Health Library
Clomiphene Citrate
Author: Dr. George Shanlikian, M.D. | Last Updated: May 28th, 2025
- Home
- >
- Health Library
- >
- Clomiphene Citrate
Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Benefits of Clomiphene Citrate
- Key Takeaways
- What is Clomiphene Citrate?
- How Clomiphene Citrate Works
- How Clomiphene Citrate Works for Women
- How Clomiphene Citrate Works for Men
- Chemical Structure of Clomiphene Citrate
- Research on Clomiphene Citrate
- Clomiphene Citrate Side Effects
- Clomiphene Citrate Uses
- Clomiphene Citrate Pills
- Clomid
- Clomid for Bodybuilding
- Clomid Testosterone Booster
- Clomiphene Mechanism of Action
- Clomiphene Dosing
- Clomiphene Citrate and Ovarian Hyperstimulation Syndrome
- Clomiphene Citrate and Menstrual Cycle
- Clomiphene Citrate and Polycystic Ovary Syndrome
- Clomiphene Citrate and Abnormal Vaginal Bleeding
- FAQ
- Reference
Table of Contents
- Potential Benefits of Clomiphene Citrate
- Key Takeaways
- What is Clomiphene Citrate?
- How Clomiphene Citrate Works
- How Clomiphene Citrate Works for Women
- How Clomiphene Citrate Works for Men
- Chemical Structure of Clomiphene Citrate
- Research on Clomiphene Citrate
- Clomiphene Citrate Side Effects
- Clomiphene Citrate Uses
- Clomiphene Citrate Pills
- Clomid
- Clomid for Bodybuilding
- Clomid Testosterone Booster
- Clomiphene Mechanism of Action
- Clomiphene Dosing
- Clomiphene Citrate and Ovarian Hyperstimulation Syndrome
- Clomiphene Citrate and Menstrual Cycle
- Clomiphene Citrate and Polycystic Ovary Syndrome
- Clomiphene Citrate and Abnormal Vaginal Bleeding
- FAQ
- Reference
Overall Benefits of Clomiphene Citrate
Clomiphene Citrate benefits include stimulating ovulation in women with infertility by increasing FSH and LH levels, which promotes egg maturation and release. It is also used in men to boost testosterone production by enhancing LH secretion, potentially improving fertility and hormonal balance.
- Boosts testosterone levels [1-7]
- Increases pregnancy rate [2-36]
- Improves sperm quality [1-7, 37-56-59]
Key Takeaways
- Ovulation Induction: Clomiphene is commonly used to treat female infertility by stimulating ovulation in women with irregular or absent menstrual cycles.
- Hormonal Regulation: It works by blocking estrogen receptors in the hypothalamus, leading to increased secretion of FSH and LH, which are essential for reproductive function.
- Male Fertility & Testosterone Boost: In men, Clomiphene can increase testosterone levels and sperm production by stimulating LH secretion, making it a potential treatment for hypogonadism and infertility.
- Potential Side Effects: Common side effects include hot flashes, mood swings, nausea, headaches, and, in rare cases, ovarian hyperstimulation syndrome (OHSS) in women.
- Limited Usage Duration: Due to the risk of side effects and diminishing effectiveness over time, Clomiphene is typically prescribed for short-term use under medical supervision.
What is Clomiphene Citrate?
Clomiphene citrate, also known by its brand name Clomid, is a U.S. Food and Drug Administration (FDA)-approved drug for infertility in both men and women. It’s touted as the “first-line” fertility medication recommended for the treatment of irregular ovulation and low sperm count. Clomiphene citrate has a proven safety record. In fact, the first successful clinical trials for this fertility medication date back to 1967 when it was used for ovulation induction. [1] People with fertility problems can benefit from clomiphene citrate by taking its oral form or via injections.
How Clomiphene Citrate Works
Clomiphene citrate stimulates the pituitary gland in the brain to increase the production of follicle-stimulating hormone (FSH) and LH (luteinizing hormone). This in turn promotes ovarian follicle growth and thus initiates ovulation.
How Clomiphene Citrate Works for Women
In women, clomiphene citrate works by blocking the production of the hormone estrogen. This mechanism stimulates the hypothalamus and pituitary glands in the brain to produce several hormones necessary for the maturation of the egg follicles, such as follicle-stimulating hormone (FSH), gonadotropin-releasing hormone (GnRH), and luteinizing hormone (LH). As the egg matures, the chances of ovulation and getting pregnant are usually higher.
How Clomiphene Citrate Works for Men
In men, clomiphene citrate also boosts the production of FSH and LH. These hormones are also present in men and are important for fertility. Specifically, LH stimulates the release of testosterone while FSH is required in the first stage of sperm production (spermatogenesis). With increased testosterone levels, the sperm can move properly through the female reproductive tract to reach the matured egg (motility). In addition, testosterone increases sex drive and sexual function.
Chemical Structure of Clomiphene Citrate
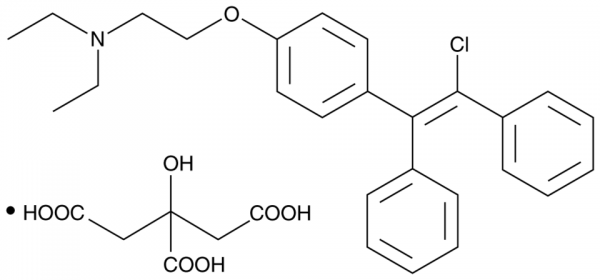
Research on Clomiphene Citrate
A. Boosts Testosterone Levels

Clomiphene citrate boosts testosterone levels by acting as a selective estrogen receptor modulator (SERM), blocking estrogen receptors in the hypothalamus. This prevents negative feedback from estrogen, leading to increased release of gonadotropin-releasing hormone (GnRH), which in turn stimulates the pituitary gland to produce more luteinizing hormone (LH) and follicle-stimulating hormone (FSH). Higher LH levels signal the testes to produce more testosterone, effectively raising endogenous levels without suppressing sperm production, making it a preferred option over traditional testosterone replacement therapy (TRT) for men who wish to maintain fertility.
- A 2007 study in the Indian Journal of Physiology and Pharmacology reported that clomiphene citrate raised sperm count in men with oligospermia by increasing testosterone, FSH, and LH levels. [1]
- In infertile men, clomiphene citrate use has been linked to higher pregnancy rates in partners and elevated levels of testosterone, LH, and FSH. [2]
- Among young, obese men at high risk for infertility due to testosterone deficiency, clomiphene citrate significantly boosted testosterone levels. [3]
- Research suggests that clomiphene citrate improves semen quality only in infertile men who experience a testosterone increase after treatment. [4-5]
- In infertile men with low testosterone, clomiphene citrate treatment led to higher sperm concentration and testosterone levels. [6-7]
B. Increases Pregnancy Rate

An overwhelming body of clinical evidence supports the beneficial effects of clomiphene citrate on fertility. Studies show that this fertility medication increases the chance of pregnancy:
- A study involving 114 961 pregnant women found that clomiphene citrate does not increase the risk for fetal malformations. [2]
- A 2004 study published in the Gynecological Endocrinology found that clomiphene citrate dose of 150-250 mg/day does not appear to increase adverse pregnancy outcomes. [8]
- In patients with polycystic ovary syndrome (PCOS), a condition characterized by irregular menstruation and difficulty getting pregnant, clomiphene citrate treatment for 3 months significantly improved ovulation rate. [9]
- A 1994 study published in the Australian & New England Journal of Obstetrics and Gynecology found that clomiphene citrate treatment is associated with higher rates of pregnancy. [10]
- In women trying to conceive, administration of clomiphene citrate for at least 6 cycles is recommended in order to get pregnant. [11]
- Data from the Yale-New Haven Medical Center showed that clomiphene citrate is associated with a 50% conception rate after 3 ovulations. [12]
- A 2016 study published in the Iranian Journal of Reproductive Medicine found that clomiphene citrate has a higher pregnancy rate compared to other fertility medications such as tamoxifen and letrozole. [13]
- Most studies recommend clomiphene citrate as first-line therapy for women with PCOS. [14-15]
- In women with PCOS, clomiphene citrate treatment is associated with 6%multiple pregnancies. [16]
- In women with anovulation (failure of ovaries to release egg), clomiphene citrate induced ovulation in 80% of the subjects but only 40% became pregnant. [17]
- A 2002 study published in Fertility and Sterility reported that clomiphene citrate treatment resulted in stimulation of ovarian folliculogenesis (maturation of the ovarian follicle) without any adverse effects on the thickness of the uterine lining. [18]
- In infertile women, administration of 100 mg clomiphene citrate for 5 days increased the pregnancy rate by 20%. [19]
- In patients with ovulatory infertility, clomiphene citrate had a higher pregnancy rate compared with letrozole. [20]
- In women with irregular ovulation, clomiphene citrate administration at doses between 50 to 250 milligrams daily effectively induced ovulation and improved fertility. [21]
- In women with anovulation associated with PCOS, clomiphene citrate treatment is associated with higher ovulation and pregnancy rates compared with metformin. [22]
- When combined with metformin, clomiphene citrate is associated with a higher pregnancy rate in women who do not respond to other fertility medications. [23-24]
- In women with unexplained infertility, administration of 100 mg of clomiphene citrate daily resulted in favorable pregnancy outcomes. [25]
- When combined with letrozole, clomiphene citrate produces a higher ovulation rate in women with polycystic ovary syndrome. [26]
- When combined with C. racemosa rhizome dry extract, clomiphene citrate induces a higher pregnancy rate and cycle outcomes. [27]
- When combined with dexamethasone, clomiphene citrate increases the number and diameter of follicles, endometrial thickness, ovulation rate, and pregnancy outcome in PCOS patients. [28]
- In women with PCOS, administration of clomiphene citrate significantly increased ovulation and pregnancy rates with no side effects. [29-39]
- Studies show that clomiphene citrate can help restore the normal ovulation cycle. [40-43]
C. Improves Sperm Quality
Clomiphene citrate can also be used as a therapeutic option for male infertility. Numerous high-quality studies show that clomiphene citrate administration has beneficial effects on sperm quality, thus increasing the chance of fertilization:
