Health Library
Tetradecylthioacetic Acid
Author: Dr. George Shanlikian, M.D. | Last Updated: May 20th, 2025
- Home
- >
- Health Library
- >
- Tetradecylthioacetic Acid
Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
Potential Tetradecylthioacetic Acid Benefits
Tetradecylthioacetic Acid benefits include enhanced fat metabolism, improved mitochondrial function, and reduced inflammation. It may also support cardiovascular health and help regulate lipid levels, making it a potential aid in managing metabolic disorders.
- Promotes fat loss [1-4]
- Improves cholesterol levels [5-12]
- Improves cardiovascular health [13-21]
- Improves blood sugar levels [2] [22-23]
- Fights inflammation [24-28]
- Prevents bone loss [29]
- Fights cancer [30-32]
- Offers Anti-oxidative Effects [33-34]
Key Takeaways
- Boosts Fat Metabolism – TTA enhances fatty acid oxidation, promoting the use of fat as an energy source.
- Improves Mitochondrial Function – It supports cellular energy production by enhancing mitochondrial efficiency.
- Reduces Inflammation – TTA exhibits anti-inflammatory effects, which may benefit metabolic and cardiovascular health.
- Regulates Lipid Levels – It can help lower triglycerides and improve lipid profiles in some individuals.
- Non-Hormonal Fat Loss Aid – TTA promotes fat loss without affecting hormone levels, making it a unique supplement in weight management.
What is Tetradecylthioacetic Acid?
Tetradecylthioacetic acid is an omega-3 fatty acid with fat-burning properties. While your body cannot use it as a source of energy, it can help regulate the amount of fat that your body can store by affecting genes associated with metabolism. In addition, tetradecylthioacetic acid is also known to improve your overall health through its antioxidant, anti-inflammatory, and anti-cancer properties. Tetradecylthioacetic acid can be taken in the form of supplements.
How Tetradecylthioacetic Acid Works?
Tetradecylthioacetic acid protects the body from excess fat by activating peroxisome proliferator-activated receptor alpha (PPAR-α). This in turn removes fat from the blood and allows them to be burnt for energy. Tetradecylthioacetic acid’s ability to clear fat from the blood also decreases the levels of bad cholesterol (low-density lipoprotein) and blood pressure.
Chemical Structure of Tetradecylthioacetic Acid
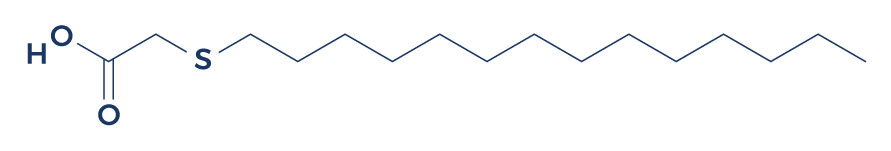
Research on Tetradecylthioacetic Acid
Promotes Fat Loss
Tetradecylthioacetic acid (TTA) is a synthetic fatty acid that has shown promise in promoting fat loss by enhancing mitochondrial fatty acid oxidation and improving lipid metabolism. Unlike traditional stimulants, TTA works at the cellular level to increase the breakdown of fat for energy, potentially leading to reductions in body fat without significantly affecting appetite or heart rate. Its non-stimulant nature makes it an attractive option for individuals seeking metabolic support for weight loss.
- In rats fed on high-fat diets, dietary supplementation of tetradecylthioacetic acid increased feed intake but reduced body weight gain and fat tissue sizes. [1]
- In Wistar rats fed with a high-fat diet, tetradecylthioacetic acid administration completely prevented diet-induced weight gain. [2]
- In rats, the administration of tetradecylthioacetic acid enhanced fatty acid metabolism. [3]
- In growing silver foxes, tetradecylthioacetic acid reduced body weight gain. [4]
Improves Cholesterol Levels
Tetradecylthioacetic acid (TTA) has been shown to improve cholesterol levels by modulating lipid metabolism and enhancing fatty acid oxidation in the liver. Studies suggest that TTA can reduce triglycerides and increase HDL (good) cholesterol while lowering LDL (bad) cholesterol, contributing to a healthier lipid profile. These effects make TTA a potential therapeutic agent for managing dyslipidemia and supporting cardiovascular health.
