Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Health Benefits of Contrave
- Key Takeaways
- What is Contrave?
- How Contrave Works
- Chemical Structure of Contrave
- Research on Contrave
- Associated Side Effects of Contrave
- Contrave Dosage
- Weight Loss Pill Contrave
- Contrave Generic
- Contrave and Alcohol
- Contrave vs Wellbutrin
- Contrave Contraindications
- Switching from Wellbutrin to Contrave
- Food to Avoid on Contrave
- Contrave Tablets
- FAQ
- References
Table of Contents
- Potential Health Benefits of Contrave
- Key Takeaways
- What is Contrave?
- How Contrave Works
- Chemical Structure of Contrave
- Research on Contrave
- Associated Side Effects of Contrave
- Contrave Dosage
- Weight Loss Pill Contrave
- Contrave Generic
- Contrave and Alcohol
- Contrave vs Wellbutrin
- Contrave Contraindications
- Switching from Wellbutrin to Contrave
- Food to Avoid on Contrave
- Contrave Tablets
- FAQ
- References
Potential Health Benefits of Contrave
Contrave benefits include appetite suppression and reduced cravings by targeting the brain’s reward system, helping with long-term weight management. It combines bupropion and naltrexone to support weight loss when paired with diet and exercise.
- Promotes weight loss [2-22]
- Wards off depression [23-36]
- Reduces blood sugar levels [2, 37-38]
- Improves sexual function [39-47]
- Improves cholesterol levels [48-50]
- Treats cigarette addiction [51-57]
Key Takeaways
- Appetite Control – Contrave helps reduce hunger and food cravings by acting on the brain’s reward and hunger-regulating centers.
- Combination Therapy – It contains bupropion and naltrexone, which work together to support weight loss efforts.
- Prescription-Only – Contrave is available by prescription and is intended for individuals with obesity or overweight conditions with weight-related health risks.
- Lifestyle Support – It is most effective when used alongside a reduced-calorie diet and increased physical activity.
- Potential Side Effects – Common side effects include nausea, headache, dizziness, and insomnia, and it may not be suitable for individuals with certain medical conditions.
What is Contrave?
Contrave is an FDA-approved drug for adults with a body mass index (BMI) of 30 or higher who wants to lose weight. In some cases, doctors recommend this drug for patients with a BMI of 27 or higher who have one or more weight-related conditions or illnesses, such as hypertension, high cholesterol, or type 2 diabetes. [1] The active ingredients in contrave are bupropion, an antidepressant, and naltrexone, a drug used to combat opioid addiction.
How Contrave Works
The exact neurochemical mechanism by which contrave exerts its weight loss effects is by affecting the function of the hypothalamus, the hunger center of the brain. This, in turn, promotes satiety, reduces food intake, and boosts energy expenditure. As one of the notable weight loss products, the combination of bupropion and naltrexone also works on the mesolimbic pathway, the brain network that provides rewarding feelings. By regulating this pathway, food-seeking behaviors will be significantly reduced.
Chemical Structure of Contrave
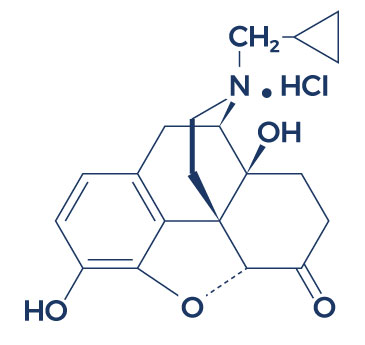
Research on Contrave
Promotes Weight Loss

Taking this medicine, Contrave, promotes weight loss by targeting the brain’s hunger and reward centers with a combination of bupropion and naltrexone. Bupropion helps reduce appetite and boost energy levels, while naltrexone curbs cravings, making it easier to maintain a calorie deficit and achieve long-term weight management. Taking this medicine consistently, as prescribed, can enhance its effectiveness, but it should be combined with a healthy diet and regular exercise for the best results.
- In overweight/obese individuals with type 2 diabetes, administration of 32 mg naltrexone sustained-release (SR)/360 mg bupropion SR (NB) resulted in significantly greater weight reduction. [2]
- In non-diabetic patients, three phase 3 trials of naltrexone SR plus bupropion SR (NB) demonstrated significant weight loss without any adverse side effects. [3-5]
- When combined with lifestyle intervention and modest calorie reduction, the naltrexone–bupropion combination appears to achieve clinically significant weight loss (over 5% of total body weight)after 6 months to 1 year of treatment. [6]
- A study found that contrave treatment was associated with 3 to 7% weight loss and improvements in obesity-related comorbidities and cardiovascular risk factors. [7]
- Patients who received 1 year of naltrexone ER/bupropion ER combination therapy with greater than 5% weight lost were likely to maintain clinically significant results. [8]
- In overweight and obese patients, naltrexone/bupropion (NB) combination therapy was associated with significantly greater weight loss. [9]
- In patients with uncomplicated obesity, combined bupropion and naltrexone therapy caused gradual sustained weight loss over 48 weeks. [10]
- In middle-aged white females, contrave treatment significantly reduced weight. [11-13]
- In obese mice, contrave significantly reduced weight by increasing the activity of POMC cells, which are peptides that play an integral role in weight regulation. [14]
- Direct injection of bupropion and naltrexone in mice produced a greater reduction in food intake. [15-16]
- In obese patients, contrave treatment reduced body fat and visceral adipose tissue. [17]
- A study found that contrave has a greater weight loss efficacy than other FDA-approved drugs for obesity, such as orlistat and lorcaserin. [18]
- Studies also found that contrave has favorable effects on obesity, medication-related weight gain, and binge eating behaviors. [19-22]
Wards Off Depression

Contrave, a combination of bupropion and naltrexone, may help ward off depression by influencing brain chemicals involved in mood regulation. Bupropion, an antidepressant, boosts dopamine and norepinephrine levels, while naltrexone may enhance endorphin activity, potentially improving mood and reducing emotional eating.
- An analysis of several studies found that there is a strong correlation between obesity and depression, suggesting that weight loss treatment with contrave can have positive effects on mood. [23-31]
- In overweight/obese women with major depressive disorder, contrave therapy for 24 weeks was associated with improvement in depressive symptoms. [32]
- A large pooled analysis of 5 clinical trials found that contrave was associated with lesser depressive symptoms and psychiatric adverse events compared to placebo. [33-34]
- In patients with major depression, treatment with contrave reduced binge eating, a common symptom of depression. [35]
- Administration of low-dose naltrexone in combination with bupropion in patients with major depressive disorder significantly reduced the incidence of relapse and recurrence. [36]
Reduces Blood Sugar Levels
Taking this medicine, Contrave, may help lower blood sugar levels by supporting weight loss, which in turn improves insulin sensitivity and glucose metabolism. The combination of bupropion and naltrexone also helps regulate appetite and energy balance, making it easier to manage blood sugar over time. By taking this medicine along with a healthy diet and regular exercise, individuals may see even greater improvements in overall metabolic health.
- In overweight and obese patients with type 2 diabetes, treatment with 32 mg naltrexone sustained-release (SR)/360 mg bupropion SR (NB) was associated with improvement in blood sugar control as evidenced by a greater reduction in hemoglobin A1c (HbA1c). [2]
- In diabetic patients, naltrexone/bupropion therapy decreased HbA1c approximately 0.5% more than placebo. [37]
- In overweight and obese patients, contrave also decreased major cardiovascular risk factors such as high blood sugar. [38]
Improves Sexual Function
Contrave (a combination of bupropion and naltrexone) may improve sexual function indirectly by promoting weight loss, enhancing mood, and increasing energy levels. Bupropion, one of its active ingredients, is known for its potential to boost libido and counteract sexual dysfunction often associated with obesity and depression.
- Studies suggest that obesity is linked with greater impairment in sexual function, suggesting that losing weight through contrave supplementation can have a positive impact on sexual health. [39-42]
- Bupropion, the component of contrave, has been reported to have pro‐sexual effects. [43-46]
- In obese patients, administration of contrave significantly improved sexual function as well as the quality of life. [47]
Improves Cholesterol Levels
Contrave may help improve cholesterol levels indirectly by promoting weight loss. As body weight decreases, levels of LDL (bad cholesterol) and triglycerides often decline, while HDL (good cholesterol) may increase, reducing the risk of cardiovascular issues.