- A 2007 study published in the Indian Journal of Physiology and Pharmacology found that clomiphene citrate increased sperm count in men with oligospermia (low sperm count) by boosting the levels of testosterone, FSH, and LH. [1]
- When administered in infertile men, clomiphene citrate is associated with a higher rate of impregnating partners and higher levels of testosterone, LH, and FSH. [2]
- In young, obese men who are at high risk for infertility related to testosterone deficiency, clomiphene citrate administration significantly increased testosterone levels. [3]
- Studies found that clomiphene citrate only improves semen quality in infertile men who had increases in testosterone levels after the treatment. [4-5]
- In infertile men with low testosterone levels, clomiphene citrate treatment increased sperm concentration as well as testosterone. [6-7]
- A review of several studies found that clomiphene citrate significantly increased sperm concentrations in infertile men. [44]
- A 2013 study published in Reproductive Science found that clomiphene citrate increased sperm parameters in males attempting to conceive. [45]
- A study reported that men treated with at least 25 mg of clomiphene citrate daily for male infertility had significant improvement in sperm concentration million and total motile count. [46]
- When administered in men with low sperm counts, clomiphene citrate appears to improve semen parameters. [47]
- In men with infertility of unknown cause, clomiphene citrate improved sperm count and sperm motility. [48-49]
- In infertile men, clomiphene citrate administration at 25 mg and 50 mg daily significantly improved semen quality. [50]
- A study comparing the efficacy of clomiphene citrate and vitamin C in treating male infertility found that both treatments improved sperm density. [51]
- When combined with vitamin E, clomiphene citrate significantly improved sperm motility in men with infertility of unknown cause. [52]
- A study found that clomiphene citrate treatment is associated with significant improvement in sperm motility percentage and sperm structure. [53]
- Studies found that clomiphene citrate treats male infertility by increasing the number of mature sperm cells (spermatogenesis). [54-55]
- Studies reported that clomiphene citrate treats male fertility by improving the quality of defective sperm. [56-59]
Clomiphene Citrate Side Effects
Clomiphene citrate side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on clomiphene citrate. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of clomiphene citrate. Despite this, it was listed as a side effect associated with clomiphene citrate even though these associated side effects are very uncommon.
Side effects associated with clomiphene citrate may include the following:
- Abdominal/pelvic fullness
- Bloating
- Breast tenderness
- Dizziness
- Flushing (“hot flashes”)
- Headache
- Stomach upset
Clomiphene Citrate Uses
Clomiphene Citrate is primarily used to treat infertility in women who have irregular or absent ovulation. It works by stimulating the release of hormones necessary for ovulation, making it a common first-line treatment for conditions like polycystic ovary syndrome (PCOS) and other ovulatory disorders. By enhancing follicle-stimulating hormone (FSH) and luteinizing hormone (LH) production, it helps trigger the growth and release of mature eggs, increasing the chances of conception.
In addition to female fertility, Clomiphene Citrate is also used in men to treat low testosterone levels and male infertility. It functions by increasing LH secretion, which stimulates the testes to produce more testosterone and improve sperm production. Unlike direct testosterone replacement therapy, Clomiphene does not suppress natural hormone production, making it a preferred option for men seeking to boost testosterone while maintaining fertility.
Beyond reproductive health, Clomiphene Citrate has been explored for off-label uses, such as treating secondary hypogonadism and certain hormonal imbalances. Some studies suggest it may help improve symptoms related to low testosterone, fatigue, and muscle loss in aging men. However, its use for these conditions requires medical supervision, as long-term safety and effectiveness are still being studied.
Clomiphene Citrate Pills
Clomiphene Citrate pills are a widely used medication for treating infertility in both women and men. In women, they stimulate ovulation by increasing the production of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which help in the maturation and release of eggs. These pills are typically prescribed to women with irregular or absent menstrual cycles due to conditions like polycystic ovary syndrome (PCOS) or other hormonal imbalances.
In men, Clomiphene Citrate pills are used to boost testosterone levels by stimulating LH secretion, which signals the testes to produce more testosterone. This makes it a potential treatment for men with low testosterone (hypogonadism) and male infertility caused by hormonal deficiencies. Unlike direct testosterone replacement therapy, Clomiphene helps maintain natural testosterone production without suppressing sperm count.
Although Clomiphene Citrate is generally well-tolerated, it can cause side effects such as hot flashes, mood swings, nausea, headaches, and vision disturbances. In women, there is also a small risk of ovarian hyperstimulation syndrome (OHSS) and multiple pregnancies. Due to these risks, the medication is usually prescribed for short-term use under a doctor’s supervision to monitor response and minimize potential complications.
Clomid
Clomid (clomiphene citrate) is a medication primarily used to treat infertility in women by stimulating ovulation. It works by blocking estrogen receptors in the hypothalamus, which triggers the release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH). These hormones help the ovaries produce and release eggs, making Clomid a first-line treatment for conditions like polycystic ovary syndrome (PCOS) and other ovulatory disorders.
Beyond female fertility, Clomid is also used in men to increase testosterone levels and improve sperm production. By stimulating LH secretion, it encourages the testes to produce more testosterone, which can be beneficial for men with hypogonadism or low sperm counts. Unlike traditional testosterone replacement therapy (TRT), Clomid helps maintain the body’s natural hormonal feedback system, reducing the risk of testicular shrinkage and infertility.
While Clomid is generally well-tolerated, it can cause side effects such as hot flashes, mood swings, headaches, nausea, and, in rare cases, ovarian hyperstimulation syndrome (OHSS) in women. In men, potential side effects include visual disturbances and changes in mood or libido. Due to these risks, Clomid should be used under medical supervision and for a limited duration to maximize benefits while minimizing adverse effects.
Clomid for Bodybuilding
Clomid (Clomiphene Citrate) is commonly used in bodybuilding as a post-cycle therapy (PCT) drug to help restore natural testosterone production after anabolic steroid use. Since steroids can suppress the body’s ability to produce testosterone, Clomid stimulates the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn boost testosterone levels. This helps bodybuilders maintain muscle mass, strength, and overall hormonal balance after a steroid cycle.
Another reason bodybuilders use Clomid is to prevent estrogen-related side effects such as gynecomastia (enlarged breast tissue in men). Since Clomid acts as a selective estrogen receptor modulator (SERM), it blocks estrogen from binding to receptors in certain tissues, reducing the risk of estrogen-driven side effects. This makes it a popular choice for PCT, as it not only supports testosterone recovery but also helps maintain a lean, dry physique.
Despite its benefits, Clomid is not without potential drawbacks. Some users report side effects such as mood swings, visual disturbances, headaches, and nausea. Additionally, excessive or prolonged use may lead to hormonal imbalances, making proper dosing and medical supervision essential. While Clomid is effective for post-cycle recovery, it should not be relied upon as a long-term solution for testosterone maintenance in bodybuilding.
Clomid Testosterone Booster
Clomid (Clomiphene Citrate) is often used off-label as a testosterone booster in men with low testosterone (low T). Unlike traditional testosterone replacement therapy (TRT), which directly introduces external testosterone into the body, Clomid works by stimulating the body’s natural production. It does this by blocking estrogen receptors in the hypothalamus, leading to increased secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which in turn stimulate the testes to produce more testosterone.
One of the key benefits of using Clomid for testosterone boosting is that it helps maintain fertility while increasing testosterone levels. Traditional TRT can suppress natural testosterone production and sperm count, potentially leading to infertility. Clomid, on the other hand, supports both testosterone and sperm production, making it a preferred option for men who want to improve their hormonal balance without compromising fertility.
Despite its benefits, Clomid is not without risks. Some men may experience side effects such as mood swings, headaches, vision disturbances, and changes in libido. Additionally, Clomid may not be as effective for all individuals, especially those with primary testicular failure, where the testes do not respond adequately to hormonal stimulation. As with any hormone-modulating therapy, Clomid should be used under medical supervision to monitor effectiveness and minimize potential risks.
Clomiphene Mechanism of Action
Clomiphene works by acting as a selective estrogen receptor modulator (SERM), primarily blocking estrogen receptors in the hypothalamus. This prevents estrogen from exerting negative feedback on the hypothalamic-pituitary-gonadal (HPG) axis, leading to an increase in gonadotropin-releasing hormone (GnRH) secretion. As a result, the pituitary gland releases higher levels of follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which are essential for reproductive function.
