tβ4) enhances the efficacy of ciprofloxacin in treating Pseudomonas aeruginosa-induced keratitis by indirectly promoting bacterial clearance. While Tβ4 does not exhibit direct bactericidal activity, it upregulates antimicrobial peptides (AMPs) in human corneal epithelial cells in response to lipopolysaccharide (LPS). Ciprofloxacin effectively kills bacteria at low concentrations, and its synergistic interaction with Tβ4 amplifies this effect. The study underscores Tβ4’s potential as an adjunct therapy with antibiotics, leveraging its ability to modulate intracellular communication and enhance immune responses during corneal infections.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC7555736/.
Shrivastava S, Srivastava D, Olson EN, DiMaio JM, Bock-Marquette I. Thymosin beta4 and cardiac repair. Annals of the New York Academy of Sciences. 2010; 1194:87-96. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20536454.
Thymosin beta4 and cardiac repair
Hypoxic heart disease is a leading cause of global morbidity and mortality, with limited options for cardiac repair after infarction in adult mammals. Thymosin beta4 (Tβ4), a small peptide, has been found to inhibit myocardial cell death, promote vessel growth, and activate endogenous cardiac progenitors by reactivating the heart’s embryonic program. This process, which includes epicardial thickening and an increase in myocardial and epicardial progenitors, occurs independently of injury. Tβ4 is the first molecule shown to simultaneously stimulate myocardial and vascular regeneration through systemic administration, offering promising potential for cardiac repair.
You can read the abstract of the article at https://nyaspubs.onlinelibrary.wiley.com/doi/10.1111/j.1749-6632.2010.05468.x.
Zhu J, Song J, Yu L. Safety and efficacy of autologous thymosin β4 pre-treated endothelial progenitor cell transplantation in patients with acute ST segment elevation myocardial infarction: A pilot study. Cytotherapy. 2016; 18(8):1037-42. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27288307.
Safety and efficacy of autologous thymosin β4 pre-treated endothelial progenitor cell transplantation in patients with acute ST segment elevation myocardial infarction: A pilot study
A pilot study investigated the feasibility and safety of thymosin β4 (Tβ4)-pretreated endothelial progenitor cell (EPC) transplantation in patients with acute STEMI. Ten patients were randomized into control (EPC-only) and experimental (Tβ4-pretreated EPC) groups. After 6 months, the experimental group showed significantly greater improvement in the 6-minute walking distance (75.7 m vs. 38.2 m increase) and left ventricular function compared to the control group. No severe complications were reported. These findings suggest that Tβ4-pretreated EPC transplantation is a safe and promising approach to enhance cardiac repair and exercise capacity in STEMI patients.
You can read the abstract of the article at https://www.isct-cytotherapy.org/article/S1465-3249(16)30380-2/abstract.
Crockford D. Development of thymosin beta4 for treatment of patients with ischemic heart disease. Annals of the New York Academy of Sciences. 2007; 1112:385-95. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17947592.
Development of thymosin beta4 for treatment of patients with ischemic heart disease
Thymosin beta 4 (Tβ4), a 43-amino acid peptide, regulates actin polymerization and plays critical roles in cell motility, organogenesis, and cardiac vessel development, including vasculogenesis, angiogenesis, and arteriogenesis. It promotes cardiomyocyte migration, survival, and protection during ischemic heart disease. RegeneRx Biopharmaceuticals is developing Tβ4 for acute myocardial infarction (AMI) treatment, focusing on drug formulation, preclinical studies, and a Phase 1 clinical trial to assess safety and pharmacokinetics. A Phase 2 trial for AMI patients is also being planned.
You can read the abstract of the article at https://nyaspubs.onlinelibrary.wiley.com/doi/10.1196/annals.1415.051.
Kumar S, Gupta S. Thymosin beta 4 prevents oxidative stress by targeting antioxidant and anti-apoptotic genes in cardiac fibroblasts. PloS one. 2011; 6(10):e26912. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22046407.
Thymosin beta 4 prevents oxidative stress by targeting antioxidant and anti-apoptotic genes in cardiac fibroblasts
Thymosin beta-4 (Tβ4) has been shown to reduce oxidative stress and prevent profibrotic changes in cardiac fibroblasts under hydrogen peroxide (H₂O₂)-induced conditions. Pre-treatment with Tβ4 decreased intracellular reactive oxygen species (ROS), upregulated antioxidant enzymes Cu/Zn superoxide dismutase (SOD) and catalase, and reduced apoptotic signaling by modifying the Bax/Bcl(2) ratio. Additionally, Tβ4 inhibited the expression of profibrotic genes such as connective tissue growth factor (CTGF) and collagen types I and III. Silencing of antioxidant genes triggered apoptosis, which Tβ4 treatment prevented, suggesting that Tβ4’s cardioprotective effects are mediated through antioxidant and anti-apoptotic mechanisms.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3201979/.