- In obese patients, naltrexone/bupropion combination significantly reduced the levels of low-density lipoprotein (bad cholesterol) and increased the levels of high-density lipoprotein (good cholesterol). [48]
- In overweight and obese patients, daily treatment with contrave increased high-density lipoprotein cholesterol and decreased triglycerides. [49]
- In obese adults undergoing weight management, contrave administration also increased high-density lipoprotein cholesterol. [50]
Treats Cigarette Addiction
Contrave may help treat cigarette addiction due to its bupropion component, which is commonly used to aid smoking cessation. Bupropion works by influencing neurotransmitters like dopamine and norepinephrine, reducing cravings and withdrawal symptoms associated with nicotine dependence. Additionally, taking this medicine can help regulate the brain’s reward system, making smoking less satisfying over time. While Contrave is primarily used for weight loss, its effects on cravings and impulse control may provide additional benefits for those trying to quit smoking.
- A preliminary investigation of the combination of naltrexone and bupropion as a treatment for cigarette addiction showed increased smoking cessation rates. [51]
- Smokers who received contrave treatment for 7 weeks reported reduced nicotine withdrawal. [52]
- A study found that both naltrexone and bupropion can help improve adherence to smoking cessation interventions. [53]
- One clinical trial found that subjects treated with low-dose naltrexone combined with bupropion stopped cigarette smoking with lesser weight gain. [54]
- In overweight or obese smokers, combination therapy of naltrexone and bupropion was associated with decreased nicotine use, limited nicotine withdrawal symptoms, and no significant weight gain. [55]
Associated Side Effects of Contrave
Taking this medicine may cause some side effects, although they are very uncommon. There have been reports of patients experiencing certain symptoms while using Contrave, but these cases were not definitively linked to the medication and could have been coincidental. However, to ensure transparency, these potential side effects are still noted. It’s important to monitor any changes while on Contrave and consult a healthcare professional if any concerns arise.
Side effects associated with contrave may include the following:
- Constipation
- Diarrhea
- Dizziness
- Dry mouth
- Headache
- Insomnia
- Nausea
- Vomiting
Contrave Dosage
The recommended Contrave dosage follows a gradual titration schedule to minimize side effects. Patients typically start with one tablet in the morning for the first week. The dosage increases weekly until reaching the maintenance dose of two tablets taken twice daily, totaling four tablets per day.
It is important to take Contrave as prescribed and avoid crushing, chewing, or splitting the tablets. The medication should be taken with food but not high-fat meals, as this can increase the risk of side effects. If a dose is missed, patients should not double up but instead take the next scheduled dose as usual.
Healthcare providers may adjust the dosage based on an individual’s response and tolerance. Patients should regularly consult their doctor to monitor progress and address any concerns. If significant side effects occur, the provider may modify or discontinue the medication as needed.
Weight Loss Pill Contrave
Contrave is a prescription weight loss pill that combines two active ingredients, bupropion and naltrexone, to help manage weight in adults with obesity or overweight conditions. It works by targeting the brain’s hunger and reward centers, reducing appetite and food cravings. When combined with a reduced-calorie diet and regular exercise, Contrave can support long-term weight management.
One of the key benefits of Contrave is its dual-action approach, which helps individuals control both emotional eating and physical hunger. Bupropion, an antidepressant, can enhance metabolism and energy levels, while naltrexone, commonly used for addiction treatment, helps regulate cravings. This combination makes it particularly useful for those who struggle with compulsive eating habits.
However, like any medication, Contrave comes with potential side effects, including nausea, headache, dizziness, and an increased heart rate. It is not suitable for individuals with uncontrolled hypertension, seizure disorders, or a history of opioid use. Consulting a healthcare provider is essential to determine if Contrave is a safe and effective option for weight loss.
Contrave Generic
Contrave is a brand-name prescription medication used for weight management, but its generic equivalent, bupropion/naltrexone, offers the same active ingredients at a potentially lower cost. The combination of these two medications helps regulate appetite and cravings by targeting the brain’s reward and hunger centers. While the generic version may not always be available due to patent protections, it provides a more affordable alternative when offered.
The generic form, like Contrave, combines bupropion, an antidepressant that also aids in weight loss, and naltrexone, which reduces cravings and addictive behaviors. Together, they help individuals struggling with obesity or overweight conditions, especially when combined with a reduced-calorie diet and increased physical activity. Patients considering the generic should ensure it is FDA-approved to guarantee the same safety and efficacy as the brand-name drug.
Cost savings are a major advantage of using a generic version, making long-term treatment more accessible to patients. However, availability may vary depending on location and pharmacy stock, so checking with a healthcare provider or pharmacist is recommended. Whether using the brand-name Contrave or its generic, proper medical supervision is essential to monitor potential side effects and ensure safe, effective weight management.
Contrave and Alcohol
Contrave is a prescription weight-loss medication that combines bupropion and naltrexone to help regulate appetite and cravings. Since bupropion affects neurotransmitters in the brain, and naltrexone is commonly used to treat alcohol dependence, combining Contrave with alcohol can pose risks. Drinking while taking Contrave may increase the likelihood of side effects such as dizziness, drowsiness, nausea, and mood changes.
Alcohol can also interfere with Contrave’s effectiveness by counteracting its appetite-suppressing effects and worsening side effects like increased blood pressure or seizures. Bupropion, one of Contrave’s active ingredients, is known to lower the seizure threshold, and alcohol withdrawal or binge drinking can further heighten this risk. Those with a history of alcohol use disorder should be particularly cautious, as naltrexone in Contrave may alter alcohol’s effects, potentially reducing tolerance and increasing the chance of intoxication.
Due to these concerns, healthcare providers often recommend limiting or avoiding alcohol while taking Contrave. If you choose to drink, it’s best to do so in moderation and monitor your body’s response carefully. Always consult with a doctor before mixing alcohol with Contrave, as individual risk factors such as medical history and current health conditions can influence safety.
Contrave vs Wellbutrin
Contrave and Wellbutrin are both medications that affect brain chemistry, but they serve different primary purposes. Contrave is a weight loss medication that combines bupropion (the active ingredient in Wellbutrin) with naltrexone to help control appetite and food cravings. Wellbutrin, on the other hand, is primarily prescribed for depression and smoking cessation, as it influences dopamine and norepinephrine levels to improve mood and motivation.
While both medications contain bupropion, their effects and uses differ significantly. Contrave’s combination with naltrexone helps regulate the brain’s reward system to reduce overeating, making it effective for weight management. In contrast, Wellbutrin is used to treat major depressive disorder (MDD) and seasonal affective disorder (SAD), with some off-label use for ADHD. Despite their different purposes, both medications can have similar side effects, including insomnia, dry mouth, and increased heart rate.
Choosing between Contrave and Wellbutrin depends on individual health goals and medical history. If weight loss is the primary concern, Contrave may be a better option due to its appetite-suppressing effects. However, if treating depression or quitting smoking is the main goal, Wellbutrin is more appropriate. Consulting a healthcare provider is essential to determine the best choice based on personal needs and potential risks.
Contrave Contraindications
Contrave contraindications include a history of seizures, as the medication lowers the seizure threshold, increasing the risk of convulsions. Individuals with eating disorders such as bulimia or anorexia nervosa should also avoid Contrave due to the heightened likelihood of seizures associated with bupropion, one of its active ingredients. Additionally, those undergoing abrupt discontinuation of alcohol, benzodiazepines, barbiturates, or antiepileptic drugs are at a higher risk of seizures and should not use Contrave.
Another key contraindication is uncontrolled hypertension, as Contrave can elevate blood pressure and heart rate. Individuals with a history of cardiovascular disease, such as recent heart attack or stroke, should also avoid this medication due to potential cardiovascular risks. Furthermore, Contrave is not recommended for those with severe liver or kidney impairment, as these conditions can alter the metabolism of the drug, leading to increased side effects.