- In mice fed with a high-fat diet, tetradecylthioacetic acid increased the uptake and transport of fatty acids and high-density lipoprotein cholesterol (good cholesterol) in the small intestine. [5]
- In cholesterol-fed hamsters, tetradecylthioacetic acid decreased the levels of low-density lipoprotein cholesterol. [6]
- In rats, tetradecylthioacetic acid significantly reduced cholesterol levels by increasing cholesterol synthesis. [7]
- A study found that prolonged supplementation of tetradecylthioacetic acid in rats reduced low-density lipoprotein cholesterol via increased fatty acid oxidation. [8]
- In male patients with type 2 diabetes mellitus, tetradecylthioacetic acid attenuated abnormal cholesterol levels via activation of PPAR-alpha/delta and increased fatty acid oxidation within the cells. [9]
- In HIV-infected patients on highly active antiretroviral therapy, tetradecylthioacetic acid administration at a dose of 1 g four times a day significantly reduced total cholesterol, low-density lipoprotein cholesterol, and triglycerides. [10]
- In rats fed with a high carbohydrate diet, tetradecylthioacetic acid reduced blood cholesterol levels significantly. [11]
- A rat study found that tetradecylthioacetic acid reduced the synthesis and secretion of lipids and cholesterol. [12]
Improves Cardiovascular Health
Tetradecylthioacetic acid (TTA) may improve cardiovascular health by reducing inflammation, enhancing lipid metabolism, and improving endothelial function. Its ability to lower triglycerides, increase HDL cholesterol, and promote fatty acid oxidation supports healthier blood vessels and reduces the risk of atherosclerosis. These combined effects position TTA as a promising compound for supporting overall heart health and reducing cardiovascular disease risk.
- Studies found that tetradecylthioacetic acid can help improve heart health through its anti-inflammatory and antioxidant properties. [13-14]
- In rats, tetradecylthioacetic acid improved the heart’s left ventricular function by increasing n-3 polyunsaturated fatty acids (PUFA) levels. [15]
- In animal models of heart failure, tetradecylthioacetic acid improved heart muscle function. [16]
- In an animal model of heart and skeletal muscle inflammation, tetradecylthioacetic acid induced heart regeneration via recruitment of immune cells. [17-19]
- In mini pigs with heart dysfunction, tetradecylthioacetic acid reduced the narrowing of the heart arteries. [20]
- In salmons, tetradecylthioacetic acid enhanced energy production in salmon hearts by stimulation of FA oxidation. [21]
Improves Blood Sugar Levels
Tetradecylthioacetic acid (TTA) may help improve blood sugar levels by enhancing insulin sensitivity and promoting more efficient glucose uptake in muscle and liver cells. By stimulating mitochondrial fatty acid oxidation and reducing lipid accumulation, TTA helps alleviate metabolic stress that can impair glucose metabolism. These effects suggest its potential as a supportive agent in managing insulin resistance and maintaining healthy blood sugar control.
- In patients with type 2 diabetes, tetradecylthioacetic acid improved blood sugar metabolism. [22]
- In male Wistar rats, tetradecylthioacetic acid increased blood sugar metabolism in the liver and reduced the plasma insulin/glucose ratio. [23]
- In Wistar rats fed with a high-fat diet, tetradecylthioacetic acid administration completely prevented diet-induced insulin resistance. [2]
Fights Inflammation
Tetradecylthioacetic acid (TTA) has demonstrated anti-inflammatory properties by modulating the expression of inflammatory cytokines and reducing oxidative stress. Through its action on mitochondrial metabolism and activation of PPARs (peroxisome proliferator-activated receptors), TTA helps lower systemic inflammation, which is linked to various chronic conditions such as cardiovascular disease, obesity, and metabolic syndrome. This makes TTA a promising candidate for reducing inflammation-related health risks.
- In a mouse model of chronic inflammation, tetradecylthioacetic acid reduced the blood levels of triacylglycerol, a substance that activates inflammation. [24]
- In Atlantic salmon cells, tetradecylthioacetic acid suppressed inflammatory status. [25]
- In psoriasis patients, the administration of 1000 mg tetradecylthioacetic acid daily for 28 days decreased the blood levels of inflammatory substances such as TNF-α, IL-8, and VCAM-1. [26]
- A study reported that tetradecylthioacetic acid exerts its anti-inflammatory effects by reducing cytokine and reactive oxygen species production, and enhancing nitric oxide levels. [27]
- A study on heart cells found that tetradecylthioacetic acid reduced the anti-inflammatory fatty acid index. [28]
Prevents Bone Loss
In one study, researchers explored the skeletal effects of tetradecylthioacetic acid in rats. [29] A group of rats had surgical removal of the ovaries to induce bone loss while another group of rats did not. Both groups then received oral tetradecylthioacetic acid daily for 4 months. The researchers then examined the bone mineral density and content of the subjects. Results showed that tetradecylthioacetic acid-treated rats maintained the normal bone mineral density of the femur (thigh bone) while the other group had a significant increase in bone mineral density.