In women, the rise in FSH stimulates the growth and maturation of ovarian follicles, promoting ovulation in those with anovulatory or irregular cycles. The surge in LH further triggers ovulation, increasing the chances of conception. This mechanism makes Clomiphene an effective first-line treatment for ovulatory dysfunction, especially in conditions like polycystic ovary syndrome (PCOS).
In men, Clomiphene enhances LH secretion, which stimulates the Leydig cells in the testes to produce more testosterone. Unlike exogenous testosterone therapy, which suppresses natural hormone production, Clomiphene maintains the body’s ability to produce its own testosterone while potentially improving sperm production. This makes it a valuable treatment option for men with secondary hypogonadism or infertility linked to low testosterone levels.
Clomiphene Dosing
Clomiphene dosing varies depending on the condition being treated. For female infertility, the typical starting dose is 50 mg daily for five days, usually beginning on the third, fourth, or fifth day of the menstrual cycle. If ovulation does not occur, the dose may be increased to 100 mg daily in subsequent cycles. Treatment should not exceed six cycles due to the risk of ovarian hyperstimulation and diminishing effectiveness over time.
In men, Clomiphene is used off-label to treat low testosterone and infertility. The typical dosage ranges from 12.5 mg to 50 mg daily or every other day, depending on individual response. Unlike in women, Clomiphene therapy for men can be continued for several months under medical supervision to maintain hormone balance and improve fertility.
Since Clomiphene affects hormone levels, regular monitoring is essential to assess its effectiveness and minimize potential side effects. Blood tests measuring testosterone, estrogen, and LH/FSH levels help guide dosage adjustments. Patients should follow their healthcare provider’s recommendations carefully to achieve the best results while reducing the risk of adverse effects.
Clomiphene Citrate and Ovarian Hyperstimulation Syndrome
Clomiphene citrate is a commonly used medication for inducing ovulation in women with infertility, particularly those with polycystic ovary syndrome (PCOS). While it effectively stimulates the ovaries by increasing FSH and LH levels, excessive stimulation can lead to ovarian hyperstimulation syndrome (OHSS), a condition where the ovaries become swollen and fluid may accumulate in the abdomen.
Mild cases of OHSS present with symptoms such as bloating, mild abdominal discomfort, and temporary weight gain due to fluid retention. However, in severe cases, women may experience severe abdominal pain, rapid weight gain, nausea, vomiting, and even life-threatening complications such as blood clots or kidney dysfunction. The risk of OHSS is higher in women with high estrogen levels, multiple ovarian follicles, or previous OHSS episodes.
To reduce the risk of OHSS, doctors carefully monitor ovarian response through ultrasound and hormone testing during clomiphene treatment. In high-risk cases, they may adjust the dosage, delay treatment cycles, or switch to alternative medications like letrozole. Staying well-hydrated and maintaining close follow-ups with a healthcare provider can also help in managing and preventing complications associated with OHSS.
Clomiphene Citrate and Menstrual Cycle
Clomiphene citrate plays a crucial role in regulating the menstrual cycle by stimulating ovulation in women with irregular or absent cycles. It works by blocking estrogen receptors in the hypothalamus, which tricks the brain into perceiving low estrogen levels. This leads to an increase in gonadotropin-releasing hormone (GnRH), prompting the pituitary gland to release more follicle-stimulating hormone (FSH) and luteinizing hormone (LH), both essential for follicle development and ovulation.
During a typical menstrual cycle, FSH stimulates the growth of ovarian follicles, while LH triggers the release of a mature egg. Clomiphene enhances this natural process by increasing the levels of these hormones, making it particularly effective for women with conditions like polycystic ovary syndrome (PCOS) or anovulation. By ensuring the release of an egg, clomiphene citrate helps improve the chances of conception in women experiencing fertility challenges.
The timing of clomiphene citrate administration is critical for its effectiveness. It is usually taken in the early follicular phase (days 3–7 or 5–9 of the cycle) to allow sufficient follicular growth before ovulation occurs. Ovulation typically happens about 5 to 10 days after the last dose, making this window ideal for conception efforts. Monitoring through ultrasound or hormone tracking helps optimize results, ensuring the treatment aligns with the body’s natural cycle.
Clomiphene Citrate and Polycystic Ovary Syndrome
Clomiphene citrate is a first-line treatment for ovulation induction in women with polycystic ovary syndrome (PCOS), a condition characterized by hormonal imbalances that lead to irregular or absent ovulation. As a selective estrogen receptor modulator (SERM), clomiphene works by blocking estrogen receptors in the hypothalamus, stimulating the release of gonadotropin-releasing hormone (GnRH), which increases follicle-stimulating hormone (FSH) and luteinizing hormone (LH) production. This process helps trigger ovulation, improving fertility in women with PCOS.
Many women with PCOS experience anovulation, making it difficult to conceive naturally. Clomiphene citrate is often prescribed as a five-day oral medication early in the menstrual cycle to stimulate follicular development. Studies have shown that clomiphene can induce ovulation in 70-80% of women with PCOS, with pregnancy success rates around 30-40% per cycle. However, some women may not respond to clomiphene alone and may require additional treatments such as metformin, letrozole, or gonadotropin therapy.
Although clomiphene citrate is effective, it has potential side effects, including hot flashes, mood swings, bloating, and an increased risk of multiple pregnancies (twins or more). Additionally, long-term use beyond six cycles is generally not recommended due to diminishing success rates and potential health risks. For women who do not conceive with clomiphene, alternative treatments like letrozole, ovarian drilling, or in vitro fertilization (IVF) may be considered to improve fertility outcomes.
Clomiphene Citrate and Abnormal Vaginal Bleeding
Clomiphene citrate is a well-established medication for stimulating ovulation in women experiencing infertility. While generally well-tolerated, some women may notice changes in their menstrual cycle, including mild spotting or breakthrough bleeding. These effects are usually temporary and indicate the body’s hormonal response to increased estrogen activity and ovulation stimulation.
For many women, clomiphene citrate helps regulate irregular cycles and improve overall reproductive health. If minor bleeding occurs, it is often a sign that the body is adjusting to hormonal changes. Most women continue treatment without significant concerns, and the benefits of improved ovulation outweigh these temporary adjustments.
In rare cases, if bleeding is persistent or unusual, consulting a healthcare provider ensures optimal treatment outcomes. However, the majority of women using clomiphene citrate experience more predictable cycles and increased fertility success, making it a valuable option for those trying to conceive.
FAQ
What is clomiphene citrate used for?
Clomiphene citrate is used to treat infertility in women by stimulating ovulation and in men to increase testosterone production and improve fertility. However, it may cause abnormal vaginal bleeding as a side effect in some women. Patients experiencing abnormal vaginal bleeding should consult their healthcare provider before starting treatment. If abnormal vaginal bleeding occurs while taking clomiphene citrate, medical evaluation is recommended to rule out underlying conditions.
What does Clomid do for men?
Clomid helps men by stimulating the release of luteinizing hormone (LH), which boosts testosterone production and may improve sperm count. A treatment cycle typically lasts several weeks and should be monitored by a doctor. The effectiveness of Clomid may vary depending on the individual’s response to the treatment cycle. If needed, adjustments to the treatment cycle may be made to optimize results.
What happens after taking clomiphene for 5 days?
In women, clomiphene stimulates follicle development, leading to ovulation within 5–10 days after the last dose. However, in rare cases, an allergic reaction may occur, causing symptoms such as rash, swelling, or difficulty breathing. If an allergic reaction is suspected, medical attention should be sought immediately. Patients with a history of an allergic reaction to similar medications should inform their healthcare provider before starting clomiphene.
What does clomiphene citrate do?
Clomiphene citrate blocks estrogen receptors in the brain, causing an increase in FSH and LH secretion, which stimulates ovulation in women and testosterone production in men. However, clomiphene may cause blurred vision, which may occur during or after treatment. If blurred vision develops, patients should seek medical advice to determine if treatment should be adjusted. In rare cases, blurred vision may persist even after discontinuing the medication.
Can clomiphene citrate cause twins?
Yes, clomiphene increases the likelihood of multiple ovulations, raising the chances of having twins or multiples. However, some women may experience pelvic pain as a side effect due to ovarian stimulation. If pelvic pain becomes severe or persistent, it is important to seek medical advice. Monitoring for pelvic pain can help detect potential complications such as ovarian hyperstimulation syndrome (OHSS).
What does clomiphene do to a man?
It increases LH and FSH levels, leading to higher testosterone production and potentially improving sperm count and fertility. However, individuals with liver disease should use caution, as the medication may not be suitable for them. Additionally, if you experience trouble breathing while taking clomiphene, it is important to seek medical attention immediately. Before taking clomiphene, those with liver disease should consult their healthcare provider to assess potential risks. In cases of liver disease or trouble breathing, alternative treatments may be considered to ensure safety and effectiveness.
What is clomiphene citrate used for in females?