Contrave should not be used by individuals taking monoamine oxidase inhibitors (MAOIs) or those who have used them within the past 14 days, as this combination can lead to dangerous increases in blood pressure. Additionally, people with a known allergy to bupropion or naltrexone, the active ingredients in Contrave, should not take this medication due to the risk of severe allergic reactions. Pregnant women should also avoid Contrave, as weight loss medications are not recommended during pregnancy and may pose risks to fetal development.
Switching from Wellbutrin to Contrave
Switching from Wellbutrin to Contrave requires careful consideration, as both medications contain bupropion, but Contrave also includes naltrexone. Since bupropion affects dopamine and norepinephrine levels, while naltrexone influences the brain’s reward system, the transition should be managed under medical supervision to minimize side effects and ensure proper dosing. Gradually adjusting Wellbutrin while introducing Contrave can help reduce the risk of overstimulation, insomnia, or nausea.
Patients switching to Contrave may notice changes in appetite, weight, and mood, as the medication is specifically designed for weight management. While Wellbutrin can sometimes lead to weight loss as a side effect, Contrave is formulated to enhance appetite control and reduce food cravings. However, side effects such as nausea, dizziness, or an increased heart rate may occur, especially during the initial adjustment period.
To ensure a smooth transition, healthcare providers typically tailor the dosing schedule based on individual response and tolerance. It’s important to monitor any changes in mood, energy levels, or potential side effects, as the combination of bupropion and naltrexone can affect each person differently. Open communication with a doctor is key to managing the switch effectively and achieving the desired health outcomes.
Food to Avoid on Contrave
When taking Contrave, it’s important to avoid high-fat meals, as they can increase the absorption of the medication and raise the risk of side effects like nausea and dizziness. Fatty foods such as fried items, creamy sauces, and fast food can also contribute to weight gain, which contradicts the purpose of using Contrave for weight management. Instead, opting for lean proteins, whole grains, and vegetables can support your weight loss goals while minimizing adverse effects.
Sugary and processed foods should also be limited while on Contrave. Excess sugar, found in sweets, sodas, and baked goods, can lead to blood sugar spikes and increased cravings, making weight loss more challenging. Highly processed foods, like chips and packaged snacks, often contain unhealthy fats and additives that can interfere with the appetite-regulating effects of the medication. Choosing nutrient-dense, whole foods can help maintain stable energy levels and support a healthier metabolism.
Alcohol is another substance to avoid while taking Contrave. Drinking alcohol can increase the risk of serious side effects, including dizziness, drowsiness, and mood changes. Since Contrave affects the brain’s reward system, alcohol consumption may worsen these effects or reduce the medication’s ability to control cravings. Staying hydrated with water, herbal teas, or other non-sugary beverages is a safer choice for overall well-being.
Contrave Tablets
Contrave tablets are a prescription medication used for weight management in adults with obesity or overweight individuals who have weight-related health conditions. They contain a combination of naltrexone and bupropion, which work together to reduce appetite and control cravings. Contrave is most effective when combined with a reduced-calorie diet and increased physical activity.
The active ingredients in Contrave target brain pathways involved in hunger and reward, helping to regulate eating behaviors. Bupropion, an antidepressant, influences dopamine and norepinephrine levels, which may help with appetite suppression, while naltrexone, typically used for addiction treatment, affects opioid receptors to reduce cravings. This dual-action mechanism makes Contrave a unique option for individuals struggling with weight loss.
Although Contrave can be effective, it may cause side effects such as nausea, constipation, headache, or dizziness. It is not suitable for individuals with uncontrolled high blood pressure, seizure disorders, or those taking opioid medications. Patients should consult their healthcare provider to determine if Contrave is the right choice for their weight loss goals.
FAQ
What does Contrave do for weight loss?
Contrave helps with weight loss by reducing appetite and controlling cravings through its combination of naltrexone and bupropion, which target brain pathways involved in hunger and reward. However, some individuals may experience an allergic reaction to its ingredients. It is important to be aware of potential side effects, including an allergic reaction, which may require immediate medical attention. If you develop symptoms of an allergic reaction, such as rash, swelling, or difficulty breathing, seek medical help promptly.
Is Contrave as effective as Ozempic?
Ozempic (semaglutide) is generally more effective for weight loss than Contrave, as it directly affects insulin regulation and appetite suppression, leading to greater weight reduction in clinical studies. However, some individuals may experience serious skin reactions, which should be monitored and reported to a healthcare provider if they occur.
Is Contrave the same as Wellbutrin?
No, Contrave is not the same as Wellbutrin. However, it contains bupropion, the active ingredient in Wellbutrin, along with naltrexone to enhance weight loss effects. If you have a missed dose, take it as soon as you remember unless it is close to your next scheduled dose. Avoid doubling up on doses to make up for a missed dose to prevent potential side effects.
How much weight can you lose on Contrave?
No, Contrave is not the same as Wellbutrin. However, it contains bupropion, the active ingredient in Wellbutrin, along with naltrexone to enhance weight loss effects. If you have a missed dose, take it as soon as you remember unless it is close to your next scheduled dose. Avoid doubling up on doses to make up for a missed dose to prevent potential side effects.
Which is better, Contrave or Ozempic?
Ozempic is generally more effective for weight loss, but Contrave may be a better option for those who cannot take GLP-1 receptor agonists or prefer an alternative approach. However, individuals with bipolar disorder should consult their healthcare provider before using Contrave, as it contains bupropion, which may affect mood stability. Additionally, those with a history of bipolar disorder need careful monitoring to prevent potential mood-related side effects.
Does Contrave really work for weight loss?
Yes, Contrave has been shown to help people lose weight, especially when combined with a healthy diet and regular exercise. However, results vary by individual, and some may experience low blood sugar as a side effect. It is important to monitor for symptoms of low blood sugar, such as dizziness, shakiness, or sweating, especially for those with diabetes or on medications that affect blood glucose levels.
What does the drug Contrave do?
Contrave is a prescription weight-loss medication that works by reducing appetite and food cravings through its combination of naltrexone and bupropion. However, some individuals may experience side effects, including a skin rash. If you notice a skin rash or other allergic reactions while taking Contrave, consult your healthcare provider promptly.
Is Contrave the same as Ozempic?
No, Contrave and Ozempic are different medications. Contrave contains naltrexone and bupropion, while Ozempic contains semaglutide, which is a GLP-1 receptor agonist. It is important to note that both medications may cause side effects, including the risk of a serious allergic reaction. If you experience symptoms of a serious allergic reaction, such as swelling, difficulty breathing, or a severe rash, seek medical attention immediately.
What is the average weight loss with Contrave?
The average weight loss with Contrave is around 5%–10% of body weight over 6–12 months, depending on diet, exercise, and adherence to the medication. It is important to discuss with your doctor how Contrave may interact with other medicines you are taking. Some other medicines could affect its effectiveness or increase the risk of side effects.
Is Contrave or Ozempic better?
Ozempic is typically more effective for weight loss, but Contrave may be a suitable alternative for individuals who cannot use GLP-1 receptor agonists. It is important to discuss with your healthcare provider how Contrave interacts with other medicines you may be taking. Additionally, some other medicines could affect the effectiveness or safety of both Ozempic and Contrave.
How much does the diet pill Contrave cost?
Contrave costs around $99 per month with a savings program, but without insurance or discounts, it can range from $200–$400 per month. It is important to consider potential drug interactions when taking Contrave, as certain medications may affect its effectiveness or increase the risk of side effects. Always consult your doctor about any drug interactions before starting treatment.
How quickly do you lose weight on Contrave?
Some people notice weight loss within the first few weeks, but significant results typically appear after 3–6 months of consistent use. However, some individuals may experience skin reactions as a side effect. If you notice any unusual skin reactions, such as rash or irritation, consult a healthcare professional.
Is Contrave better than Ozempic for weight loss?
No, Ozempic is generally more effective for weight loss than Contrave due to its stronger appetite-suppressing and metabolic effects. However, some individuals may experience chest pain as a side effect, which should not be ignored. If you develop chest pain while using Ozempic, seek medical attention immediately.
Do you gain the weight back after Contrave?
Weight regain is possible after stopping Contrave if lifestyle changes are not maintained, as with most weight-loss medications. Additionally, some individuals may experience suicidal thoughts, especially when first starting the medication or adjusting the dose. It is important to monitor for suicidal thoughts and seek medical attention if any concerning symptoms arise.