Fights Cancer
Tetradecylthioacetic acid (TTA) has shown potential in fighting cancer by modulating cellular metabolism, reducing inflammation, and influencing gene expression related to cell growth and apoptosis. Its ability to activate PPARs and alter mitochondrial function may help inhibit cancer cell proliferation and promote the death of abnormal cells. While more research is needed, early studies suggest that TTA could be a promising adjunct in cancer prevention and therapy.
- In human leukemia cells, the administration of tetradecylthioacetic acid 1000 mg/day for 7 consecutive days inhibited the growth of cancer cells. [30]
- A cell study also found that tetradecylthioacetic acid prevented the growth and multiplication of human SW620 colon cancer cells. [31]
- In mice, dietary tetradecylthioacetic acid produced inhibitory effects on colon cancer growth. [32]
Offers Anti-oxidative Effects
Tetradecylthioacetic acid (TTA) offers notable anti-oxidative effects by reducing oxidative stress and enhancing mitochondrial function. It helps neutralize harmful reactive oxygen species (ROS), which can damage cells and contribute to aging and chronic diseases. By supporting the body’s antioxidant defenses and improving metabolic efficiency, TTA contributes to cellular protection and overall metabolic health.
- A study showed that tetradecylthioacetic acid and tetradecylselenoacetic acid exerted their antioxidant capacity via interaction with copper or iron ions. [33]
- In rats, tetradecylthioacetic acid reduced colonic oxidative damage and the levels of the pro-inflammatory substance known as cytokines. [34]
Tetradecylthioacetic Acid Side Effects
Tetradecylthioacetic acid side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on tetradecylthioacetic acid. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of tetradecylthioacetic acid. Despite this, it was listed as a side effect associated with tetradecylthioacetic acid even though these associated side effects are very uncommon.
Side effects associated with tetradecylthioacetic acid may include the following:
- Cramps
- Headaches
FAQ
What is TTA supplement?
TTA (Tetradecylthioacetic acid) is a synthetic fatty acid supplement often used to support fat metabolism and improve mitochondrial function. It has been researched for its potential to increase fat oxidation, reduce inflammation, and enhance metabolic health.
What is the benefit of rumenic acid?
Rumenic acid, a type of conjugated linoleic acid (CLA), has antioxidant and anti-carcinogenic properties. It may help reduce body fat, improve immune function, and support heart health, though clinical results are mixed.
What is the benefit of thioacetic acid?
Thioacetic acid itself is primarily a chemical reagent in organic synthesis, and it is not typically used as a dietary supplement. Its biological benefits are not well-established or studied in the context of human health.
What does decanoic acid do?
Decanoic acid (capric acid), a medium-chain fatty acid found in coconut oil, supports energy metabolism, exhibits anticonvulsant properties (especially in ketogenic diets), and may benefit brain function and mitochondrial health.
Are hyaluronic acid supplements good for you?
Yes, hyaluronic acid supplements are generally considered beneficial for skin hydration, joint health, and reducing symptoms of osteoarthritis. They are well-tolerated with minimal side effects for most people.
What supplement makes you lose body fat?
Supplements like green tea extract, caffeine, conjugated linoleic acid (CLA), L-carnitine, and forskolin have been associated with fat loss, but results vary and should be combined with proper diet and exercise for effectiveness.
What is a linoleic acid supplement?
A linoleic acid supplement provides omega-6 essential fatty acids, which are important for cell membrane integrity, skin health, and immune function. It is commonly found in vegetable oils, safflower oil, and evening primrose oil.
What are the side effects of fatty acid supplements?
Side effects can include gastrointestinal discomfort (bloating, diarrhea), increased bleeding risk (especially with omega-3s), fishy aftertaste (in fish oil), and potential interactions with medications like blood thinners.
What are the benefits of Pentadecylic acid?
Pentadecylic acid (C15:0), a saturated fatty acid, may support metabolic, cardiovascular, and immune health. Recent studies suggest it may help improve cellular function and reduce inflammation, acting similarly to essential fatty acids.
What are the benefits of nervonic acid?
Nervonic acid is a monounsaturated fatty acid involved in brain and nerve cell membrane development. It may support cognitive function, myelin repair, and has potential benefits for neurological disorders like Alzheimer’s and multiple sclerosis.
What are the benefits of amlexanox?