It is primarily used to induce ovulation in women with irregular or absent menstrual cycles due to conditions like polycystic ovary syndrome (PCOS). However, some women may experience abnormal uterine bleeding as a side effect. If abnormal uterine bleeding occurs, it is important to seek medical advice to determine the cause. Patients with a history of abnormal uterine bleeding should discuss their condition with a healthcare provider before starting treatment.
When should clomiphene citrate be taken?
For women, it is typically taken on days 3–7 or 5–9 of the menstrual cycle, but caution is advised for those with ovarian cysts. A pelvic examination is recommended to check for any ovarian abnormalities before starting treatment. For men, it is usually taken daily or every other day as prescribed by a doctor. Women with ovarian cysts should consult their healthcare provider before using clomiphene, as it may exacerbate the condition. If ovarian cysts develop or enlarge during treatment, a pelvic examination may be necessary, and discontinuation may be required.
What is clomiphene citrate tablets used for?
It is used for treating female infertility and male hypogonadism by stimulating hormone production. Patients should carefully follow the prescription label instructions to ensure safe and effective use, as improper use may lead to an increased risk of side effects.
What happens to your body when you take Clomid?
In women, Clomid stimulates egg development and ovulation. In men, it enhances testosterone production, potentially improving energy, mood, and libido. However, there may be an increased risk of multiple pregnancies when taking clomiphene for ovulation induction.
Can you get clomiphene over the counter?
No, clomiphene requires a prescription from a healthcare provider and should be taken as directed, especially regarding timing in relation to sexual intercourse for optimal effectiveness. There is an increased risk of multiple pregnancies when taking clomiphene, so medical supervision is essential. Additionally, it is not recommended for use during breastfeeding, as the drug may pass into breast milk. Women who are breastfeeding should discuss alternative treatment options with their healthcare provider.
What does Clomid do exactly?
Clomid blocks estrogen receptors in the hypothalamus, increasing FSH and LH secretion, which promotes ovulation in women and testosterone production in men. Some women may experience side effects like breast pain as a result of taking clomiphene and its hormonal effects. It is important to note that breastfeeding may be affected while taking Clomid, as the medication can alter hormone levels. Women who are breastfeeding should consult their healthcare provider before using clomiphene to assess any potential risks.
How soon do you get pregnant on Clomid?
Some women conceive in the first cycle, but it may take 3–6 cycles for pregnancy to occur after egg ovulation occurs. However, there is a risk of ovarian cyst formation with prolonged use of fertility treatments like clomiphene citrate. If you experience any concerning symptoms, call your doctor for further guidance and evaluation, especially if related to egg ovulation.
Why would a man take Clomid?
Men take Clomid to boost testosterone levels, improve sperm production, and treat conditions like secondary hypogonadism. Some men may experience abdominal pain as a side effect during treatment. If you experience persistent or severe abdominal pain, call your doctor for further evaluation. Additionally, if you experience signs of a serious allergic reaction, such as difficulty breathing, swelling of the face or throat, or hives, seek immediate medical attention.
What are the major side effects of Clomid?
Common side effects include hot flashes, mood swings, headaches, nausea, breast tenderness, and ovarian hyperstimulation syndrome (OHSS) in women. Certain medications or health conditions can affect clomiphene and increase the risk of these side effects, so it’s important to inform your doctor about any other treatments or conditions.
What is the most common side effect of clomiphene?
Hot flashes are the most commonly reported side effects, and they may occur around the time of your menstrual period in women taking clomiphene citrate. While rare, there is a potential risk of birth defects associated with clomiphene citrate use, particularly when taken in high doses or for extended periods.
Does Clomid cause you to gain weight?
Weight gain is not a common side effect, but some people report fluid retention or bloating, which can sometimes be influenced by the use of human chorionic gonadotropin alongside treatments like clomiphene citrate. Additionally, a missed dose of clomiphene citrate can affect the treatment’s effectiveness and should be addressed according to the prescribed schedule.
What is the risk of taking Clomid?
Potential risks include ovarian hyperstimulation syndrome (OHSS), multiple pregnancies, mood changes, vision disturbances, and complications related to uterine fibroids in some women.
Does Clomid help men build muscle?
Clomid can increase testosterone, which may help with muscle growth, but it is not a primary muscle-building drug. Additionally, some users have reported rapid weight gain as a side effect, though it is not common. There have also been concerns about a potential increased risk of ovarian cancer with long-term use of Clomid, though further research is needed to establish a clear link. Rapid weight gain may occur in some individuals, and it is important to monitor for any significant changes while using the medication.
Does Clomid make men feel good?
Some men experience improved energy, mood, and libido due to increased testosterone levels, but others may have mood swings or irritability. Taking double or extra doses of Clomid can increase the risk of side effects, including mood changes, and should be avoided unless advised by a healthcare provider. In cases of infertility, intrauterine insemination may be considered in conjunction with Clomid treatment to enhance the chances of conception. However, intrauterine insemination should be discussed with a fertility specialist to determine the best course of action.
How long do men stay on Clomid?
Treatment duration varies but typically lasts between 3–6 months under medical supervision. It is important to avoid alcoholic beverages during treatment, as they can interfere with the medication’s effectiveness. If you miss a dose, follow your healthcare provider’s instructions on how to proceed, and refrain from consuming alcoholic beverages until you have received further guidance.
Is Clomid or Nolvadex better?
Clomid is better for stimulating testosterone and fertility, while Nolvadex is more commonly used for post-cycle therapy (PCT) in bodybuilding. To ensure proper treatment, your healthcare provider may recommend certain medical tests before and during Clomid use. If you miss a dose of Clomid, it’s important to follow your healthcare provider’s instructions on how to proceed, which may include additional medical tests to monitor hormone levels.
Is Clomid a good testosterone booster?
Yes, Clomid can effectively raise testosterone levels in men with low testosterone. However, some men may experience visual symptoms such as blurred vision or light sensitivity, and should seek medical advice if these occur. It’s important to follow the prescribed dose of clomiphene to minimize side effects. If symptoms persist, the dose of clomiphene may need to be adjusted by a healthcare provider.
How long until Clomid raises testosterone?
Testosterone levels usually start increasing within 2–4 weeks of use, but individuals with a pituitary tumor may experience different responses due to hormonal imbalances caused by the tumor. As a fertility drug, clomiphene citrate can help stimulate testosterone production, particularly in men with low levels. It is important to monitor hormone levels when using this fertility drug to ensure effective treatment.
Does Clomid increase testicular size?
Yes, in men with low testosterone, Clomid can lead to testicular enlargement due to increased hormone production. However, men with a pituitary tumor should be cautious, as it may affect hormone regulation and interfere with Clomid’s effectiveness. If any adverse effects occur, it may be necessary to discontinue treatment and consult a doctor. In such cases, healthcare providers might recommend discontinuing treatment and exploring alternative therapies for better hormone regulation.
How long should a man take Clomid?
Men typically take Clomid for 3–6 months, but long-term use should be monitored by a doctor. Common side effects include nausea and vomiting, which may occur during treatment, so it’s important to discuss any concerns with a healthcare provider. If nausea or vomiting becomes severe, dosage adjustments or an alternative treatment may be necessary.
What is the mechanism of action of clomiphene?
Clomiphene blocks estrogen receptors in the hypothalamus, stimulating the pituitary gland to release more FSH and LH, which promotes ovulation in women and testosterone production in men. However, it may reduce breast milk production in breastfeeding women.
Is clomiphene a GnRH agonist?
No, clomiphene is a selective estrogen receptor modulator (SERM), not a GnRH agonist. It may also reduce breast milk production in some women. There is no direct evidence linking clomiphene use to neural tube defects, but it’s important for women who are planning to conceive to discuss any potential risks with their doctor. While studies on neural tube defects and clomiphene are limited, careful monitoring during early pregnancy is advised.
How does clomiphene induce ovulation?
By blocking estrogen receptors in the hypothalamus, clomiphene increases FSH and LH levels, which stimulate the growth and release of an egg. It is important to be aware of how certain medications may clomiphene interact with each other, as drug interactions can affect its effectiveness. Always inform your healthcare provider about any other medications you are taking to avoid potential clomiphene interaction effects that could interfere with its action.
What is clomiphene and how does it work?
Clomiphene is a SERM that increases FSH and LH secretion, leading to ovulation in women and testosterone production in men. However, it may also cause ovarian enlargement as a side effect in some women undergoing treatment. It is important to be aware of potential drug interactions, as Clomiphene may interact with other medications. Always discuss possible drug interactions with a healthcare provider before starting or adjusting Clomiphene treatment.
What is the Clomid 5-day rule?
It refers to taking Clomid for 5 consecutive days early in the menstrual cycle (typically days 3–7 or 5–9) to stimulate ovulation, which may sometimes lead to ovarian enlargement as a side effect. Clomid is the common brand of clomiphene citrate, widely prescribed for ovulation induction. As a common brand, Clomid is known for its effectiveness in treating infertility but should be used under medical supervision to minimize potential risks.
What is normal Clomid dosing?
The typical starting dose is 50 mg per day for 5 days in women. In men, doses range from 25–50 mg daily or every other day. One potential side effect in women is ovarian enlargement, which should be monitored by a healthcare provider. Clomiphene citrate also increases the risk of multiple births, including twins and higher-order multiples. Women using Clomid should be aware of the risk of multiple births and discuss it with their healthcare provider.