Is there a generic for Contrave?
No, there is currently no generic version of Contrave available. If an overdose occurs, contact a poison control center immediately for guidance. In case of severe reactions, seeking help from a poison control center can be crucial for proper medical intervention.
Is there a cheaper alternative to Contrave?
A potential alternative is taking generic naltrexone and bupropion separately, but this should only be done under a doctor’s supervision. Individuals with kidney disease should exercise caution and consult their healthcare provider before starting these medications. Since kidney disease can affect how drugs are processed in the body, dosage adjustments may be necessary.
What is the cheapest way to get Contrave?
The cheapest way is to use the Contrave Savings Program, which offers the medication for around $99 per month. Some online telehealth services also offer discounts, but Contrave is not available over the counter. If you’re looking for weight loss options over the counter, consider discussing alternatives with your healthcare provider.
What are three common side effects?
Common side effects include nausea, constipation, and headache. Although Contrave is not a narcotic medicine, it contains bupropion, which affects brain chemistry. Unlike a narcotic medicine, it does not carry the same risk of dependency but should still be used under medical supervision.
What to avoid while on Contrave?
Avoid high-fat meals when taking Contrave, as they can increase the risk of side effects, including trouble sleeping. Also, avoid alcohol and opioid medications, as they may worsen side effects or lead to complications. If you experience trouble sleeping, consider taking Contrave earlier in the day or consulting your healthcare provider for guidance.
How much alcohol can I drink on Contrave?
It is best to avoid alcohol while taking Contrave, as it can increase the risk of seizures and other side effects. Additionally, it is not known whether Contrave passes into breast milk, so nursing mothers should consult their doctor before using it. If you are breastfeeding, discuss the potential risks of breast milk exposure to the medication with your healthcare provider.
Can you drink alcohol while taking bupropion?
Drinking alcohol while taking bupropion is not recommended, as it can increase the risk of seizures and mood disturbances. Always read the prescription label carefully for warnings related to alcohol consumption. If you have any concerns, consult your healthcare provider and follow the instructions on the prescription label to ensure safe use of the medication.
Is Contrave hard on the liver?
Contrave can affect liver function, particularly in people with preexisting liver disease, so liver function should be monitored while taking it. Some individuals may experience flu-like symptoms, such as fatigue and body aches, as a side effect. If you notice persistent flu-like symptoms, consult your healthcare provider to rule out any serious complications.
Are Contrave and Wellbutrin the same thing?
No, but Contrave contains bupropion, which is the active ingredient in Wellbutrin. Wellbutrin is used primarily for depression and smoking cessation, while Contrave is for weight loss. In some cases, dark urine may be a sign of a serious side effect and should be reported to a healthcare provider. If you notice dark urine along with other symptoms like yellowing of the skin or eyes, seek medical attention promptly.
What is better than Contrave for weight loss?
Ozempic, Wegovy, and other GLP-1 receptor agonists are often more effective for weight loss than Contrave. However, some users may experience unusual tiredness as a side effect. If you notice persistent unusual tiredness, it is advisable to consult your healthcare provider.
How much Wellbutrin is in one Contrave pill?
Each Contrave tablet contains 90 mg of bupropion, which is lower than standard Wellbutrin dosages. If you have a history of a head injury, consult your doctor before using Contrave, as bupropion may increase the risk of seizures. Additionally, individuals recovering from a head injury should be cautious when taking medications that affect the central nervous system.
Has anyone lost weight on Wellbutrin?
Yes, some people experience weight loss on Wellbutrin due to its appetite-suppressing effects, though it is not specifically approved for weight loss.
What medications should not be taken with Contrave?
Avoid taking opioid medications, MAO inhibitors, seizure medications, and other drugs that interact with bupropion or naltrexone. If you miss a dose, take it as soon as you remember, unless it is close to your next scheduled dose. Do not double up on doses if you miss a dose, as this may increase the risk of side effects.
Who shouldn't use Contrave?
People with uncontrolled high blood pressure, seizure disorders, opioid use, or a history of eating disorders should not take Contrave. This medication comes in extended release tablets, which affect how the drug is absorbed in the body. It is important to take extended release tablets as prescribed to ensure safe and effective treatment.
What is the black box warning for Contrave?
Contrave carries a black box warning for an increased risk of suicidal thoughts and behaviors due to its bupropion component. It may also interact with certain medicines, potentially increasing the risk of side effects. Before starting Contrave, inform your doctor if you are taking certain medicines that could affect its safety and effectiveness.
What condition is this major contraindication for bupropion use?
Seizure disorders are a major contraindication for bupropion use, as it can lower the seizure threshold. Additionally, certain medicines may interact with bupropion, increasing the risk of seizures. It is important to consult a healthcare provider before taking bupropion, especially if you are using certain medicines that affect the central nervous system.
What happens if you take Contrave without food?
Taking Contrave without food is recommended, as high-fat meals can increase the risk of side effects. Additionally, consuming alcoholic beverages while taking Contrave may heighten the risk of adverse reactions. It is advisable to limit or avoid alcoholic beverages to ensure the medication’s effectiveness and safety.
How do I get the best results from Contrave?
For the best results, take Contrave as prescribed, follow a reduced-calorie diet, exercise regularly, and avoid high-fat meals. Contrave works to decrease appetite, helping individuals manage their food intake more effectively. By targeting brain pathways, it helps decrease appetite and control cravings for improved weight loss results.
Should you take Contrave before or after eating?
Contrave should be taken in the morning and evening, but not with high-fat meals to avoid increased side effects. Some users may experience blurred vision as a side effect, which can affect daily activities. If blurred vision persists or worsens, consult a healthcare professional promptly.
What not to eat while taking Contrave?
Avoid high-fat foods, as they can increase drug absorption and raise the risk of side effects like nausea and eye pain. If you experience symptoms such as severe eye pain, vision changes, or discomfort, consult a healthcare professional promptly.
Does Contrave cause hair thinning?
Hair thinning is not a commonly reported side effect of Contrave, but some individuals may experience hair loss as a rare reaction to bupropion, one of its active ingredients. Additionally, bupropion may contribute to physical dependence in some users. It is important to consult a healthcare provider if concerns about physical dependence arise while taking Contrave.
What is the most common side effect of Contrave?
The most common side effect of Contrave is nausea, especially when first starting the medication. It is important to consult a doctor before using Contrave during pregnancy, as it may affect an unborn baby. Research on its safety for an unborn baby is limited, so medical guidance is essential.
Does bupropion cause hair loss?
Bupropion has been associated with hair loss in some individuals, though it is considered an uncommon side effect. It is also important to store Contrave properly to maintain its effectiveness. Be sure to store Contrave according to the manufacturer’s guidelines to prevent degradation.
Is Contrave better than Wellbutrin?
Contrave and Wellbutrin serve different purposes; Contrave is used for weight loss, while Wellbutrin is primarily prescribed for depression and smoking cessation. Contrave contains bupropion (the active ingredient in Wellbutrin) along with naltrexone, making it more effective for weight loss than Wellbutrin alone.
Can you take Contrave while on Wellbutrin?
No, taking Contrave with Wellbutrin is not recommended because both contain bupropion, increasing the risk of side effects such as seizures. Contrave acts as an appetite suppressant, helping with weight loss by reducing cravings. Combining multiple medications that affect neurotransmitters involved in appetite suppressant mechanisms can heighten the risk of adverse effects.
How many mg of Wellbutrin is in Contrave?
Contrave contains 90 mg of bupropion per tablet, with a maximum daily dose of 360 mg (four tablets). If a dose is missed, it is generally recommended to take the next dose at the scheduled time rather than doubling up. Always follow your healthcare provider’s instructions on when to take the next dose to ensure safe and effective use.
How long does it take your body to get used to Contrave?
Most people adjust to Contrave within a few weeks as their body adapts to the medication and side effects become less noticeable. It is important to read the medication guide provided with your prescription to understand potential risks and usage instructions. Always follow the medication guide and consult your doctor if you have concerns about side effects.
How does Contrave make you lose weight?