Retrieved from https://transmedcomms.biomedcentral.com/articles/10.1186/s41231-016-0008-y.
- Thymosin beta 4 treatment improves left ventricular function after myocardial infarction and is related to Up-regulation of chitinase 3-like-1 in miceThymosin beta 4 (Tβ4) demonstrated mild cardioprotective effects in a mouse model of myocardial infarction, improving left ventricular function and reducing cardiac remodeling. The treatment led to increased activity of the soluble purinergic enzyme CD73 and up-regulation of chitinase 3-like-1, which were identified as potential novel molecules involved in cardioprotection. While Tβ4 did not significantly affect apoptosis or inflammation, it did show beneficial effects on heart function, suggesting its role in promoting repair after myocardial injury.You can read the full article at
Evans MA, Smart N, Dubé KN, et al. Thymosin β4-sulfoxide attenuates inflammatory cell infiltration and promotes cardiac wound healing. Nature communications. 2013;4:2081. doi:10.1038/ncomms3081. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22046407.
hymosin β4-sulfoxide attenuates inflammatory cell infiltration and promotes cardiac wound healing. Nature communications
Thymosin β4-sulfoxide, downstream of hydrogen peroxide, plays a key role in resolving inflammation by depleting inflammatory macrophages, promoting wound healing, and reducing scarring in both zebrafish and mice. It also modulates immune responses in human cells, making it a potential therapeutic target for cardiac repair and fibrosis resolution.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/23820300/.Postrach J, Schmidt M, Thormann M. Adeno-associated viral vector 2.9 thymosin ß4 application attenuates rejection after heart transplantation: results of a preclinical study in the pig. Transplantation. 2014; 98(8):835-43. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25321165.
Adeno-associated viral vector 2.9 thymosin ß4 application attenuates rejection after heart transplantation: results of a preclinical study in the pig
This study demonstrates that pretransplant gene therapy using AAV2.9-mediated thymosin β4 (Tß4) delivery significantly reduces graft rejection, improves capillary density, and enhances myocardial function in a porcine cardiac transplantation model, thereby prolonging graft survival. This approach highlights the potential of perioperative gene therapy for improving outcomes in cardiac allotransplantation.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/25321165/.
Bock-Marquette, I., Saxena, A., White, M. D., Dimaio, J. M., & Srivastava, D. (2004). Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature, 432(7016), 466–472. https://doi.org/10.1038/nature03000.
Thymosin beta4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair
Thymosin beta4 enhances cardiomyocyte migration, survival, and repair by forming a functional complex with PINCH and ILK, activating the survival kinase Akt. This pathway shows potential as a therapeutic target for improving cardiac function and repair following acute myocardial damage.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/15565145/.
Kim J, Jung Y. Potential Role of Thymosin Beta 4 in Liver Fibrosis. Haybaeck J, ed. International Journal of Molecular Sciences. 2015;16(5):10624-10635. doi:10.3390/ijms160510624. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4463665/.
Special Issue of International Journal of Molecular Sciences (IJMS) “Purinergic P2 Receptors: Structure and Function”
This Special Issue of International Journal of Molecular Sciences features 7 reviews and 12 original research articles by experts, focusing on recent advancements in the molecular structure and cellular functions of purinergic P2 receptors.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/33396540/.
Jiang Y, Han T, Zhang Z-G, et al. Potential role of thymosin beta 4 in the treatment of nonalcoholic fatty liver disease. Chronic Diseases and Translational Medicine. 2017;3(3):165-168. doi:10.1016/j.cdtm.2017.06.003. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5643779/.
Potential role of thymosin beta 4 in the treatment of nonalcoholic fatty liver disease. Chronic Diseases and Translational Medicine
Non-alcoholic fatty liver disease (NAFLD) is a leading cause of chronic liver disease due to rising obesity rates, with some cases progressing to severe conditions like cirrhosis or cancer. This abstract hypothesizes that thymosin beta 4 (Tβ4), known for its anti-inflammatory and anti-fibrotic properties, could be a potential treatment for NAFLD.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/29063072/.
Schirmacher P., Geerts A., Pietrangelo A., Dienes H.P., Rogler C.E. Hepatocyte growth factor/hepatopoietin a is expressed in fat-storing cells from rat liver but not myofibroblast-like cells derived from fat-storing cells. Hepatology. 1992;15:5–11. doi: 10.1002/hep.1840150103. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/1530788.