Can I take 100mg of Clomid per day?
Yes, but higher doses should only be used under medical supervision if 50 mg is ineffective, as it may increase the chances of conceiving multiple babies. It’s important to be aware that higher doses may also increase the risk of vision problems, such as blurriness or disturbances. If vision problems occur, it’s essential to contact a healthcare provider for further evaluation.
Is 50mg of Clomid too much?
No, 50 mg is the standard starting dose for both men and women, but in women, it may increase the chances of conceiving multiple babies. However, some individuals may experience vision problems as a side effect, such as blurred vision or spots. If vision problems persist or worsen, it is important to seek medical advice.
Reference
Sharma D, Zillioux J, Khourdaji I, et al. Improvements in semen parameters in men treated with clomiphene citrate-A retrospective analysis. Andrologia. 2019;51(5):e13257.
Improvements in semen parameters in men treated with clomiphene citrate-A retrospective analysis. Andrologia
Clomiphene citrate (CC) is commonly used off-label for male infertility, but there is limited data on patient selection. A study of 77 men treated with CC showed significant improvements in sperm concentration and motility, though no specific predictors of treatment response were identified, offering useful response rates for decision-making.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/30779195/.Sokol RZ, Steiner BS, Bustillo M, Petersen G, Swerdloff RS. A controlled comparison of the efficacy of clomiphene citrate in male infertility. FertilSteril. 1988;49(5):865-70.
A controlled comparison of the efficacy of clomiphene citrate in male infertility
A study on clomiphene citrate (CC) in oligospermic men found no significant improvement in pregnancy rates or semen parameters compared to placebo, though CC did increase levels of certain hormones. The authors concluded that CC is not effective for treating male infertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/3129318/.Bendre SV, Murray PJ, Basaria S. Clomiphene Citrate Effectively Increases Testosterone in Obese, Young, Hypogonadal Men. ReprodSyst Sex Disord. 2015;4(4):155. doi:10.4172/2161-038X.1000155.
Clomiphene Citrate Effectively Increases Testosterone in Obese, Young, Hypogonadal Men
You can read the abstract of this article at
This study examines the effectiveness of clomiphene citrate (CC) in increasing testosterone (T) levels in obese, hypogonadal males aged 18-21 years. After three months of treatment, testosterone, LH, and FSH levels significantly increased, with no correlation to weight or BMI. It concludes that CC is effective in boosting testosterone in young obese hypogonadal men, warranting further research on its safety and potential use.
https://pubmed.ncbi.nlm.nih.gov/26844009/.Emperaire JC, Riviere J, Ruffie A, Audebert AJ. Clomiphene test and clomiphene therapy in idiopathic male infertility. Arch Androl. 1979;2(3):223-31.
Clomiphene test and clomiphene therapy in idiopathic male infertility
Clomiphene citrate (50 mg/day) was administered to 105 patients with idiopathic azoospermia or oligoasthenospermia, showing three types of responses: complete positive, dissociated positive, and negative. Hormonal responses on day 15 did not reliably predict improvements in sperm counts, with only 11 out of 54 completing treatment showing elevated sperm counts.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/485645/.Krzastek SC, Sharma D, Abdullah N, et al. Long-Term Safety and Efficacy of Clomiphene Citrate for the Treatment of Hypogonadism. J Urol. 2019;202(5):1029-1035.
Long-Term Safety and Efficacy of Clomiphene Citrate for the Treatment of Hypogonadism
Clomiphene citrate is an effective and safe long-term treatment for hypogonadism, with minimal side effects. In a study of 400 patients treated for up to 7 years, 88% of those treated for more than 3 years achieved normal testosterone levels and 77% reported symptom improvement. The most common side effects were mild, and there were no significant adverse events.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/31216250/.Surbone A, Vaucher L, Primi MP, et al. Clomiphene citrate effect on testosterone level and semen parameters in 18 infertile men with low testosterone level and normal/low gonadotropines level. Eur J ObstetGynecolReprod Biol. 2019;238:104-109.
Clomiphene citrate effect on testosterone level and semen parameters in 18 infertile men with low testosterone level and normal/low gonadotropines level
A 3-month course of clomiphene citrate (CC) increased plasma testosterone levels and sperm concentration in infertile men with low testosterone and normal or low gonadotropin levels, though it had no significant effect on sperm motility. Three natural pregnancies occurred during treatment. Further research is needed to confirm its effects on sperm parameters and pregnancy rates.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/31128532/.Patel DP, Brant WO, Myers JB, et al. The safety and efficacy of clomiphene citrate in hypoandrogenic and subfertile men. Int J Impot Res. 2015;27(6):221-4.
The safety and efficacy of clomiphene citrate in hypoandrogenic and subfertile men. Int J Impot Res
A retrospective study evaluated the safety and efficacy of clomiphene citrate (CC) in infertile, hypoandrogenic men, showing significant increases in testosterone and estradiol after two weeks, with potential improvements in semen parameters, though a small percentage of men did not respond positively.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/26289907/.Beall SA, DeCherney A. History and challenges surrounding ovarian stimulation in the treatment of infertility. Fertil Steril. 2012;97(4):795–801. doi:10.1016/j.fertnstert.2012.02.030.
History and challenges surrounding ovarian stimulation in the treatment of infertility
Superovulation has revolutionized infertility treatment by enabling ovulation induction and IVF but has also led to increased multiple births. Advances in monitoring and regulations have reduced higher-order multiples, yet twin rates remain high, with future efforts aimed at achieving singleton live births.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/22463773/.Weller A, Daniel S, Koren G, Lunenfeld E, Levy A. The fetal safety of clomiphene citrate: a population-based retrospective cohort study. BJOG. 2017;124(11):1664-1670.
The fetal safety of clomiphene citrate: a population-based retrospective cohort study}
A large population-based study found no increased risk of major or specific fetal malformations following exposure to clomiphene citrate for ovulation induction.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/28334503/.Jain JK, Kuo J. Pregnancy outcomes with increased clomiphene citrate dose. GynecolEndocrinol. 2004;19(3):141-5.
Pregnancy outcomes with increased clomiphene citrate dose
Increasing the clomiphene citrate dose to 150-250 mg/day did not significantly affect pregnancy outcomes compared to lower doses (50-100 mg/day). The rates of spontaneous abortion, ectopic pregnancy, congenital malformations, stillbirth, and normal gestation were similar between both groups.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/15697075/.Elkhateeb RR, Mahran AE, Kamel HH. Long-term use of clomiphene citrate in induction of ovulation in PCO patients with clomiphene citrate resistance. J GynecolObstet Hum Reprod. 2017;46(7):575-577.
Long-term use of clomiphene citrate in induction of ovulation in PCO patients with clomiphene citrate resistance
Extended clomiphene citrate (CC) treatment significantly improves ovulation and pregnancy rates in CC-resistant PCOS patients. Further multi-center studies are needed for stronger evidence.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/28549986/.Venn A, Lumley J. Clomiphene citrate and pregnancy outcome. Aust N Z J ObstetGynaecol. 1994;34(1):56-66.
Clomiphene citrate and pregnancy outcome
Assisted conception methods like IVF and GIFT are linked to higher risks of adverse perinatal outcomes, including preterm birth and perinatal mortality. This review examines the impact of ovulation induction with clomiphene citrate, highlighting inconsistencies in data on risks such as ectopic pregnancy and congenital malformations, while recognizing multiple pregnancy as a known complication.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/8053878/.Kousta E, White DM, Franks S. Modern use of clomiphene citrate in induction of ovulation. Hum Reprod Update. 1997;3(4):359-65.
Modern use of clomiphene citrate in induction of ovulation
Clomiphene citrate is the first-line treatment for infertility in anovulatory women with normal estrogen levels, with success rates similar to the fertile population. Weight reduction improves response, and ultrasound monitoring helps minimize risks like ovarian hyperstimulation and multiple pregnancies. Treatment should be limited to 12 cycles due to potential long-term risks.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/9459281/.Gorlitsky GA, Kase NG, Speroff L. Ovulation and pregnancy rates with clomiphene citrate. Obstet Gynecol. 1978;51(3):265-9.
Ovulation and pregnancy rates with clomiphene citrate
High-dose clomiphene citrate (150–200 mg) effectively induces ovulation in women who do not respond to lower doses, with treatment starting at 50 mg and increasing if necessary. Children from clomiphene-induced pregnancies develop normally, and about 50% of patients conceive after three ovulations, aligning with general population statistics.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/628527/.Seyedoshohadaei F, Zandvakily F, Shahgeibi S. Comparison of the effectiveness of clomiphene citrate, tamoxifen and letrozole in ovulation induction in infertility due to isolated unovulation. Iran J Reprod Med. 2012;10(6):531–536.
Comparison of the effectiveness of clomiphene citrate, tamoxifen and letrozole in ovulation induction in infertility due to isolated inoculation
This study compared the effectiveness of clomiphene, tamoxifen, and letrozole in inducing ovulation in non-PCOS anovulatory women. Clomiphene had the highest pregnancy rate, but tamoxifen and letrozole were associated with lower miscarriage rates.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/25246922/.SOGC clinical practice guideline. Ovulation induction in polycystic ovarian syndrome. Ottawa, ON: Society of Obstetricians and Gynaecologists of Canada; 2010. Available from: https://docs.google.com/viewerng/viewer?url=http://sogc.org/wp-content/uploads/2013/01/gui242CPG1005E_000.pdf.