Contrave affects the brain’s hunger and reward centers, reducing appetite and cravings, which helps control food intake and promotes weight loss. The medication is a combination of two drugs, naltrexone and bupropion, which work together to influence these brain pathways. By using two drugs with complementary effects, Contrave provides a unique approach to weight management.
How to make Contrave more effective?
To maximize the effectiveness of Contrave, combine it with a healthy diet, regular exercise, and behavioral changes such as mindful eating and portion control. However, be aware that in rare cases, Contrave may cause life-threatening side effects. If you experience severe reactions, seek immediate medical attention, as some complications can be life-threatening.
How long does it take for Contrave to work?
Some young adults may start noticing reduced appetite and cravings within the first few weeks, but significant weight loss results typically occur after 12 weeks of use. In clinical studies, young adults and other age groups experienced varying degrees of weight loss depending on factors like adherence and lifestyle changes.
What is the average weight loss on Contrave?
Clinical studies show that people taking Contrave can lose an average of 5% to 10% of their body weight over six months to a year when combined with diet and exercise. However, some individuals may experience side effects, including irregular heartbeat, which should be monitored closely. If you notice symptoms such as dizziness or irregular heartbeat, consult your healthcare provider immediately.
Who should not take Contrave?
Contrave is not recommended for individuals with uncontrolled high blood pressure, seizure disorders, a history of eating disorders, opioid use, or those who are pregnant or breastfeeding.
Is Contrave the same as Ozempic?
No, Contrave and Ozempic are different medications. Contrave is a combination of bupropion and naltrexone, while Ozempic is a GLP-1 receptor agonist (semaglutide) used for diabetes and weight loss.
What does Contrave do to your body?
Contrave influences brain chemicals that regulate appetite and cravings, helping individuals eat less and lose weight over time.
What foods should you not eat while taking Contrave?
It’s best to avoid high-fat meals, as they can increase the absorption of Contrave and the risk of side effects like nausea.
Why can't you eat eggs on Contrave?
There is no strict restriction on eggs, but some people report nausea when consuming high-fat foods, including eggs, especially if cooked in oil or butter.
How to maximize weight loss on Contrave?
For the best results, follow a structured meal plan, exercise regularly, stay hydrated, and track your progress to maintain motivation and consistency.
Can I eat pizza while taking Contrave?
Yes, but it’s best to choose a healthier version with whole-grain crust, lean protein, and vegetables while avoiding excessive cheese and processed meats.
What does Contrave tablets do?
Contrave tablets help with weight loss by reducing appetite and controlling food cravings through their effects on brain neurotransmitters.
How fast do you lose weight on Contrave?
Weight loss varies, but some people start noticing changes within a few weeks, with more significant results seen over several months.
How long does it take Contrave to start working?
Contrave starts affecting appetite and cravings within the first few weeks, but noticeable weight loss usually takes a few months.
Should I take Contrave on an Empty Stomach?
Contrave should not be taken on an empty stomach. The medication contains naltrexone and bupropion, both of which can cause nausea if taken without food. To minimize stomach discomfort and improve absorption, it is recommended to take Contrave with a meal, preferably one that contains some fat.
Does contrave cause hair loss?
Contrave is a prescription weight-loss medication that combines bupropion and naltrexone. While hair loss is not a commonly reported side effect, some users have experienced it. This could be due to the effects of weight loss itself, changes in metabolism, or individual reactions to the drug’s active ingredients.
Reference
Contrave–a combination of bupropion and naltrexone for weight loss. Med Lett Drugs Ther. 2014;56(1455):112-4.
Hollander P, Gupta AK, Plodkowski R, et al. Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes [published correction appears in Diabetes Care. 2014 Feb;37(2):587]. Diabetes Care. 2013;36(12):4022–4029. doi:10.2337/dc13-0234.
Effects of naltrexone sustained-release/bupropion sustained-release combination therapy on body weight and glycemic parameters in overweight and obese patients with type 2 diabetes
A 56-week, placebo-controlled study found that naltrexone/bupropion (NB) significantly reduced weight and improved glycemic control in overweight/obese individuals with type 2 diabetes, with more patients achieving ≥5% weight loss and HbA1c <7% compared to placebo, though NB was associated with more gastrointestinal side effects.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3836105/.
Apovian CM, Aronne LJ, Rubino DM, et al. COR-II Study Group A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring) 2013;21:935–943.
A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II)
The COR-II study found that naltrexone/bupropion (NB32) led to significantly greater weight loss, improved cardiometabolic markers, and enhanced quality of life in overweight and obese individuals compared to placebo, with mild nausea as the most common side effect, supporting NB as a promising obesity treatment.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3739931/.Greenway FL, Fujioka K, Plodkowski RA, et al. COR-I Study Group Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2010;376:595–605.
Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial
The COR-I study found that a combination of sustained-release naltrexone and bupropion significantly reduced bodyweight in obese individuals over 56 weeks, with 48% achieving at least a 5% weight loss, while nausea was the most common side effect.
You can read the abstract of the article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(10)60888-4/fulltext.Wadden TA, Foreyt JP, Foster GD, et al. Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial. Obesity (Silver Spring) 2011;19:110–120.
Weight loss with naltrexone SR/bupropion SR combination therapy as an adjunct to behavior modification: the COR-BMOD trial
A 56-week trial with 793 participants (mean BMI 36.5) found that naltrexone SR (32 mg/day) plus bupropion SR (360 mg/day) combined with intensive behavior modification (BMOD) led to significantly greater weight loss and improved cardiometabolic markers compared to placebo with BMOD, though nausea was more common; overall, the combination was well-tolerated and effective for obesity management.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4459776/.Tek C. Naltrexone HCI/bupropion HCI for chronic weight management in obese adults: patient selection and perspectives. Patient Prefer Adherence. 2016;10:751–759. Published 2016 May 4. doi:10.2147/PPA.S84778.
Naltrexone HCI/bupropion HCI for chronic weight management in obese adults: patient selection and perspectives
A sustained-release combination of naltrexone and bupropion has been approved for obesity treatment, as it effectively enhances weight loss by modulating food reward systems, especially when combined with lifestyle changes. While bupropion alone promotes weight loss, naltrexone’s impact is limited. This review examines clinical trials, patient selection, and practical applications for optimizing outcomes.You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4862388/.
Padwal R. Contrave, a bupropion and naltrexone combination therapy for the potential treatment of obesity. CurrOpinInvestig Drugs. 2009;10(10):1117-25.
Contrave, a bupropion and naltrexone combination therapy for the potential treatment of obesity
Contrave, a combination of bupropion and naltrexone, targets obesity by increasing energy expenditure and reducing appetite. Phase III trials showed 3–7% weight loss and health improvements, though nausea was a common side effect. If successful, it could become a leading anti-obesity treatment.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/19777400/.
ujioka K, Plodkowski R, O’neil PM, Gilder K, Walsh B, Greenway FL. The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy. Int J Obes (Lond). 2016;40(9):1369-75.
The relationship between early weight loss and weight loss at 1 year with naltrexone ER/bupropion ER combination therapy
Early weight loss with naltrexone/bupropion (≥5% by Week 16) predicts sustained weight reduction at Week 56, with 85% maintaining significant loss, though further research is needed on cardiovascular and metabolic benefits.
You can read the full article at https://www.nature.com/articles/ijo201667.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II). Obesity (Silver Spring). 2013;21(5):935–943. doi:10.1002/oby.20309.
A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II)
The COR-II study found that naltrexone/bupropion (NB32) significantly improved weight loss, cardiometabolic risk markers, and eating control in overweight and obese participants, with mild to moderate nausea as the main side effect and no increased risk of depression or suicidality.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3739931/.
Greenway FL, Dunayevich E, Tollefson G, et al. Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo. J ClinEndocrinolMetab. 2009;94(12):4898-906.
Comparison of combined bupropion and naltrexone therapy for obesity with monotherapy and placebo
A randomized, double-blind trial with 419 obese patients found that combining naltrexone and bupropion led to significant weight loss compared to placebo or monotherapy, with N eks, with mild transient nausea as the most common side effect, warranting further long-term studies.
You can read the full article at https://academic.oup.com/jcem/article/94/12/4898/2596850?login=false.Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142:532–546.