Hepatocyte growth factor/hepatopoietin A is expressed in fat-storing cells from rat liver but not myofibroblast-like cells derived from fat-storing cells
Hepatocyte growth factor (HGF) is expressed by fat-storing cells in the liver, particularly in periportal regions, and plays a role in maintaining the balance between cell death and regeneration. In chronic liver disease, the transition of these cells to a myofibroblast-like state leads to a loss of HGF expression, potentially impairing liver regeneration.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/1530788/.`
Philp D, Kleinman HK. Animal studies with thymosin beta, a multifunctional tissue repair and regeneration peptide. Annals of the New York Academy of Sciences. 2010; 1194:81-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20536453.
Animal studies with thymosin beta, a multifunctional tissue repair and regeneration peptide
Thymosin beta-4 (Tβ4), a key actin-sequestering molecule, has shown promising wound-healing properties in animal studies by modulating inflammation, promoting cell migration and survival, and enhancing blood vessel and stem cell development. This review explores its topical and systemic applications, forming the basis for ongoing clinical trials in dermal, corneal, and cardiac repair.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/20536453/.,
Liang J., Cai W.J., Han T., Jing L., Ma Z., Gao Y. The expression of thymosin β4 in chronic hepatitis B combined nonalcoholic fatty liver disease. Medicine (Baltimore) 2016;95:e5763. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28033294.
The expression of thymosin β4 in chronic hepatitis B combined nonalcoholic fatty liver disease
The study investigated thymosin β4 (Tβ4) expression in serum and tissues of patients with chronic hepatitis B (CHB) combined with nonalcoholic fatty liver disease (NAFLD). Tβ4 levels negatively correlated with inflammation, fibrosis, and TNF-α expression, suggesting its potential role in regulating chronic inflammation and fibrosis in CHB-NAFLD progression.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/28033294/.
Mann J., Oakley F., Akiboye F., Elsharkawy A., Thorne A.W., Mann D.A. Regulation of myofibroblasttransdifferentiation by DNA methylation and mecp2: Implications for wound healing and fibrogenesis. Cell Death Differ. 2007;14:275–285. doi: 10.1038/sj.cdd.4401979. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16763620.
Regulation of myofibroblast transdifferentiation by DNA methylation and MeCP2: implications for wound healing and fibrogenesis
Myofibroblasts play a key role in wound healing, with hepatic stellate cells (HSC) transdifferentiating into hepatic myofibroblasts during liver injury. The study reveals that DNA methylation and MeCP2 regulate this process by epigenetically repressing IkappaBalpha and PPARgamma, while treatment with 5-azadC reverses these effects, highlighting potential targets for controlling liver fibrosis.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/16763620/.
Philp D, Goldstein AL, Kleinman HK. Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mechanisms of ageing and development. 2004; 125(2):113-5. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15037013.
Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development
Thymosin beta(4) is a novel small molecule that enhances angiogenesis, wound repair, and hair growth in both normal and aged rodents by promoting cell migration and blood vessel formation. It is currently undergoing clinical trials for wound repair applications.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/15037013/.
Malinda KM, Sidhu GS, Mani H. Thymosin beta4 accelerates wound healing. The Journal of investigative dermatology. 1999; 113(3):364-8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10469335.
Thymosin beta4 accelerates wound healing
Thymosin beta4 (Tβ4) significantly enhances wound healing in a rat model by promoting reepithelialization, wound contraction, collagen deposition, angiogenesis, and keratinocyte migration. These findings highlight Tβ4’s potential as a therapeutic agent for improving wound repair.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/10469335/.
Ti D, Hao H, Xia L. Controlled release of thymosin beta 4 using a collagen-chitosan sponge scaffold augments cutaneous wound healing and increases angiogenesis in diabetic rats with hindlimb ischemia. Tissue engineering. Part A. 2015; 21(3-4):541-9. Retrieves from https://www.ncbi.nlm.nih.gov/pubmed/25204972.
Controlled release of thymosin beta 4 using a collagen-chitosan sponge scaffold augments cutaneous wound healing and increases angiogenesis in diabetic rats with hindlimb ischemia
This study demonstrates that a collagen-chitosan sponge scaffold encapsulated with thymosin beta 4 (CCSS-eTβ4) promotes effective wound healing in diabetic rats with hindlimb ischemia by enhancing vascularization, reepithelialization, and dermal reorganization, while modulating inflammatory and angiogenic gene expression through VEGF/AKT pathway activation. These findings highlight the potential of CCSS-eTβ4 for treating diabetic cutaneous wounds.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/25204972/.
Kim S, Kwon J. Thymosin beta 4 improves dermal burn wound healing via downregulation of receptor of advanced glycation end products in db/db mice. Biochimicaetbiophysicaacta. 2014; 1840(12):3452-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25230158.