Amlexanox is an anti-inflammatory and immunomodulatory drug used for conditions like aphthous ulcers and asthma. It also shows promise in treating obesity, type 2 diabetes, and non-alcoholic fatty liver disease by reducing inflammation and improving metabolic parameters.
Reference
Wensaas AJ, Rustan AC, Rokling-Andersen MH, Caesar R, Jensen J, Kaalhus O, Graff BA, Gudbrandsen OA, Berge RK, Drevon CA. Dietary supplementation of tetradecylthioacetic acid increases feed intake but reduces body weight gain and adipose depot sizes in rats fed on high-fat diets. Diabetes ObesMetab. 2009 Nov;11(11):1034-49. doi: 10.1111/j.1463-1326.2009.01092.x. Epub 2009 Sep 9. PMID: 19740081.
Dietary supplementation of tetradecylthioacetic acid increases feed intake but reduces body weight gain and adipose depot sizes in rats fed on high-fat diets
Tetradecylthioacetic acid (TTA), a pan-PPAR ligand and fatty acid analogue, was shown to reduce body weight gain, adipose tissue mass, and plasma lipids in rats fed a high-fat diet, despite increased food intake. Over 7 weeks, TTA enhanced fatty acid β-oxidation in the liver and heart, significantly reduced fat depots, and increased the expression of thermogenic genes like UCP3 in the liver and Ucp1 in fat tissue. These findings suggest TTA promotes fat loss and energy expenditure by boosting lipid metabolism and reducing energy efficiency.
You can read the abstract of the article at https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/j.1463-1326.2009.01092.x.Madsen L, Guerre-Millo M, Flindt EN, Berge K, Tronstad KJ, Bergene E, Sebokova E, Rustan AC, Jensen J, Mandrup S, Kristiansen K, Klimes I, Staels B, Berge RK. Tetradecylthioacetic acid prevents high fat diet induced adiposity and insulin resistance. J Lipid Res. 2002 May;43(5):742-50. PMID: 11971945.
Tetradecylthioacetic acid prevents high fat diet induced adiposity and insulin resistance
Tetradecylthioacetic acid (TTA), a non-beta-oxidizable fatty acid analog, effectively prevents diet-induced and genetic obesity and insulin resistance in rodents by activating PPAR receptors, especially PPARα. In high-fat-fed Wistar rats and obese Zucker rats, TTA reduced fat mass and improved insulin sensitivity. It enhanced hepatic expression of genes involved in fatty acid metabolism, increased mitochondrial β-oxidation, and lowered plasma fatty acid/ketone body ratios, suggesting that TTA promotes fat clearance and metabolic health primarily through PPARα-dependent pathways.
You can read the full article at https://www.jlr.org/article/S0022-2275(20)30116-4/fulltext.Vaagenes H, Madsen L, Dyrøy E, Elholm M, Stray-Pedersen A, Frøyland L, Lie O, Berge RK. Methylatedeicosapentaenoic acid and tetradecylthioacetic acid: effects on fatty acid metabolism. BiochemPharmacol. 1999 Oct 1;58(7):1133-43. doi: 10.1016/s0006-2952(99)00198-7. PMID: 10484071.
Methylatedeicosapentaenoic acid and tetradecylthioacetic acid: effects on fatty acid metabolism
Methylation of eicosapentaenoic acid (EPA) at the 2- or 3-position enhances its hypolipidemic effects in rats by boosting hepatic mitochondrial and peroxisomal β-oxidation, reducing plasma lipids, and downregulating lipogenic enzymes. The most effective derivative contained two methyl groups at the 2-position and one at the 3-position, which increased fatty acid oxidation and decreased triglyceride secretion in hepatocytes. In contrast, 2-methyl-tetradecylthioacetic acid increased only peroxisomal oxidation without lowering plasma lipids, highlighting the key role of mitochondrial oxidation in EPA’s lipid-lowering action.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0006295299001987?via%3Dihub.Available from https://nmbu.brage.unit.no/nmbu-xmlui/handle/11250/2570197.
The effect of tetradecylthioacetic acid (TTA) on body weight management in growing silver foxes (vulpes vulpes) as a model for dogs (Canis familiaris)
This thesis explores obesity-related health issues in dogs and evaluates tetradecylthioacetic acid (TTA) as a dietary supplement to manage obesity, using growing silver foxes as a model. Over an 85-day study, TTA supplementation reduced feed intake and body weight gain at higher doses, while its effects on plasma lipids—particularly triglycerides, LDL-cholesterol, and free fatty acids—were dose-dependent and most notable at lower doses. The findings suggest TTA may enhance lipid metabolism and reduce plasma lipid levels, although changes in fat deposition were more influenced by energy intake than TTA alone.