Ovulation induction in polycystic ovarian syndrome
This review suggests a rational treatment order for anovulatory women with PCOS, emphasizing weight reduction in obese/overweight patients and recommending inositol, insulin sensitizers, selective estrogen receptor modulators, and aromatase inhibitors as first-line options. Second-line treatments include ovarian electrocautery and low-dose FSH, while IVF is a last resort due to the risk of ovarian hyperstimulation syndrome.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29889977/.Legro RS, Arslanian SA, Ehrmann DA, Hoeger KM, Murad MH, Pasquali R, et al. Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J ClinEndocrinolMetab. 2013;98(12):4565–92.
Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline
The Endocrine Society developed evidence-based guidelines for diagnosing and treating polycystic ovary syndrome (PCOS) using the GRADE system. The recommendations include using the Rotterdam criteria for diagnosis, hormonal contraceptives for menstrual and androgen-related symptoms, and clomiphene for infertility, while metformin is beneficial for metabolic issues but has limited effects on hirsutism, acne, or infertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/24151290/.Legro RS, Barnhart HS, Schlaff WD, Carr BR, Diamond MP, Carson SA, et al. Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome. N Engl J Med. 2007;356(6):551–66.
Clomiphene, metformin, or both for infertility in the polycystic ovary syndrome
Clomiphene is more effective than metformin in achieving live births in women with polycystic ovary syndrome, though it carries a higher risk of multiple pregnancies. Combination therapy with metformin and clomiphene offers no significant advantage over clomiphene alone.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/17287476/.Richard JP, Steiner AZ, Terplan M. Comparison of tamoxifen and clomiphene citratefor ovulation induction: a meta-analysis. Hum Reprod. 2005;20:1511–1515.
Comparison of tamoxifen and clomiphene citratefor ovulation induction: a meta-analysis. Hum Reprod
This meta-analysis found that tamoxifen and clomiphene citrate are equally effective in inducing ovulation in patients with anovulatory infertility. There is no significant difference between the two in achieving pregnancy per cycle or per ovulatory cycle.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/15845599/.Fisher SA, Reid RL, Van vugt DA, Casper RF. A randomized double-blind comparison of the effects of clomiphene citrate and the aromatase inhibitor letrozole on ovulatory function in normal women. Fertil Steril. 2002;78(2):280-5.
A randomized double-blind comparison of the effects of clomiphene citrate and the aromatase inhibitor letrozole on ovulatory function in normal women
A randomized controlled trial compared letrozole and clomiphene citrate in ovulatory women, finding both drugs stimulated folliculogenesis similarly without affecting endometrial thickness or ovulation patterns. Letrozole led to lower estrogen levels than clomiphene.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/12137863/.Sipe CS, Davis WA, Maifeld M, Van voorhis BJ. A prospective randomized trial comparing anastrozole and clomiphene citrate in an ovulation induction protocol using gonadotropins. Fertil Steril. 2006;86(6):1676-81.
A prospective randomized trial comparing anastrozole and clomiphene citrate in an ovulation induction protocol using gonadotropins
This study compared the effects of anastrozole and clomiphene citrate with gonadotropins on ovarian and endometrial response in infertile women. Anastrozole resulted in fewer follicles and lower estrogen levels than clomiphene, with similar pregnancy and cycle cancellation rates, suggesting it may be a safer option for patients at risk of hyperstimulation and multiple births.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/17007849/.Bayar U, Tanriverdi HA, Barut A, Ayoğlu F, Ozcan O, Kaya E. Letrozole vs. clomiphene citrate in patients with ovulatory infertility. Fertil Steril. 2006;85(4):1045-8.
Letrozole vs. clomiphene citrate in patients with ovulatory infertility
Letrozole and clomiphene citrate (CC) showed similar effectiveness in ovulatory patients with borderline male factor infertility, early-stage endometriosis, and unexplained infertility, with comparable ovulation and pregnancy rates.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/16580393/.Hughes E, Collins J, Vandekerckhove P. WITHDRAWN: Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea. Cochrane Database Syst Rev. 1996;(1):CD000056.
Clomiphene citrate for ovulation induction in women with oligo-amenorrhoea
Clomiphene citrate stimulates pituitary hormone release, promoting ovulation and improving fertility in women with oligo-ovulatory subfertility. While effective, it carries risks such as multiple pregnancies and a potential link to ovarian cancer.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/10796477/Zain MM, Jamaluddin R, Ibrahim A, Norman RJ. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial. FertilSteril. 2009;91(2):514-21.
Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction, achievement of pregnancy, and live birth in Asian women with polycystic ovary syndrome: a randomized controlled trial
Clomiphene citrate (CC) is the preferred first-line treatment for ovulation induction in anovulatory PCOS patients, as it leads to higher ovulation, pregnancy, and live birth rates compared to metformin. Combination therapy with metformin and CC showed slightly better outcomes but without significant statistical differences.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/18321486/.Abu hashim H, Shokeir T, Badawy A. Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a randomized controlled trial. FertilSteril. 2010;94(4):1405-9.
Letrozole versus combined metformin and clomiphene citrate for ovulation induction in clomiphene-resistant women with polycystic ovary syndrome: a randomized controlled trial
Letrozole is more effective and acceptable than clomiphene citrate (CC) with metformin for inducing ovulation in CC-resistant polycystic ovary syndrome (PCOS), showing higher ovulation and clinical pregnancy rates, better endometrial thickness, fewer adverse effects, and a higher multiple pregnancy rate.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29076376/.Ganesh A, Goswami SK, Chattopadhyay R, Chaudhury K, Chakravarty B. Comparison of letrozole with continuous gonadotropins and clomiphene-gonadotropin combination for ovulation induction in 1387 PCOS women after clomiphene citrate failure: a randomized prospective clinical trial. J Assist Reprod Genet. 2009;26:19–24.
Comparison of letrozole with continuous gonadotropins and clomiphene-gonadotropin combination for ovulation induction in 1387 PCOS women after clomiphene citrate failure: a randomized prospective clinical trial
Letrozole was compared with rFSH and CC/rFSH for ovarian stimulation in IUI cycles among PCOS women with CC failure. It showed higher ovulation and pregnancy rates than CC/rFSH and was most effective when baseline estradiol was >60 pg/ml.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/19127427/.Badawy A, Shokeir T, Allam AF, Abdelhady H. Pregnancy outcome after ovulation induction with aromatase inhibitors or clomiphene citrate in unexplained infertility. Acta ObstetGynecol Scand. 2009;88(2):187-91.
Pregnancy outcome after ovulation induction with aromatase inhibitors or clomiphene citrate in unexplained infertility
Aromatase inhibitors and clomiphene citrate (CC) were effective for ovulation induction, leading to comparable pregnancy and miscarriage rates. No significant safety concerns were found for mothers or fetuses.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/19089782/.Mejia RB, Summers KM, Kresowik JD, Van Voorhis BJ. A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil Steril. 2019;111(3):571-578.e1. doi:10.1016/j.fertnstert.2018.11.030.
A randomized controlled trial of combination letrozole and clomiphene citrate or letrozole alone for ovulation induction in women with polycystic ovary syndrome. Fertil Steril
A randomized controlled trial found that a combination of letrozole and clomiphene citrate (CC) resulted in a significantly higher ovulation rate (77%) compared to letrozole alone (43%) in women with PCOS-related infertility. Both treatments had similar side effects, with no serious adverse events or multiple-gestation pregnancies.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/30683591/.Shahin AY, Ismail AM, Zahran KM, Makhlouf AM. Adding phytoestrogens to clomiphene induction in unexplained infertility patients–a randomized trial. Reprod Biomed Online. 2008;16(4):580-588. doi:10.1016/s1472-6483(10)60465-8.
Adding phytoestrogens to clomiphene induction in unexplained infertility patients–a randomized trial
This study found that adding Cimicifuga racemosa (a phytoestrogen) to clomiphene citrate treatment improved pregnancy rates, endometrial thickness, and hormone levels in patients with unexplained infertility and recurrent induction failure. The combination led to significantly better cycle outcomes compared to clomiphene citrate alone.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/18413068/.Esmaeilzadeh S, Amiri MG, Basirat Z, Shirazi M. Does Adding Dexamethasone to Clomiphene Citrate Improve Ovulation in PCOS Patients? A Triple – Blind Randomized Clinical Trial Study. Int J Fertil Steril. 2011;5(1):9-12.
Does Adding Dexamethasone to Clomiphene Citrate Improve Ovulation in PCOS Patients? A Triple – Blind Randomized Clinical Trial Study
Adding dexamethasone to clomiphene citrate in CC-resistant PCOS patients significantly increased the number of mature follicles but did not significantly improve ovulation or pregnancy rates.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC4040244/.Badawy A, Allam A, Abulatta M. Extending clomiphene treatment in clomiphene-resistant women with PCOS: a randomized controlled trial. Reprod Biomed Online. 2008 Jun;16(6):825-9. doi: 10.1016/s1472-6483(10)60148-4. PMID: 18549692.