Thymosin beta 4 improves dermal burn wound healing via downregulation of receptor of advanced glycation end products in db/db mice
Thymosin β4 (Tβ4) has been shown to improve healing of diabetic burn wounds by promoting wound closure, granulation, and vascularization while reducing receptor for advanced glycation end products (RAGE) levels, suggesting its potential as a therapeutic agent for impaired burn wound healing in diabetes.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/25230158/.
Available from https://clinicaltrials.gov/ct2/show/study/NCT00598871.
A Phase 2 Study of the Safety and Efficacy of Thymosin Beta 4 for Treating Corneal Wounds
Diabetic retinopathy (DR) often leads to severe visual impairment in diabetic patients, who face challenges in wound healing due to compromised organ systems. A therapeutic agent that accelerates wound closure and reduces healing time could significantly decrease infection risks and corneal scarring, offering a major clinical and economic benefit in managing this condition.
You can read the full article at https://clinicaltrials.gov/ct2/show/study/NCT00598871.
Dunn, S. P., Heidemann, D. G., Chow, C. Y., Crockford, D., Turjman, N., Angel, J., Allan, C. B., & Sosne, G. (2010). Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin beta4. Annals of the New York Academy of Sciences, 1194, 199–206. https://doi.org/10.1111/j.1749-6632.2010.05471.x.
Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin beta4
Thymosin beta 4 (Tbeta4) eye drops were used to treat nine patients with chronic neurotrophic corneal defects, leading to significant healing in those with geographic defects and reduced ocular irritation in all patients. These findings suggest Tbeta4 could be a promising topical treatment for nonhealing corneal ulcers.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/20536469/.
Kleinman, H. K., & Sosne, G. (2016). Thymosin β4 Promotes Dermal Healing. Vitamins and hormones, 102, 251–275. https://doi.org/10.1016/bs.vh.2016.04.005.
Thymosin β4 Promotes Dermal Healing
Thymosin beta 4 (Tβ4) is a naturally occurring regenerative protein with angiogenic and anti-inflammatory properties that accelerates dermal wound healing in various preclinical models and phase 2 trials for chronic wounds, demonstrating safety and potential for broader tissue repair applications.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/27450738/.
Xu, T. J., Wang, Q., Ma, X. W., Zhang, Z., Zhang, W., Xue, X. C., Zhang, C., Hao, Q., Li, W. N., Zhang, Y. Q., & Li, M. (2013). A novel dimeric thymosin beta 4 with enhanced activities accelerates the rate of wound healing. Drug design, development and therapy, 7, 1075–1088. https://doi.org/10.2147/DDDT.S50183.
A novel dimeric thymosin beta 4 with enhanced activities accelerates the rate of wound healing
Thymosin beta 4 (Tβ4) is a peptide critical for tissue repair, and a novel, cost-effective Tβ4 dimer demonstrated superior activity in promoting wound healing and cell migration compared to native Tβ4, with potential applications in treating injuries like heart attacks and chronic wounds. The dimer was efficiently produced via genetic engineering and showed enhanced efficacy in both in vitro and in vivo models.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/24109178/.
Brady, R. D., Grills, B. L., Schuijers, J. A., Ward, A. R., Tonkin, B. A., Walsh, N. C., & McDonald, S. J. (2014). Thymosin β4 administration enhances fracture healing in mice. Journal of orthopaedic research : official publication of the Orthopaedic Research Society, 32(10), 1277–1282. https://doi.org/10.1002/jor.22686.
Thymosin β4 administration enhances fracture healing in mice
Thymosin β4 (Tβ4), a regenerative peptide, was found to significantly enhance fracture healing in mice by improving biomechanical properties and promoting new bone formation. Tβ4-treated calluses demonstrated increased strength, stiffness, and mineralized tissue, highlighting its potential as a therapeutic agent for bone fractures.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/25042765/.
Sosne G, Qiu P, Kurpakus-Wheater M. Thymosin beta 4: A novel corneal wound healing and anti-inflammatory agent. Clinical ophthalmology (Auckland, NZ). 2007;1(3):201-207. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25230158.
Thymosin beta 4: A novel corneal wound healing and anti-inflammatory agent
Thymosin beta 4 (Tβ4) is a multifunctional protein that plays a key role in promoting cell migration, wound healing, anti-inflammatory effects, and apoptosis suppression, particularly in the cornea. This review highlights its potential clinical applications as a therapeutic agent for maintaining corneal health and facilitating tissue repair.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/19668473/.
Sosne G, Chan CC, Thai K. Thymosin beta 4 promotes corneal wound healing and modulates inflammatory mediators in vivo. Experimental eye research. 2001; 72(5):605-8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11311052.