Meta-analysis: pharmacologic treatment of obesity
This study assessed the efficacy and safety of FDA-approved and commonly used weight loss medications, finding modest benefits for drugs like sibutramine, phentermine, orlistat, and others, though long-term health data were limited and side effects varied.
You can read the full article at https://www.acpjournals.org/doi/full/10.7326/0003-4819-142-7-200504050-00012?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Rucker D, Padwal R, Li SK, Curioni C, Lau DC. Long term pharmacotherapy for obesity and overweight: updated meta-analysis. BMJ. 2007;335:1194–1199.
Long term pharmacotherapy for obesity and overweight: updated meta-analysis
This meta-analysis of 30 trials (1–4 years) assessed the long-term efficacy of orlistat, sibutramine, and rimonabant in weight loss and health outcomes. Compared to placebo, weight reductions were 2.9 kg (orlistat), 4.2 kg (sibutramine), and 4.7 kg (rimonabant), with varying effects on cardiovascular risk factors. Orlistat improved cholesterol, blood pressure, and diabetes risk but caused gastrointestinal issues, sibutramine improved lipids, and rimonabant enhanced cardiovascular markers but increased mood disorder risk.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2128668/.Health I. Plymouth, PA: The National Disease and Therapeutic Index; pp. 1998–2003.
Greenway FL, Whitehouse MJ, Guttadauria M, et al. Rational design of a combination medication for the treatment of obesity. Obesity (Silver Spring). 2009;17(1):30-9.
Rational design of a combination medication for the treatment of obesity
This study found that combining bupropion and naltrexone stimulated POMC neurons in mice, reducing food intake and leading to sustained weight loss in obese adults without a plateau effect, offering a promising alternative to monotherapie
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1038/oby.2008.461.
Available from https://www.scienceopen.com/document?vid=675a2d4d-d825-45be-bf9c-716f23852839.
Wright FL, Rodgers RJ. Acute behavioural effects of bupropion and naltrexone, alone and in combination, in non-deprived male rats presented with palatable mash. Psychopharmacology (Berl). 2013;228(2):291-307.
Acute behavioural effects of bupropion and naltrexone, alone and in combination, in non-deprived male rats presented with palatable mash
This study examined the behavioral effects of bupropion, naltrexone, and their combination on appetite in rats, finding that bupropion disrupted satiety and increased activity, naltrexone accelerated satiety without affecting movement, and their low-dose combination enhanced appetite suppression while reducing bupropion’s side effects.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s00213-013-3036-6.
Smith SR, Fujioka K, Gupta AK, et al. Combination therapy with naltrexone and bupropion for obesity reduces total and visceral adiposity. Diabetes ObesMetab. 2013;15(9):863-6.
Combination therapy with naltrexone and bupropion for obesity reduces total and visceral adiposity
Naltrexone/bupropion therapy significantly reduced body fat and visceral adipose tissue in obese subjects, outperforming placebo and monotherapies, with changes proportional to weight loss and no excessive lean mass reduction.
You can read the abstract of the article at https://dom-pubs.onlinelibrary.wiley.com/doi/10.1111/dom.12095.Verpeut JL, Bello NT. Drug safety evaluation of naltrexone/bupropion for the treatment of obesity. Expert Opin Drug Saf. 2014;13(6):831-41.
Drug safety evaluation of naltrexone/bupropion for the treatment of obesity
This review examines the combination of naltrexone and bupropion as an anti-obesity therapy, highlighting its promising weight loss effects and favorable metabolic changes based on four Phase III trials. Compared to other FDA-approved options, it offers good efficacy with a relatively mild side effect profile and no abuse potential.
You can read the abstract of the article at https://www.tandfonline.com/doi/abs/10.1517/14740338.2014.909405.Howland RH. Melatonin, Liraglutide, and Naltrexone/Bupropion for the Treatment of Obesity and Medication-Related Weight Gain. J PsychosocNursMent Health Serv. 2015;53(6):19-22.
Melatonin, Liraglutide, and Naltrexone/Bupropion for the Treatment of Obesity and Medication-Related Weight Gain
Melatonin, liraglutide, and naltrexone/bupropion offer potential benefits for managing obesity in psychiatric patients, though bupropion may pose risks for bipolar disorder or schizophrenia; further research is needed.
You can read the abstract of the article at https://journals.healio.com/doi/10.3928/02793695-20150526-02?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Padwal R. Contrave, a bupropion and naltrexone combination therapy for the potential treatment of obesity. CurrOpinInvestig Drugs. 2009;10(10):1117-1125.
Contrave, a bupropion and naltrexone combination therapy for the potential treatment of obesity
Contrave, developed by Orexigen Therapeutics for obesity treatment, combines bupropion and naltrexone to reduce appetite and increase energy expenditure. In phase III trials, it showed 3–7% placebo-subtracted weight loss and health benefits, though nausea was a common side effect. Pending successful trials, it could become a leading anti-obesity therapy.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/19777400/,
Caixàs A, Albert L, Capel I, Rigla M. Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date. Drug Des Devel Ther. 2014;8:1419-1427. Published 2014 Sep 18. doi:10.2147/DDDT.S55587.
Naltrexone sustained-release/bupropion sustained-release for the management of obesity: review of the data to date
Obesity is a global health issue linked to lifestyle changes and chronic disease risks, prompting research into treatments like Contrave® (naltrexone SR/bupropion SR), which achieves around 4.5% placebo-subtracted weight loss with a generally acceptable safety profile, though cardiovascular effects need further study.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4174046/.
-
An open-label trial on the efficacy and tolerability of naltrexone/bupropion SR for treating altered eating behaviours and weight loss in binge eating disorder
The study found that a 16-week treatment with naltrexone-bupropion (NB) alongside lifestyle changes led to significant weight loss (~8% BMI) and improved pathological eating behaviors, especially in obese patients with binge eating disorder (BED), with good tolerability and a low dropout rate.
You can read the full article at https://www.researchgate.net/publication/341060191_An_open-label_trial_on_the_efficacy_and_tolerability_of_naltrexonebupropion_SR_for_treating_altered_eating_behaviours_and_weight_loss_in_binge_eating_disorder.
de Wit L, Luppino F, van Straten A, et al. Depression and obesity: a meta-analysis of community-based studies. Psychiatry Res. 2010;178(2):230–235.
Depression and obesity: a meta-analysis of community-based studies
A meta-analysis of 17 studies (204,507 participants) found a strong overall association between depression and obesity, with a notable gender difference—significant in females but not in males—highlighting the need for further research on underlying factors and causality.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S016517810900170X?via%3Dihub.
Luppino FS, de Wit LM, Bouvy PF, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67(3):220–229.
Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies
This meta-analysis of 15 studies (58,745 participants) found that obesity and overweight increase the risk of developing depression, especially among Americans and adults, while depression raises the likelihood of obesity but not overweight, highlighting their complex reciprocal relationship.
You can read the full article at https://jamanetwork.com/journals/jamapsychiatry/fullarticle/210608.Blaine B. Does depression cause obesity? a meta-analysis of longitudinal studies of depression and weight control. J Health Psychol. 2008;13(8):1190–1197.
Does depression cause obesity? a meta-analysis of longitudinal studies of depression and weight control
A meta-analysis of 16 studies found that depression significantly increases the risk of developing obesity, especially in adolescent females (OR = 2.57), highlighting the need for early screening and intervention to prevent adult obesity.
You can read the abstract of the article at https://journals.sagepub.com/doi/10.1177/1359105308095977?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Khan A, Schwartz KA, Kolts RL, et al. BMI, sex, and antidepressant response. J Affect Disord. 2007;99(1–3):101–106.
BMI, sex, and antidepressant response
Obese men with depression showed the least benefit from SSRIs compared to placebo, suggesting potential limitations in SSRI effectiveness for this group and highlighting the need for further research on treatment strategies.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0165032706003612?via%3Dihub.
Kloiber S, Ising M, Reppermund S, et al. Overweight and obesity affect treatment response in major depression. Biol Psychiatry. 2007;62(4):321–326.
Overweight and obesity affect treatment response in major depression
Overweight and obese MDD patients show slower clinical response, poorer neuroendocrine and cognitive improvements, and less weight gain during treatment, suggesting the need for tailored interventions.