You can read the full article at https://nmbu.brage.unit.no/nmbu-xmlui/handle/11250/2570197.Lundåsen T, Pedrelli M, Bjørndal B, Rozell B, Kuiper RV, Burri L, Pavanello C, Turri M, Skorve J, Berge RK, Alexson SEH, Tillander V. The PPAR pan-agonist tetradecylthioacetic acid promotes redistribution of plasma cholesterol towards large HDL. PLoS One. 2020 Mar 16;15(3):e0229322. doi: 10.1371/journal.pone.0229322. PMID: 32176696; PMCID: PMC7075573.
The PPAR pan-agonist tetradecylthioacetic acid promotes redistribution of plasma cholesterol towards large HDL
Tetradecylthioacetic acid (TTA), a synthetic fatty acid that activates PPARα and resists complete β-oxidation, significantly reduced body weight and plasma triglycerides in high-fat diet-fed mice while increasing liver TAG levels. TTA shifted plasma lipoprotein profiles toward larger HDL particles and decreased lipid droplet size in intestinal cells. It also enhanced intestinal expression of fatty acid transporters and cholesterol efflux genes like Abca1, suggesting improved lipid handling in the gut and altered lipoprotein metabolism without affecting total cholesterol levels.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC7075573/.Frøyland L, Asiedu DK, Vaagenes H, Garras A, Lie O, Totland GK, Berge RK. Tetradecylthioacetic acid incorporated into very low density lipoprotein: changes in the fatty acid composition and reduced plasma lipids in cholesterol-fed hamsters. J Lipid Res. 1995 Dec;36(12):2529-40. PMID: 8847479.
Tetradecylthioacetic acid incorporated into very low density lipoprotein: changes in the fatty acid composition and reduced plasma lipids in cholesterol-fed hamsters
Tetradecylthioacetic acid (CMTTD), a non-β-oxidizable fatty acid, significantly lowered plasma cholesterol and triglycerides in hyperlipidemic hamsters by reducing VLDL and LDL cholesterol levels without affecting HDL cholesterol. Its hypolipidemic effects were associated with increased mitochondrial and peroxisomal fatty acid oxidation, and it altered the lipid composition of plasma, liver, and heart by accumulating preferentially in heart and VLDL. Despite these changes, key genes involved in cholesterol synthesis and uptake remained unchanged, suggesting CMTTD’s effects occur through enhanced lipid oxidation and redistribution rather than gene regulation of cholesterol pathways.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/8847479/.Asiedu DK, al-Shurbaji A, Rustan AC, Björkhem I, Berglund L, Berge RK. Hepatic fatty acid metabolism as a determinant of plasma and liver triacylglycerol levels. Studies on tetradecylthioacetic and tetradecylthiopropionic acids. Eur J Biochem. 1995 Feb 1;227(3):715-22. doi: 10.1111/j.1432-1033.1995.tb20193.x. PMID: 7867630.
Hepatic fatty acid metabolism as a determinant of plasma and liver triacylglycerol levels. Studies on tetradecylthioacetic and tetradecylthiopropionic acids
Tetradecylthioacetic acid (TTA) significantly reduced plasma triglycerides and cholesterol in rats by enhancing mitochondrial and peroxisomal fatty acid oxidation, lowering hepatic triglyceride synthesis and secretion, and suppressing HMG-CoA reductase activity. These changes led to lower VLDL and LDL cholesterol and improved the HDL/LDL cholesterol ratio, while HDL cholesterol remained unchanged. In contrast, tetradecylthiopropionic acid had opposite effects, increasing triglyceride levels and decreasing mitochondrial oxidation. The study highlights how modulating fatty acid availability and oxidation influences lipoprotein metabolism and lipid profiles.
You can read the abstract of the article at https://febs.onlinelibrary.wiley.com/doi/full/10.1111/j.1432-1033.1995.0715p.x?sid=nlm%3Apubmed.Asiedu DK, Frøyland L, Vaagenes H, Lie O, Demoz A, Berge RK. Long-term effect of tetradecylthioacetic acid: a study on plasma lipid profile and fatty acid composition and oxidation in different rat organs. BiochimBiophysActa. 1996 Apr 19;1300(2):86-96. doi: 10.1016/0005-2760(95)00235-9. PMID: 8652642.