Thymosin beta 4 promotes corneal wound healing and modulates inflammatory mediators in vivo
No abstract availableYou can read the full article at https://pubmed.ncbi.nlm.nih.gov/11311052/.
Dunn SP, Heidemann DG, Chow CY. Treatment of chronic nonhealingneurotrophic corneal epithelial defects with thymosin beta4. Annals of the New York Academy of Sciences. 2010; 1194:199-206. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20536469.
Treatment of chronic nonhealing neurotrophic corneal epithelial defects with thymosin beta4
Thymosin beta 4 (Tβ4) eye drops demonstrated significant healing effects in six out of nine patients with chronic nonhealing neurotrophic corneal epithelial defects, reducing ocular irritation and promoting recovery without significant neovascularization. These findings suggest Tβ4 may offer a promising topical treatment for such corneal ulcers.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/20536469/.
Sosne G, Szliter EA, Barrett R, Kernacki KA, Kleinman H, Hazlett LD. Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury. Experimental eye research. 2002; 74(2):293-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/11950239.
Thymosin beta 4 promotes corneal wound healing and decreases inflammation in vivo following alkali injury
Thymosin beta 4 (Tbeta(4)) accelerates corneal wound healing and reduces inflammation in mice with alkali-induced eye injuries, promoting faster re-epithelialization, decreased immune cell infiltration, and lower inflammatory cytokine and chemokine levels compared to controls. These findings suggest Tbeta(4) as a potential clinical treatment for severe corneal wounds.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/11950239/.Sosne G, Ousler GW. Thymosin beta 4 ophthalmic solution for dry eye: a randomized, placebo-controlled, Phase II clinical trial conducted using the controlled adverse environment (CAETM) model. Clinical Ophthalmology (Auckland, NZ). 2015;9:877-884. doi:10.2147/OPTH.S80954. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4445951/.
Thymosin beta 4 ophthalmic solution for dry eye: a randomized, placebo-controlled, Phase II clinical trial conducted using the controlled adverse environment (CAE™) model
This Phase II study evaluated the safety and efficacy of 0.1% thymosin beta 4 (Tβ4) ophthalmic solution in treating moderate to severe dry eye. While no significant improvements were observed in primary endpoints, Tβ4-treated subjects showed significant reductions in discomfort scores and improvements in corneal staining, with no adverse events reported, indicating its potential as a safe and effective treatment for dry eye.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/26056426/.
Zhang J, Zhang ZG, Morris D, et al. Neurological Functional Recovery After Thymosin Beta4 Treatment in Mice with Experimental Auto Encephalomyelitis. Neuroscience. 2009;164(4):1887-1893. doi:10.1016/j.neuroscience.2009.09.054. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2784109/.
Neurological functional recovery after thymosin beta4 treatment in mice with experimental auto encephalomyelitis
The study suggests that thymosin beta 4 (Tbeta4) may be an effective therapy for multiple sclerosis (MS), as it improved functional recovery in a mouse model by reducing inflammation and stimulating the proliferation of oligodendrocyte progenitor cells (OPCs) and mature oligodendrocytes. The results indicate that Tbeta4 treatment could potentially enhance oligodendrogenesis and promote recovery in MS.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/19782721/.
Cheng P, Kuang F, Zhang H, Ju G, Wang J. Beneficial effects of thymosin β4 on spinal cord injury in the rat. Neuropharmacology. 2014; 85:408-16. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24937047.
Beneficial effects of thymosin β4 on spinal cord injury in the rat
This study explored the therapeutic potential of Thymosin β4 (Tβ4) in spinal cord injury (SCI), showing that Tβ4 improves locomotor recovery, promotes neuronal survival, reduces inflammation, and enhances vasculoprotective properties in rats. The findings suggest Tβ4 as a promising treatment for SCI in humans due to its neuroprotective effects and safety in clinical trials.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/24937047/.
Goldstein AL, Kleinman HK. Advances in the basic and clinical applications of thymosin β4. Expert opinion on biological therapy. 2015; 15 Suppl 1:S139-45. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26096726.
Thymosin β4 (Tβ4) is a multifunctional peptide that plays a key role in tissue regeneration and repair, with clinical applications in eye injuries, dermal wounds, heart and brain healing, and more. Ongoing research suggests its potential in treating kidney, liver, spinal cord, and bone damage, as well as aging and viral infections.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/26096726/.
Zhang, J., Zhang, Z. G., Li, Y., Lu, M., Zhang, Y., Elias, S. B., & Chopp, M. (2016). Thymosin beta4 promotes oligodendrogenesis in the demyelinating central nervous system. Neurobiology of disease, 88, 85–95. https://doi.org/10.1016/j.nbd.2016.01.010.