You can read the abstract of the article at https://www.biologicalpsychiatryjournal.com/article/S0006-3223(06)01240-6/fulltext.
Papakostas GI, Petersen T, Iosifescu DV, et al. Obesity among outpatients with major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):59–63.
Obesity among outpatients with major depressive disorder
The study found that over half of MDD outpatients were overweight, with 20% being obese, and while obesity was linked to poorer somatic well-being, it did not affect depression severity or anxiety. However, higher body weight, rather than obesity itself, was associated with a greater risk of non-response to fluoxetine, suggesting weight may influence treatment resistance.
You can read the abstract of the article at https://academic.oup.com/ijnp/article/8/1/59/698700?login=false.
Uher R, Mors O, Hauser J, et al. Body weight as a predictor of antidepressant efficacy in the GENDEP project. J Affect Disord. 2009;118(1–3):147–154.
Body weight as a predictor of antidepressant efficacy in the GENDEP project
Higher BMI and obesity were linked to a poorer response to nortriptyline, especially in obese men, while obese women showed reduced response to both nortriptyline and escitalopram. Body weight influenced improvements in sleep and appetite, highlighting its importance in antidepressant selection.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0165032709000834?via%3Dihub.
Oskooilar N, Wilcox CS, Tong ML, et al. Body mass index and response to antidepressants in depressed research subjects. J Clin Psychiatry. 2009;70(11):1609–1610.
Murphy JM, Horton NJ, Burke JD, Jr, et al. Obesity and weight gain in relation to depression: findings from the Stirling County Study. Int J Obes (Lond) 2009;33(3):335–341.
Obesity and weight gain in relation to depression: findings from the Stirling County Study
Obese individuals in the community do not have a direct link to depression but are more likely to experience severe depressive episodes, marked by overeating, weight gain, longer durations, and greater preoccupation with death.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2656591/.
Mcelroy SL, Guerdjikova AI, Kim DD, et al. Naltrexone/Bupropion combination therapy in overweight or obese patients with major depressive disorder: results of a pilot study. Prim Care Companion CNS Disord. 2013;15(3).
Naltrexone/Bupropion combination therapy in overweight or obese patients with major depressive disorder: results of a pilot study
NB32 (32 mg naltrexone SR + 360 mg bupropion SR) significantly reduced depressive symptoms and promoted weight loss in overweight and obese women with MDD over 24 weeks, with 95% showing clinical improvement and 70% achieving remission, while maintaining a safety profile consistent with its components.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3795584/.
Pi-sunyer X, Apovian CM, Mcelroy SL, Dunayevich E, Acevedo LM, Greenway FL. Psychiatric adverse events and effects on mood with prolonged-release naltrexone/bupropion combination therapy: a pooled analysis. Int J Obes (Lond). 2019.
Psychiatric adverse events and effects on mood with prolonged-release naltrexone/bupropion combination therapy: a pooled analysis
This post hoc analysis of five clinical trials found that naltrexone/bupropion therapy in individuals with overweight or obesity was associated with mild to moderate psychiatric adverse events, mainly sleep disorders, anxiety, and depression, occurring mostly during dose escalation but not linked to suicidal ideation; mood changes were minimal and similar to placebo.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7111229/.
Available from https://www.nature.com/articles/s41366-018-0302-z.
Psychiatric adverse events and effects on mood with prolonged-release naltrexone/bupropion combination therapy: a pooled analysis
This post hoc analysis of five clinical trials found that naltrexone/bupropion (NB) for weight management was associated with mild to moderate psychiatric adverse events, mainly sleep disorders, anxiety, and depression, occurring mostly during dose escalation, but showed no significant impact on depressive symptoms or suicidal ideation compared to placebo.
You can read the full article at https://www.nature.com/articles/s41366-018-0302-z..
Guerdjikova AI, Walsh B, Shan K, Halseth AE, Dunayevich E, Mcelroy SL. Concurrent Improvement in Both Binge Eating and Depressive Symptoms with Naltrexone/Bupropion Therapy in Overweight or Obese Subjects with Major Depressive Disorder in an Open-Label, Uncontrolled Study. AdvTher. 2017;34(10):2307-2315.
Concurrent Improvement in Both Binge Eating and Depressive Symptoms with Naltrexone/Bupropion Therapy in Overweight or Obese Subjects with Major Depressive Disorder in an Open-Label, Uncontrolled Study
A 24-week trial of naltrexone ER/bupropion ER in 25 women with MDD and overweight/obesity found significant improvements in binge eating behavior, with 83% reporting minimal issues by week 24. These improvements were linked to better depressive symptoms and eating control, suggesting NB’s potential for treating binge eating disorder.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5656719/.
Available from https://clinicaltrials.gov/ct2/show/NCT01874951.
Low-Dose Naltrexone (LDN) for Depression Relapse and RecurrenceA pilot double-blind, randomized study tested low-dose naltrexone (1 mg b.i.d.) versus placebo in patients with major depressive disorder who relapsed on dopaminergic agents, assessing its impact on depressive symptoms without altering existing antidepressant regimens. Conducted over three weeks in Boston, the study monitored adherence, side effects, and suicidal ideation, with responders and non-responders given an optional open-label LDN phase. Statistical analyses evaluated response and remission rates, side effects, and effect sizes.
You can read the full article at https://classic.clinicaltrials.gov/ct2/show/NCT01874951.
Makowski CT, Gwinn KM, Hurren KM. Naltrexone/bupropion: an investigational combination for weight loss and maintenance. Obes Facts. 2011;4(6):489-94.
Naltrexone/bupropion: an investigational combination for weight loss and maintenance
Naltrexone/bupropion is an investigational weight-loss therapy that led to a mean 4.7% weight reduction over a year in obese or overweight patients with comorbidities, significantly outperforming placebo. It also improved metabolic markers and lowered HbA1c in diabetics by 0.5%, though common side effects include nausea and dizziness. Further research is needed on cardiovascular effects.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6444555/.
Nissen SE, Wolski KE, Prcela L, et al. Effect of Naltrexone-Bupropion on Major Adverse Cardiovascular Events in Overweight and Obese Patients With Cardiovascular Risk Factors: A Randomized Clinical Trial. JAMA. 2016;315(10):990-1004.
Effect of Naltrexone-Bupropion on Major Adverse Cardiovascular Events in Overweight and Obese Patients With Cardiovascular Risk Factors: A Randomized Clinical Trial
This study investigated the cardiovascular safety of naltrexone-bupropion in overweight and obese patients but was terminated early due to the release of interim data. Preliminary results showed a lower incidence of major adverse cardiovascular events (MACE) in the treatment group, but the trial’s early termination left its cardiovascular safety inconclusive, necessitating further research.You can read the full article at https://jamanetwork.com/journals/jama/fullarticle/2499275.
Sarwer DB, Steffen KJ. Quality of life, body image and sexual functioning in bariatric surgery patients. Eur Eat Disord Rev 2015; 23: 504–508.
Quality of life, body image and sexual functioning in bariatric surgery patients
Bariatric surgery improves quality of life, body image, and sexual functioning in individuals with extreme obesity, often before maximum weight loss, though the long-term sustainability of these benefits remains uncertain.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1002/erv.2412.
Steffen KJ, King WC, White GE, et al. Sexual functioning of men and women with severe obesity before bariatric surgery. SurgObesRelat Dis 2017; 13: 334–343.
Sexual functioning of men and women with severe obesity before bariatric surgery
This study of adults with severe obesity before bariatric surgery found high rates of sexual inactivity, low desire, and dissatisfaction, with physical health constraints limiting sexual activity. Poorer sexual function was linked to age, urinary incontinence, depression, and antidepressant use, with ethnicity affecting women and marital status affecting men.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6067110/.
Sarwer DB, Spitzer JC, Wadden TA, et al. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surg 2014; 149: 26–33.
Changes in sexual functioning and sex hormone levels in women following bariatric surgery
Bariatric surgery led to significant improvements in sexual functioning, reproductive hormones, and psychosocial well-being in 106 women, alongside substantial weight loss (over 32% at two years). Enhancements in sexual health, body image, and depressive symptoms emerged within the first year and persisted into the second, reinforcing the procedure’s broad benefits.