Extending clomiphene treatment in clomiphene-resistant women with PCOS: a randomized controlled trial
This study compared extended clomiphene citrate treatment with gonadotrophin therapy in 318 women with clomiphene-resistant PCOS. While gonadotrophins led to significantly higher ovulation (57.6% vs. 28.1%) and pregnancy rates (20.2% vs. 11.4%), extended clomiphene citrate treatment provided a safer, more cost-effective alternative with modest success.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/18549692/.Dasari P, Pranahita G. The efficacy of metformin and clomiphene citrate combination compared with clomiphene citrate alone for ovulation induction in infertile patients with PCOS. J Hum Reprod Sci. 2009;2(1):18-22. doi:10.4103/0974-1208.51337.
The efficacy of metformin and clomiphene citrate combination compared with clomiphene citrate alone for ovulation induction in infertile patients with PCOS
A randomized controlled trial compared Clomiphene Citrate (CC) alone versus CC with metformin in infertile women with PCOS. The combination therapy significantly improved ovulation, regular cycles, and conception rates compared to CC alone.
You can read the abstract of this article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC3725449/.Neveu N, Granger L, St-Michel P, Lavoie HB. Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction and achievement of pregnancy in 154 women with polycystic ovary syndrome. Fertil Steril. 2007 Jan;87(1):113-20. doi: 10.1016/j.fertnstert.2006.05.069. Epub 2006 Nov 1. PMID: 17081535.
Comparison of clomiphene citrate, metformin, or the combination of both for first-line ovulation induction and achievement of pregnancy in 154 women with polycystic ovary syndrome. Fertil Steril
Metformin is more effective than clomiphene citrate (CC) for ovulation induction in PCOS patients, with similar pregnancy rates across treatments. Metformin’s efficacy is independent of weight and insulin levels, making it a suitable first-line option.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/17081535/.Dehbashi S, Vafaei H, Parsanezhad MD, Alborzi S. Time of initiation of clomiphene citrate and pregnancy rate in polycystic ovarian syndrome. Int J Gynaecol Obstet. 2006 Apr;93(1):44-8. doi: 10.1016/j.ijgo.2005.10.015. Epub 2006 Mar 10. PMID: 16530767.
Time of initiation of clomiphene citrate and pregnancy rate in polycystic ovarian syndrome
Starting clomiphene citrate (CC) earlier in the menstrual cycle (days 1-5) results in higher pregnancy rates compared to starting later (days 5-9), despite similar ovulation rates.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/16530767/.Moll E, Bossuyt PM, Korevaar JC, Lambalk CB, van der Veen F. Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial. BMJ. 2006;332(7556):1485. doi:10.1136/bmj.38867.631551.55.
Effect of clomifene citrate plus metformin and clomifene citrate plus placebo on induction of ovulation in women with newly diagnosed polycystic ovary syndrome: randomised double blind clinical trial
A randomized clinical trial in 20 Dutch hospitals compared clomifene citrate plus metformin to clomifene citrate plus placebo in 228 women with polycystic ovary syndrome. The study found no significant difference in ovulation, pregnancy, or miscarriage rates between the groups, but more women in the metformin group discontinued due to side effects. Metformin was not an effective addition for inducing ovulation.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/16769748/.Kar S. Clomiphene citrate or letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trial. J Hum Reprod Sci. 2012;5(3):262-265. doi:10.4103/0974-1208.106338.
Clomiphene citrate or letrozole as first-line ovulation induction drug in infertile PCOS women: A prospective randomized trial
This study compares letrozole (5 mg) and clomiphene citrate (100 mg) for ovulation induction in 103 infertile women with PCOS. While ovulation rates were similar, letrozole showed significantly higher monofollicular development and pregnancy rates, suggesting it should be considered on par with clomiphene citrate as a first-line treatment.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/23531705/.Brown J, Farquhar C. Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome. Cochrane Database Syst Rev. 2016;12(12):CD002249. Published 2016 Dec 15. doi:10.1002/14651858.CD002249.pub5.
Clomiphene and other antioestrogens for ovulation induction in polycystic ovarian syndrome
Clomiphene citrate increases the chance of clinical pregnancy compared to placebo but shows no clear advantage over tamoxifen and may reduce live birth rates compared to gonadotropins. Evidence on adjunctive treatments and alternative regimens is limited and of low quality, requiring further research.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/27976369/.Legro RS, Brzyski RG, Diamond MP, et al. Letrozole versus clomiphene for infertility in the polycystic ovary syndrome [published correction appears in N Engl J Med. 2014 Oct 9;317(15):1465]. N Engl J Med. 2014;371(2):119-129. doi:10.1056/NEJMoa1313517.
Letrozole versus clomiphene for infertility in the polycystic ovary syndrome [published correction appears in N Engl J Med
A study comparing letrozole and clomiphene for infertility treatment in women with polycystic ovary syndrome found that letrozole resulted in higher live-birth and ovulation rates. Both treatments had similar safety profiles, but letrozole was linked to higher fatigue and dizziness, while clomiphene caused more hot flashes.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/25006718/.Mukherjee S, Sharma S, Chakravarty BN. Comparative evaluation of pregnancy outcome in gonadotrophin-clomiphene combination vs clomiphene alone in polycystic ovarian syndrome and unexplained infertility-A prospective clinical trial. J Hum Reprod Sci. 2010;3(2):80-84. doi:10.4103/0974-1208.69341.
Comparative evaluation of pregnancy outcome in gonadotrophin-clomiphene combination vs clomiphene alone in polycystic ovarian syndrome and unexplained infertility-A prospective clinical trial
A clinical trial compared the efficacy of a single dose of uFSH combined with clomiphene citrate versus clomiphene citrate alone for ovulation induction in women with PCOS and unexplained infertility. The results showed significantly higher pregnancy rates in women with PCOS in the uFSH + clomiphene group, but no significant difference in pregnancy rates for women with unexplained infertility. Miscarriage rates were similar between the two groups. The study concluded that adding uFSH improves pregnancy outcomes in anovulatory infertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/21209751/.Hajishafiha M, Dehghan M, Kiarang N, Sadegh-Asadi N, Shayegh SN, Ghasemi-Rad M. Combined letrozole and clomiphene versus letrozole and clomiphene alone in infertile patients with polycystic ovary syndrome [published correction appears in Drug Des Devel Ther. 2017 Apr 28;11:1367]. Drug Des Devel Ther. 2013;7:1427-1431. Published 2013 Dec 3. doi:10.2147/DDDT.S50972.
Combined letrozole and clomiphene versus letrozole and clomiphene alone in infertile patients with polycystic ovary syndrome [published correction appears in Drug Des Devel Ther
This study explores the use of a combination of letrozole and clomiphene in women with polycystic ovary syndrome (PCOS) who are resistant to both drugs individually. The results show a 42% pregnancy rate and suggest that this combination may be a viable first-line therapy to induce ovulation in PCOS patients before resorting to more aggressive treatments or surgery.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/24348019/.Bae SA, Joo JK, Choi JR, Kim SS, Lee KS. Clinical outcomes of three- or five-day treatment with clomiphene citrate combined with gonadotropins and a timed intercourse cycle in polycystic ovary syndrome patients. Clin Exp Reprod Med. 2015;42(3):106-110. doi:10.5653/cerm.2015.42.3.106.
Clinical outcomes of three- or five-day treatment with clomiphene citrate combined with gonadotropins and a timed intercourse cycle in polycystic ovary syndrome patients
This study investigated the effect of a new clomiphene citrate (CC) regimen on endometrial thickness and pregnancy rates in PCOS patients undergoing timed intercourse cycles. The results showed that a three-day CC treatment significantly increased endometrial thickness and pregnancy rates compared to the standard five-day treatment.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/26473110/.Nasseri S, Ledger WL. Clomiphene citrate in the twenty-first century. Hum Fertil (Camb). 2001;4(3):145-51. doi: 10.1080/1464727012000199212. PMID: 11591271.
Clomiphene citrate in the twenty-first century
Clomiphene citrate is the first-line treatment for ovulation induction in women with polycystic ovary syndrome, typically used for up to 12 cycles, with careful monitoring due to risks like multiple pregnancies and ovarian hyperstimulation. Alternatives for clomiphene-resistant patients include gonadotropin injections, metformin, and laparoscopic ovarian drilling.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/11591271/.Hammond MG, Halme JK, Talbert LM. Factors affecting the pregnancy rate in clomiphene citrate induction of ovulation. Obstet Gynecol. 1983 Aug;62(2):196-202. PMID: 6866363.
Factors affecting the pregnancy rate in clomiphene citrate induction of ovulation. Obstet Gynecol
Clomiphene citrate induced ovulation in 86% of anovulatory and oligomenorrheic patients, with 49% of those who ovulated conceiving. Factors like abnormal semen analysis and pelvic abnormalities impacted conception rates, while patient discontinuation of therapy was the most significant contributor to reduced pregnancy rates. Continuing treatment for 10-12 cycles corrected for other infertility factors led to near 100% cumulative pregnancy rates.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/6866363/.Willets AE, Corbo JM, Brown JN. Clomiphene for the treatment of male infertility. Reprod Sci. 2013;20(7):739-44.