Long-term effect of tetradecylthioacetic acid: a study on plasma lipid profile and fatty acid composition and oxidation in different rat organs
Tetradecylthioacetic acid (TTA) enhances mitochondrial and peroxisomal β-oxidation, lowers plasma free fatty acids and VLDL-triglycerides, and reduces LDL and HDL cholesterol levels, while increasing lipogenic enzyme activities and upregulating LDL receptor and HMG-CoA reductase gene expression. It alters fatty acid composition by increasing monounsaturated fats in plasma and liver and raising the omega-3/omega-6 ratio in the heart, potentially influencing membrane function. These sustained hypolipidemic effects suggest TTA’s impact is driven by enhanced fatty acid oxidation and lipid metabolism modulation.
You can read the abstract of the article at https://www.academia.edu/29116801/Long_term_effect_of_tetradecylthioacetic_acid_a_study_on_plasma_lipid_profile_and_fatty_acid_composition_and_oxidation_in_different_rat_organs.Løvås K, Røst TH, Skorve J, Ulvik RJ, Gudbrandsen OA, Bohov P, Wensaas AJ, Rustan AC, Berge RK, Husebye ES. Tetradecylthioacetic acid attenuates dyslipidaemia in male patients with type 2 diabetes mellitus, possibly by dual PPAR-alpha/delta activation and increased mitochondrial fatty acid oxidation. Diabetes ObesMetab. 2009 Apr;11(4):304-14. doi: 10.1111/j.1463-1326.2008.00958.x. PMID: 19267708.
Tetradecylthioacetic acid attenuates dyslipidaemia in male patients with type 2 diabetes mellitus, possibly by dual PPAR-alpha/delta activation and increased mitochondrial fatty acid oxidation
In a pilot study, tetradecylthioacetic acid (TTA) safely reduced LDL cholesterol and improved HDL-related markers in men with type 2 diabetes, without affecting glucose metabolism; mechanistic studies showed TTA acted as a pan-PPAR agonist—mainly activating PPAR-α and PPAR-δ—to enhance mitochondrial fatty acid oxidation in liver and muscle cells, supporting its potential as a lipid-modulating therapy.
You can read the abstract of the article at https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/j.1463-1326.2008.00958.x.Fredriksen J, Ueland T, Dyrøy E, Halvorsen B, Melby K, Melbye L, Skalhegg BS, Bohov P, Skorve J, Berge RK, Aukrust P, Frøland SS. Lipid-lowering and anti-inflammatory effects of tetradecylthioacetic acid in HIV-infected patients on highly active antiretroviral therapy. Eur J Clin Invest. 2004 Oct;34(10):709-15. doi: 10.1111/j.1365-2362.2004.01410.x. PMID: 15473896.
Lipid-lowering and anti-inflammatory effects of tetradecylthioacetic acid in HIV-infected patients on highly active antiretroviral therapy
In HIV-infected patients on HAART with hyperlipidaemia, tetradecylthioacetic acid (TTA) combined with a cholesterol-lowering diet significantly reduced total cholesterol, LDL, triglycerides, and LDL/HDL ratio, while also lowering plasma TNF-α levels—suggesting both lipid-lowering and anti-inflammatory effects potentially mediated by upregulation of scavenger and LDL receptors.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1365-2362.2004.01410.x?sid=nlm%3Apubmed.Skorve J, Berge RK. The hypocholesterolemic effect of sulfur-substituted fatty acid analogues in rats fed a high carbohydrate diet. BiochimBiophysActa. 1993 Apr 7;1167(2):175-81. doi: 10.1016/0005-2760(93)90159-7. PMID: 8466946.
The hypocholesterolemic effect of sulfur-substituted fatty acid analogues in rats fed a high carbohydrate diet
In rats fed a high-carbohydrate diet, sulfur-substituted fatty acids—particularly 3-thiadicarboxylic acid and tetradecylthioacetic acid—significantly lowered plasma cholesterol by reducing lipogenic enzyme activities and acetyl-CoA availability, with 3-thiadicarboxylic acid being more potent; this effect may also involve reduced cholesterol esterification due to decreased ACAT activity, despite an increase in HMG-CoA reductase.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/0005276093901597.Skorve J, Rustan AC, Berge RK. Effects of non-beta-oxidizablesulfur-substituted fatty acid analogues on synthesis and secretion of triacylglycerol and cholesterol in cultured rat hepatocytes. Lipids. 1995 Nov;30(11):987-94. doi: 10.1007/BF02536282. PMID: 8569438.