Thymosin beta4 promotes oligodendrogenesis in the demyelinating central nervous system
Thymosin beta4 (Tβ4) promotes remyelination in multiple sclerosis (MS) models by enhancing oligodendrocyte progenitor cell differentiation and maturation, with the epidermal growth factor receptor (EGFR) pathway playing a key role in this process. These findings suggest that Tβ4 could be a potential therapy for MS and other demyelinating disorders.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/26805386/.
Morris DC, Chopp M, Zhang L, Zhang ZG. Thymosin beta4: a candidate for treatment of stroke? Annals of the New York Academy of Sciences. 2010; 1194:112-7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20536457.
Thymosin beta4: a candidate for treatment of stroke?
Neurorestorative therapy aims to enhance recovery after stroke by targeting brain cells to promote neurogenesis, angiogenesis, and axonal sprouting. Research suggests that thymosin beta4 (Tbeta4) could be a promising agent for stimulating these processes and improving functional outcomes.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/20536457/.
Morris DC, Chopp M, Zhang L, Lu M, Zhang ZG. Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke. Neuroscience. 2010; 169(2):674-82. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/20627173.
Thymosin beta4 improves functional neurological outcome in a rat model of embolic stroke
The study investigates the effects of Thymosin beta4 (Tbeta4) on neurological recovery in a rat model of embolic stroke, finding that Tbeta4 significantly improves functional outcomes, promotes axonal remodeling, and increases myelination and vessel density in the ischemic area, likely through mobilization of oligodendrocyte progenitor cells.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/20627173/.
Morris DC, Cui Y, Cheung WL. A dose-response study of thymosin β4 for the treatment of acute stroke. Journal of the neurological sciences. 2014; 345(1-2):61-7. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25060418.
A dose-response study of thymosin β4 for the treatment of acute stroke
This study evaluated the effects of different doses of Thymosin β4 (Tβ4) on neurological recovery in a rat model of embolic stroke, finding that doses of 2 and 12 mg/kg significantly improved long-term neurological function, with an optimal dose of 3.75 mg/kg providing the best results. These findings support the use of Tβ4 for clinical trials targeting neurological restoration after stroke.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/25060418/.
Morris DC, Zhang ZG, Zhang J, Xiong Y, Zhang L, Chopp M. Treatment of neurological injury with thymosin β4. Annals of the New York Academy of Sciences. 2012; 1269:110-6. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23045978.
Treatment of neurological injury with thymosin β4
Thymosin β4 (Tβ4) has shown promise as a neurorestorative agent, improving neurological outcomes in models of embolic stroke, multiple sclerosis, and traumatic brain injury by promoting oligodendrogenesis and other recovery processes. Preclinical data support its potential for clinical trials targeting neurological diseases and injuries.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/23045978/
Henry Ford Health System. “Researchers propose novel new treatment of stroke, other neurological diseases.” ScienceDaily. ScienceDaily, 2 March 2015. Retrieved from https://www.sciencedaily.com/releases/2015/03/150302123301.htm.
“Researchers propose novel new treatment of stroke, other neurological diseases.”
Ischemic stroke causes long-term disability, and while neurogenesis plays a key role in recovery, the complex mechanisms behind it remain under extensive research. Therapeutic strategies, such as cell therapy, rehabilitation, and pharmacotherapy, have shown promise in enhancing brain repair and recovery through neurogenesis, though further studies are needed to translate these findings into effective clinical treatments.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/37893146/.
Xiong Y, Mahmood A, Meng Y. Neuroprotective and neurorestorative effects of thymosin β4 treatment following experimental traumatic brain injury. Annals of the New York Academy of Sciences. 2012; 1270:51-8. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23050817.
Neuroprotective and neurorestorative effects of thymosin β4 treatment following experimental traumatic brain injury
Traumatic brain injury (TBI) remains a major cause of mortality and disability, with no effective pharmacological treatments available; however, thymosin beta 4 (Tβ4) shows promise as a neuroprotective and neurorestorative candidate, promoting recovery through anti-apoptotic, anti-inflammatory, and neurogenesis-supporting properties.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/23050817/.
Conte E, Genovese T, Gili E. Protective effects of thymosin β4 in a mouse model of lung fibrosis. Annals of the New York Academy of Sciences. 2012; 1269:69-73. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23045972.
Protective effects of thymosin β4 in a mouse model of lung fibrosis
Thymosin β4 (Tβ4) has demonstrated anti-inflammatory and antifibrotic effects in various tissues, including the eye, skin, heart, liver, and lungs. Recent studies show Tβ4’s potential protective role in lung injury, notably reducing inflammation and histological damage in a bleomycin-induced lung damage model.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/23045972/.