Clomiphene for the treatment of male infertility
Male infertility, caused by factors like low sperm production or blockages, is often treated with clomiphene citrate, which may improve sperm parameters. A review of 9 clinical studies showed mixed results, with only one study showing a significant increase in pregnancy rates, while most showed improved sperm concentrations. Overall, clomiphene was well-tolerated, but there is insufficient evidence to support its effectiveness in treating male infertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/23202725/.Patankar SS, Kaore SB, Sawane MV, Mishra NV, Deshkar AM. Effect of clomiphene citrate on sperm density in male partners of infertile couples. Indian J PhysiolPharmacol. 2007;51(2):195-8.
Effect of clomiphene citrate on sperm density in male partners of infertile couples
Clomiphene citrate, a nonsteroidal agent, stimulates pituitary gonadotropin release and enhances spermatogenesis by increasing testosterone levels. A study of 65 men with oligozoospermia showed significant improvements in sperm count and motility after 3 months of treatment with clomiphene citrate, suggesting its effectiveness in improving male fertility in the absence of end-organ pathology.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/18175667/.Schellen TM. Clomiphene treatment in male infertility. Int J Fertil. 1982;27(3):136-45.
Clomiphene treatment in male infertility
Clomiphene citrate improves sperm concentration and motility in infertile men, making it a potentially effective and safe treatment for male infertility, though it does not affect sperm morphology.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/36680549/.Ghanem H, Shaeer O, El-segini A. Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial. FertilSteril. 2010;93(7):2232-5.
Combination clomiphene citrate and antioxidant therapy for idiopathic male infertility: a randomized controlled trial
This study evaluated the effects of clomiphene citrate and vitamin E on pregnancy rates and sperm quality in men with idiopathic oligozoospermia. Results showed a significantly higher pregnancy rate and improvements in sperm count and motility in the treatment group compared to the placebo group.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/19268928/.Hayashi N, Sugimura Y, Hori N, et al. [Clomiphene citrate therapy in idiopathic male infertility]. Hinyokika Kiyo. 1988;34(5):847-50.
[Clomiphene citrate therapy in idiopathic male infertility]
Clomiphene citrate therapy improved sperm count and motility in 36.7% of 30 male infertility patients, with 4 wives becoming pregnant. It also increased serum levels of key hormones. However, the therapy’s significant increase in testosterone could affect androgen-dependent organs like the prostate.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/3140618/.Roth LW, Ryan AR, Meacham RB. Clomiphene citrate in the management of male infertility. SeminReprod Med. 2013;31(4):245-50.
Clomiphene citrate in the management of male infertility
Clomiphene citrate has been used for 50 years to treat male infertility, with mixed results. More research is needed to determine its specific indications in male infertility management.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/23775379/.Allag IS, Alexander NJ. Clomiphene citrate therapy for male infertility. Urology. 1979;14(5):500-3.
Clomiphene citrate therapy for male infertility
Clomiphene citrate improves sperm concentration and motility in infertile men, making it a potential safe therapy, though it has no effect on sperm morphology. It also increases hormonal levels without serious side effects, with pregnancy rates ranging from 0% to 40%.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/36680549/.Abel BJ, Carswell G, Elton R, et al. Randomised trial of clomiphene citrate treatment and vitamin C for male infertility. Br J Urol. 1982;54(6):780-4.
Randomised trial of clomiphene citrate treatment and vitamin C for male infertility
A randomized trial of 179 men with infertility found no significant difference in pregnancy rates between those treated with clomiphene citrate or vitamin C. Vitamin C appears to be a more cost-effective alternative for male infertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/6817845/.Elsheikh MG, Hosny MB, Elshenoufy A, Elghamrawi H, Fayad A, Abdelrahman S. Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: A prospective, randomized trial. Andrology. 2015;3(5):864-7.
Combination of vitamin E and clomiphene citrate in treating patients with idiopathic oligoasthenozoospermia: A prospective, randomized trial
This study evaluated the effects of combining vitamin E and clomiphene citrate on sperm concentration and motility in men with idiopathic oligoasthenozoospermia. Results showed significant improvements in both parameters with combination therapy, compared to either treatment alone.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/26235968/.Moradi M, Moradi A, Alemi M, et al. Safety and efficacy of clomiphene citrate and L-carnitine in idiopathic male infertility: a comparative study. Urol J. 2010;7(3):188-93.
Safety and efficacy of clomiphene citrate and L-carnitine in idiopathic male infertility: a comparative study
A randomized controlled trial compared the effects of L-carnitine and clomiphene citrate on sperm parameters in men with idiopathic infertility, finding both medications improved sperm count and motility, with L-carnitine enhancing semen volume and clomiphene improving motility percentage and morphology. Both treatments are safe and effective for initial male infertility treatment.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/20845296/.Paulson DF, Wacksman J. Clomiphene citrate in the management of male infertility. J Urol. 1976;115(1):73-6.
Clomiphene citrate in the management of male infertility
Clomiphene citrate has been used for 50 years to treat male infertility, including unexplained infertility, oligospermia, hypogonadism, and azoospermia, with mixed results. More research is needed to determine its specific indications for male infertility treatment.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/23775379/.Wu C, Forbes E, Jarvi KA. Clomiphene citrate rescue of spermatogenesis in men with infertility while remaining on finasteride: A case report. Can UrolAssoc J. 2017;11(3-4):E122–E123. doi:10.5489/cuaj.4191.
Clomiphene citrate rescue of spermatogenesis in men with infertility while remaining on finasteride: A case report
Finasteride, used for benign prostatic hyperplasia and male pattern hair loss, may adversely affect fertility in some men, even at low doses. This case report is the first to document the use of clomiphene citrate to improve fertility in a man taking finasteride.
You can read the full article at
https://pmc.ncbi.nlm.nih.gov/articles/PMC5365389/.Check JH, Chase JS, Nowroozi K, Wu CH, Adelson HG. Empirical therapy of the male with clomiphene in couples with unexplained infertility. Int J Fertil. 1989;34(2):120-2.
Empirical therapy of the male with clomiphene in couples with unexplained infertility
Clomiphene therapy for men with unexplained infertility, despite normal semen parameters, significantly improved fertility, with 58% of couples achieving pregnancy compared to 16% with ascorbic acid treatment. However, no measurable changes in sperm quality or semen parameters were observed, suggesting the improvement might be due to an unmeasurable sperm defect or a psychogenic effect.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/2565314/.Soares AH, Horie NC, Chiang LAP, Caramelli B, Matheus MG, Campos AH, Marti LC, Rocha FA, Mancini MC, Costa EMF, Cercato C. Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study. Int J Obes (Lond). 2018 Jun;42(5):953-963. doi: 10.1038/s41366-018-0105-2. Epub 2018 May 17. PMID: 29777228.
Effects of clomiphene citrate on male obesity-associated hypogonadism: a randomized, double-blind, placebo-controlled study
A randomized controlled trial found that clomiphene citrate (CC) improved testosterone levels, body composition, and some sexual symptoms in men with obesity-associated secondary hypogonadism (MOSH), though it reduced HDL and had no significant effect on endothelial function. CC may be a viable alternative to testosterone therapy for MOSH.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/29777228/.Panner Selvam MK, Baskaran S, Tannenbaum J, Greenberg J, Shalaby HY, Hellstrom WJG, Sikka SC. Clomiphene Citrate in the Management of Infertility in Oligospermic Obese Men with Hypogonadism: Retrospective Pilot Study. Medicina (Kaunas). 2023 Oct 26;59(11):1902. doi: 10.3390/medicina59111902. PMID: 38003951; PMCID: PMC10673313.
Clomiphene Citrate in the Management of Infertility in Oligospermic Obese Men with Hypogonadism: Retrospective Pilot Study
Clomiphene citrate (CC) treatment significantly improves serum testosterone levels and semen parameters in oligospermic obese hypogonadal men. This study suggests CC as an effective alternative to testosterone replacement therapy for enhancing male fertility.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/38003951/.Pelusi C, Giagulli VA, Baccini M, Fanelli F, Mezzullo M, Fazzini A, Bianchi N, Carbone MD, De Pergola G, Mastroroberto M, Morselli Labate AM, Pasquali R. Clomiphene citrate effect in obese men with low serum testosterone treated with metformin due to dysmetabolic disorders: A randomized, double-blind, placebo-controlled study. PLoS One. 2017 Sep 8;12(9):e0183369. doi: 10.1371/journal.pone.0183369. PMID: 28886024; PMCID: PMC5590732.
Clomiphene citrate effect in obese men with low serum testosterone treated with metformin due to dysmetabolic disorders: A randomized, double-blind, placebo-controlled study
Clomiphene citrate (CC) significantly increased testosterone levels in obese men with impaired glucose tolerance (IGT) or type 2 diabetes (T2DM) on metformin, compared to placebo. CC also improved fasting glucose, insulin levels, and insulin resistance, suggesting potential metabolic benefits.
You can read the abstract of this article at
https://pubmed.ncbi.nlm.nih.gov/28886024/.
Other Peptides
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