Effects of non-beta-oxidizablesulfur-substituted fatty acid analogues on synthesis and secretion of triacylglycerol and cholesterol in cultured rat hepatocytes
In cultured hepatocytes, tetradecylthioacetic acid (TTA) reduced triacylglycerol and diacylglycerol synthesis and secretion by enhancing fatty acid oxidation, while 3-thiadicarboxylic acid lowered both glycerolipid and cholesterol synthesis and secretion without increasing fatty acid oxidation, indicating distinct hypolipidemic mechanisms for each compound.
You can read the abstract of the article at https://aocs.onlinelibrary.wiley.com/doi/abs/10.1007/BF02536282?sid=nlm%3Apubmed.Wrzesinski K, R León I, Kulej K, Sprenger RR, Bjørndal B, Christensen BJ, Berge RK, Jensen ON, Rogowska-Wrzesinska A. Proteomics identifies molecular networks affected by tetradecylthioacetic acid and fish oil supplemented diets. J Proteomics. 2013 Jun 12;84:61-77. doi: 10.1016/j.jprot.2013.03.027. Epub 2013 Apr 6. PMID: 23568020.
Proteomics identifies molecular networks affected by tetradecylthioacetic acid and fish oil supplemented diets
This study shows that fish oil and tetradecylthioacetic acid (TTA), especially TTA, significantly alter mitochondrial metabolism in rats fed high-fat diets, affecting fatty acid and amino acid metabolism, lipid oxidation, and oxidative phosphorylation—largely through PPAR signaling—while TTA’s strong antioxidant effects may stem from downregulation of respiratory chain components, providing valuable insight into their potential to counter metabolic syndrome.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S1874391913001747?via%3Dihub.Berge RK, Skorve J, /Tronstad KJ, Berge K, Gudbrandsen OA, Grav H. Metabolic effects of thia fatty acids. CurrOpinLipidol. 2002 Jun;13(3):295-304. doi: 10.1097/00041433-200206000-00010. PMID: 12045400.
Metabolic effects of thia fatty acids
Tetradecylthioacetic acid (TTA), a sulfur-substituted saturated fatty acid, exhibits diverse biological effects including enhanced mitochondrial activity, increased fatty acid catabolism, improved insulin sensitivity, antioxidant and anti-inflammatory properties, and reduced cell proliferation, largely through PPAR activation, though some benefits may occur via PPAR-independent pathways.
You can read the abstract of the article at https://journals.lww.com/co-lipidology/abstract/2002/06000/metabolic_effects_of_thia_fatty_acids.10.aspx.Strand E, Bjorndal B, Nygard O, Burri L, Berge C, Bohov P, Christensen BJ, Berge K, Wergedahl H, Viste A, Berge RK. Long-term treatment with the pan-PPAR agonist tetradecylthioacetic acid or fish oil is associated with increased cardiac content of n-3 fatty acids in rat. Lipids Health Dis. 2012 Jun 27;11:82. doi: 10.1186/1476-511X-11-82. PMID: 22738017; PMCID: PMC3459737.
Long-term treatment with the pan-PPAR agonist tetradecylthioacetic acid or fish oil is associated with increased cardiac content of n-3 fatty acids in rat
Long-term treatment with tetradecylthioacetic acid (TTA) and/or high-dose fish oil (FO) in rats led to significant changes in cardiac fatty acid metabolism, including increased heart n-3 PUFA levels, enhanced fatty acid oxidation, and upregulation of key metabolic enzymes, highlighting distinct effects on heart versus liver lipid processing.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3459737/.Øie E, Berge RK, Ueland T, Dahl CP, Edvardsen T, Beitnes JO, Bohov P, Aukrust P, Yndestad A. Tetradecylthioacetic acid increases fat metabolism and improves cardiac function in experimental heart failure. Lipids. 2013 Feb;48(2):139-54. doi: 10.1007/s11745-012-3749-z. Epub 2012 Dec 25. PMID: 23266898.
Tetradecylthioacetic acid increases fat metabolism and improves cardiac function in experimental heart failure
In a rat model of post-myocardial infarction heart failure, tetradecylthioacetic acid (TTA) improved cardiac function without altering heart remodeling, likely by lowering plasma free fatty acids and increasing myocardial n-3 PUFA levels, suggesting TTA may support heart health by modulating fatty acid availability and composition.
You can read the full article at https://link.springer.com/article/10.1007/s11745-012-3749-z.Grammes F, Rørvik KA, Takle H. Tetradecylthioacetic acid modulates cardiac transcription in Atlantic salmon, Salmosalar L., suffering heart and skeletal muscle inflammation. J Fish Dis. 2012 Feb;35(2):109-17. doi: 10.1111/j.1365-2761.2011.01326.x. PMID: 22233512.