You can read the full article at https://jamanetwork.com/journals/jamasurgery/fullarticle/1764857.
Sarwer DB, Lavery M, Spitzer JC. A review of the relationships between extreme obesity, quality of life, and sexual function. ObesSurg 2012; 22: 668–676.
A review of the relationships between extreme obesity, quality of life, and sexual function
Extreme obesity affects both physical health and psychosocial well-being, yet its impact on sexual functioning is often overlooked. This paper highlights the need to study quality of life and sexual health in obese individuals, especially changes following significant weight loss through bariatric surgery.
You can read the abstract of the article article at https://link.springer.com/article/10.1007/s11695-012-0588-1.
Patel K, Allen S, Haque MN, Angelescu I, Baumeister D, Tracy DK. Bupropion: a systematic review and meta‐analysis of effectiveness as an antidepressant. TherAdvPsychopharmacol 2016; 6: 99–144.
Bupropion: a systematic review and meta‐analysis of effectiveness as an antidepressant
Bupropion, an antidepressant affecting noradrenaline and dopamine reuptake, has shown effectiveness over placebo and comparable efficacy to other antidepressants, especially when combined with another drug. It is generally well-tolerated, with minimal sexual side effects and a tendency for weight loss. However, research gaps remain, particularly in treatment-naïve depression and its augmentation potential.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4837968/.
Gardner EA, Johnson JA. Bupropion: an antidepressant without sexual pathophysiological action. J ClinPsychopharmacol 1985; 5: 24–9.
Bupropion: an antidepressant without sexual pathophysiological action
In an open clinical study of 40 male outpatients, bupropion (300–600 mg/day) improved or resolved antidepressant-induced sexual dysfunction in 24 of 28 affected patients (p < 0.001), while the 12 without prior dysfunction maintained normal function, suggesting bupropion has a low risk of sexual side effects.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/3919069/.
Walker PW, Cole JO, Gardner EA, et al. Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion. J Clin Psychiatry 1993; 54: 459–65.
Improvement in fluoxetine-associated sexual dysfunction in patients switched to bupropion
Bupropion improved orgasm function, libido, and sexual satisfaction in fluoxetine-treated patients with anorgasmia or delayed orgasm, with 94% experiencing resolution of dysfunction and 81% reporting higher satisfaction. Depression scores also improved, and bupropion was well tolerated, suggesting its suitability for patients with fluoxetine-induced sexual dysfunction.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/8276736/.
Labbate LA, Pollack MH. Treatment of fluoxetine-induced sexual dysfunction with bupropion: a case report. Ann Clin Psychiatry 1994; 6: 13–5.
Treatment of fluoxetine-induced sexual dysfunction with bupropion: a case report
Bupropion successfully resolved fluoxetine-induced sexual dysfunction in a 50-year-old man with recurrent major depression, marking the first reported case. Unlike fluoxetine, which affects serotonin, bupropion’s minimal sexual side effects and mild dopamine reuptake blockade may explain its effectiveness.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/7951639/.
Halseth A, Shan K, Gilder K, Malone M, Acevedo L, Fujioka K. Quality of life, binge eating and sexual function in participants treated for obesity with sustained release naltrexone/bupropion. ObesSciPract. 2018;4(2):141–152. Published 2018 Feb 23. doi:10.1002/osp4.156.
Quality of life, binge eating and sexual function in participants treated for obesity with sustained release naltrexone/bupropion
A 26-week trial found that naltrexone SR/bupropion SR with lifestyle intervention (NB + CLI) led to significantly greater weight loss (9.46% vs. 0.94%), improved weight-related quality of life, better control over eating, and enhanced sexual function compared to usual care, though some participants discontinued due to nausea, anxiety, or headache.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5893468/.
Christou GA, Kiortsis DN. The efficacy and safety of the naltrexone/bupropion combination for the treatment of obesity: an update. Hormones (Athens). 2015;14(3):370-5.
The efficacy and safety of the naltrexone/bupropion combination for the treatment of obesity: an update
NB32 (32 mg naltrexone and 360 mg bupropion prolonged release) is FDA- and EMA-approved as an adjunct to lifestyle interventions for weight loss, showing 5.0%-9.3% total weight loss (3.2%-5.2% vs. placebo) over 56 weeks, with benefits on metabolic markers; common side effects include nausea and constipation, while serious adverse effects are rare.
You can read the abstract of the article at https://link.springer.com/article/10.14310/horm.2002.1600.
Yanovski SZ, Yanovski JA. Naltrexone extended-release plus bupropion extended-release for treatment of obesity. JAMA. 2015;313(12):1213–1214. doi:10.1001/jama.2015.1617.
Naltrexone extended-release plus bupropion extended-release for treatment of obesity
In 2014, the FDA approved Contrave (naltrexone-bupropion) and Saxenda (liraglutide) for long-term obesity treatment. While naltrexone and bupropion were previously approved for other conditions, their combination was developed for weight management, showing greater efficacy than either drug alone. Contrave, not a controlled substance, is prescribed based on BMI criteria and costs around $200 per month.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4993523/.
Sherman MM, Ungureanu S, Rey JA. Naltrexone/Bupropion ER (Contrave): Newly Approved Treatment Option for Chronic Weight Management in Obese Adults. P T. 2016;41(3):164–172.
Naltrexone/Bupropion ER (Contrave): Newly Approved Treatment Option for Chronic Weight Management in Obese Adults
Naltrexone/bupropion extended-release, marketed as Contrave, is a recently approved therapy for chronic weight management in obese adults.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4771085/.
Toll, B. A., Leary, V., Wu, R., Salovey, P., Meandzija, B., & O’Malley, S. S. (2008). A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain. Addictive Behaviors, 33, 173-179. doi:10.1016/j.addbeh.2007.05.012.
A preliminary investigation of naltrexone augmentation of bupropion to stop smoking with less weight gain
A study on smoking cessation compared naltrexone (25 mg/day) plus sustained-release bupropion (300 mg/day) to bupropion alone in 40 weight-concerned smokers, finding no significant difference in quit rates but a trend toward less weight gain in the combination group, warranting further research.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2488403/.
Mooney ME, Schmitz JM, Allen S, et al. Bupropion and naltrexone for smoking cessation: A double-blind randomized placebo-controlled clinical trial. ClinPharmacolTher. 2016;100(4):344-52.
Bupropion and naltrexone for smoking cessation: A double-blind randomized placebo-controlled clinical trial
A randomized, double-blind study of 121 smokers found that combining bupropion (BUP) with naltrexone (NTX) significantly improved short-term smoking abstinence compared to BUP alone (54.1% vs. 33.3%), but this effect was not sustained at six months. Continuous abstinence rates did not differ, and while BUP + NTX reduced withdrawal symptoms, it caused more side effects.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5017904/.
Pacek LR, Mcclernon FJ, Bosworth HB. Adherence to Pharmacological Smoking Cessation Interventions: A Literature Review and Synthesis of Correlates and Barriers. Nicotine Tob Res. 2018;20(10):1163-1172.
Adherence to Pharmacological Smoking Cessation Interventions: A Literature Review and Synthesis of Correlates and Barriers
This review of 50 studies highlights factors influencing nonadherence to smoking cessation pharmacotherapies, distinguishing between nonpreventable (e.g., sociodemographics) and preventable (e.g., forgetfulness) barriers. It emphasizes the need for standardized adherence definitions, objective adherence measures, and improved reporting to enhance treatment effectiveness, especially for vulnerable populations.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6121917/.Wilcox CS, Oskooilar N, Erickson JS, Billes SK, Katz BB, Tollefson G, et al. An open‐label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects. Addictive Behaviors 2010;35(3):229‐34.
An open‐label study of naltrexone and bupropion combination therapy for smoking cessation in overweight and obese subjects
A 24-week study on overweight or obese smokers found that combining naltrexone (32 mg/day) and bupropion SR (360 mg/day) with behavioral counseling led to a 48% abstinence rate, significant reductions in smoking and cotinine levels, and stable body weight, with mild and transient withdrawal symptoms and common side effects like nausea, insomnia, and constipation.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0306460309002962?via%3Dihub.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