Wang L, Chopp M, Szalad A. Thymosin β4 promotes the recovery of peripheral neuropathy in type II diabetic mice. Neurobiology of disease. 2012; 48(3):546-55. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22922221.
Thymosin β4 promotes the recovery of peripheral neuropathy in type II diabetic mice
Thymosin β4 (Tβ4) treatment in a diabetic mouse model improved sciatic nerve vascular function and nerve recovery by upregulating angiopoietin-1 (Ang1) and activating the PI3K/Akt pathway, suggesting its potential as a treatment for diabetic peripheral neuropathy. Tβ4 also reversed high glucose-induced vascular dysfunction in endothelial and Schwann cells in vitro.You can read the full article at https://pubmed.ncbi.nlm.nih.gov/22922221/.
Zhu J, Su LP, Ye L, Lee KO, Ma JH. Thymosin beta 4 ameliorates hyperglycemia and improves insulin resistance of KK Cg-Ay/J mouse. Diabetes research and clinical practice. 2012; 96(1):53-9. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22217673.
Thymosin beta 4 ameliorates hyperglycemia and improves insulin resistance of KK Cg-Ay/J mouse
Thymosin beta 4 (Tβ4) improved glucose tolerance, reduced HbA1c and triglyceride levels, increased adiponectin, and enhanced insulin sensitivity in a KK mouse model of type 2 diabetes. Tβ4 also elevated phosphorylated AKT levels in skeletal muscle, suggesting its potential as an alternative insulin sensitizer for managing type 2 diabetes.
You can read the abstract of the article at https://www.diabetesresearchclinicalpractice.com/article/S0168-8227(11)00677-2/abstract.
Retrieved from https://www.google.com/patents/US4444757.
Use of thymosin as an anti-diabetes and anti-hypertensive disease agent
Thymus gland extracts, such as Thymosin fraction 5, can be used to treat autoimmune diseases like hypertension, lupus, and diabetes. These extracts stimulate the development of thymus suppressor cells, which help reduce autoimmune activity and alleviate disease symptoms.
You can read the abstract of the article at https://patents.google.com/patent/US4444757.
Philp, D., Nguyen, M., Scheremeta, B., St-Surin, S., Villa, A. M., Orgel, A., Kleinman, H. K., & Elkin, M. (2004). Thymosin beta4 increases hair growth by activation of hair follicle stem cells. FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 18(2), 385–387. https://doi.org/10.1096/fj.03-0244fje.
Thymosin beta4 increases hair growth by activation of hair follicle stem cells
Thymosin beta 4 (Tβ4) promotes hair growth by accelerating key events in the hair follicle cycle, including stem cell migration from the bulge region, differentiation, and extracellular matrix remodeling. In rats and mice, Tβ4 enhances the activity of hair follicle keratinocytes and increases the expression of matrix metalloproteinase-2, supporting its role in stimulating hair growth through stem cell activation and tissue remodeling.
You can read the full article at https://faseb.onlinelibrary.wiley.com/doi/epdf/10.1096/fj.03-0244fje.
Gao, X. Y., Hou, F., Zhang, Z. P., Nuo, M. T., Liang, H., Cang, M., Wang, Z. G., Wang, X., Xu, T., Yan, L. Y., Guo, X. D., & Liu, D. J. (2016). Role of thymosin beta 4 in hair growth. Molecular genetics and genomics: MGG, 291(4), 1639–1646. https://doi.org/10.1007/s00438-016-1207-y.
Role of thymosin beta 4 in hair growth
Thymosin beta 4 (Tβ4) promotes hair growth by influencing hair follicle structure, shaft number, and gene expression through the Wnt/β-catenin/Lef-1 signaling pathway. In Tβ4 over-expressing mice, increased expression of VEGF and MMP-2 enhances blood vessel growth and cell migration around hair follicles, while Tβ4 knockout reduces these effects. These findings suggest Tβ4’s potential as a therapeutic agent for hair growth issues, warranting further investigation.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s00438-016-1207-y.
Philp, D., St-Surin, S., Cha, H. J., Moon, H. S., Kleinman, H. K., & Elkin, M. (2007). Thymosin beta 4 induces hair growth via stem cell migration and differentiation. Annals of the New York Academy of Sciences, 1112, 95–103. https://doi.org/10.1196/annals.1415.009.
Thymosin beta 4 induces hair growth via stem cell migration and differentiation
Thymosin beta 4, a 43-amino-acid molecule with diverse biological functions, promotes hair growth in rodent models, including transgenic mice overexpressing thymosin beta 4. It enhances follicle stem cell growth, migration, differentiation, and protease production, revealing its mechanism for stimulating hair follicle activity and hair growth.
You can read the abstract of the article at https://nyaspubs.onlinelibrary.wiley.com/doi/10.1196/annals.1415.009.
Gao, X., Liang, H., Hou, F., Zhang, Z., Nuo, M., Guo, X., & Liu, D. (2015). Thymosin Beta-4 Induces Mouse Hair Growth. PloS one, 10(6), e0130040. https://doi.org/10.1371/journal.pone.0130040.
Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development
Thymosin beta 4 is a small molecule that promotes angiogenesis, wound repair, and hair growth in both normal and aged rodents by enhancing blood vessel formation and cell migration. Its effectiveness in addressing reduced angiogenesis in aged animals has led to ongoing clinical trials for wound repair applications.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0047637403002252?via%3Dihub.
Philp, D., Goldstein, A. L., & Kleinman, H. K. (2004). Thymosin beta4 promotes angiogenesis, wound healing, and hair follicle development. Mechanisms of ageing and development, 125(2), 113–115. https://doi.org/10.1016/j.mad.2003.11.005.
You can read the full article at
Dai, B., Sha, R. N., Yuan, J. L., & Liu, D. J. (2021). Multiple potential roles of thymosin β4 in the growth and development of hair follicles. Journal of cellular and molecular medicine, 25(3), 1350–1358. https://doi.org/10.1111/jcmm.16241.
Multiple potential roles of thymosin β4 in the growth and development of hair follicles
Thymosin β4 (Tβ4), a key actin-sequestering protein, plays a crucial role in hair follicle (HF) growth and development by promoting the migration and differentiation of HF stem cells and their progeny. It activates HF cycle transitions, accelerates hair growth in mice, and increases secondary HFs in cashmere goats, enhancing cashmere production. While Tβ4’s functions in HF growth are evident, its molecular mechanisms remain underexplored, warranting further investigation.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC7875905/.
Dai, B., Sha, R. N., Yuan, J. L., & Liu, D. J. (2021). Multiple potential roles of thymosin β4 in the growth and development of hair follicles. Journal of cellular and molecular medicine, 25(3), 1350–1358. https://doi.org/10.1111/jcmm.16241.
Multiple potential roles of thymosin β4 in the growth and development of hair follicles
Thymosin β4 (Tβ4), a key actin-sequestering protein, plays a crucial role in hair follicle (HF) growth and development by promoting the migration and differentiation of HF stem cells and their progeny. It activates HF cycle transitions, accelerates hair growth in mice, and increases secondary HFs in cashmere goats, enhancing cashmere production. While Tβ4’s functions in HF growth are evident, its molecular mechanisms remain underexplored, warranting further investigation.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC7875905/.
Cha, H. J., Philp, D., Lee, S. H., Moon, H. S., Kleinman, H. K., & Nakamura, T. (2010). Over-expression of thymosin beta 4 promotes abnormal tooth development and stimulation of hair growth. The International journal of developmental biology, 54(1), 135–140. https://doi.org/10.1387/ijdb.082735hc.
Over-expression of thymosin beta 4 promotes abnormal tooth development and stimulation of hair growth
Thymosin beta 4 plays key roles in wound healing, hair growth, angiogenesis, and tumor progression. In transgenic mice overexpressing thymosin beta 4 under a skin-specific promoter, accelerated hair growth, upregulated laminin-5 expression in skin, and abnormalities in tooth development, such as white, dull-shaped teeth, were observed. These findings highlight its significant physiological roles in hair and tooth development.
You can read the abstract of the article at https://ijdb.ehu.eus/article/082735hc.
Rinaldi, F., Marzani, B., Pinto, D., & Sorbellini, E. (2019). Randomized controlled trial on a PRP-like cosmetic, biomimetic peptides based, for the treatment of alopecia areata. The Journal of dermatological treatment, 30(6), 588–593. https://doi.org/10.1080/09546634.2018.1544405.
Randomized controlled trial on a PRP-like cosmetic, biomimetic peptides based, for the treatment of alopecia areata
A randomized double-blinded study demonstrated that a PRP-mimicking cosmetic product (TR-M-PRP plus) significantly improved hair growth in patients with alopecia areata (AA) over three months of treatment, as measured by the SALT score (p < .001). Hair growth continued to improve after a one-month follow-up, suggesting the product’s potential as a safer and effective alternative to autologous PRP therapy for AA treatment.
You can read the full article at https://www.tandfonline.com/doi/10.1080/09546634.2018.1544405?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed#d1e196.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