Tetradecylthioacetic acid modulates cardiac transcription in Atlantic salmon, Salmosalar L., suffering heart and skeletal muscle inflammation
Tetradecylthioacetic acid (TTA) pre-feeding in Atlantic salmon enhances cardiac immune gene expression and activates heart growth pathways during HSMI infection, potentially increasing cardiac resilience and reducing mortality without affecting viral load.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2761.2011.01326.x.Alne H, Thomassen MS, Takle H, Terjesen BF, Grammes F, Oehme M, Refstie S, Sigholt T, Berge RK, Rørvik KA. Increased survival by feeding tetradecylthioacetic acid during a natural outbreak of heart and skeletal muscle inflammation in S0 Atlantic salmon, Salmosalar L. J Fish Dis. 2009 Nov;32(11):953-61. doi: 10.1111/j.1365-2761.2009.01078.x. Epub 2009 Jul 10. PMID: 19602091.
Increased survival by feeding tetradecylthioacetic acid during a natural outbreak of heart and skeletal muscle inflammation in S0 Atlantic salmon, Salmosalar L
Feeding Atlantic salmon a TTA-supplemented diet before a natural outbreak of HSMI significantly reduced mortality, improved growth, increased cardiac lipid oxidation gene expression, and lowered plasma urea levels, suggesting TTA enhances survival by modulating inflammation and promoting efficient energy use.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2761.2009.01078.x.Grammes F, Rørvik KA, Thomassen MS, Berge RK, Takle H. Genome wide response to dietary tetradecylthioacetic acid supplementation in the heart of Atlantic Salmon (Salmosalar L). BMC Genomics. 2012 May 11;13:180. doi: 10.1186/1471-2164-13-180. PMID: 22577878; PMCID: PMC3483216.
Genome wide response to dietary tetradecylthioacetic acid supplementation in the heart of Atlantic Salmon (Salmosalar L)
Tetradecylthioacetic acid (TTA) feeding in Atlantic salmon induces long-lasting cardiac gene expression changes associated with improved heart performance, including enhanced fatty acid metabolism, glycolysis, TCA cycle activity, and physiological heart growth, suggesting potential benefits for heart efficiency and survival in aquaculture.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3483216/Pettersen RJ, Muna ZA, Kuiper KK, Svendsen E, Müller F, Aukrust P, Berge RK, Nordrehaug JE. Sustained retention of tetradecylthioacetic acid after local delivery reduces angioplasty-induced coronary stenosis in the minipig. Cardiovasc Res. 2001 Nov;52(2):306-13. doi: 10.1016/s0008-6363(01)00404-7. PMID: 11684079.
Sustained retention of tetradecylthioacetic acid after local delivery reduces angioplasty-induced coronary stenosis in the minipig
Local delivery of tetradecylthioacetic acid (TTA) significantly reduced coronary artery stenosis in minipigs following angioplasty by promoting favorable vessel remodeling rather than inhibiting intimal hyperplasia, likely through its antioxidant and anti-inflammatory actions, including reduced LDL oxidation and modulation of cytokine release.
You can read the abstract of the article at https://academic.oup.com/cardiovascres/article-abstract/52/2/306/260668?redirectedFrom=fulltext&login=false.Arge R, Dessen JE, Østbye TK, Ruyter B, Thomassen MS, Rørvik KA. Effects of tetradecylthioacetic acid (TTA) treatment on lipid metabolism in salmon hearts-in vitro and in vivo studies. Fish Physiol Biochem. 2018 Apr;44(2):703-716. doi: 10.1007/s10695-018-0466-4. Epub 2018 Jan 19. PMID: 29349633.
Effects of tetradecylthioacetic acid (TTA) treatment on lipid metabolism in salmon hearts-in vitro and in vivo studies
Tetradecylthioacetic acid (TTA) enhances fatty acid oxidation and energy production in the hearts of Atlantic salmon, as shown through in vivo and in vitro experiments that revealed increased cardiac fatty acid uptake, upregulation of genes for peroxisomal oxidation and lipid metabolism, and improved cardiac performance indicators during early sea transfer.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s10695-018-0466-4.Wensaas AJ, Rustan AC, Just M, Berge RK, Drevon CA, Gaster M. Fatty acid incubation of myotubes from humans with type 2 diabetes leads to enhanced release of beta-oxidation products because of impaired fatty acid oxidation: effects of tetradecylthioacetic acid and eicosapentaenoic acid. Diabetes. 2009 Mar;58(3):527-35. doi: 10.2337/db08-1043. Epub 2008 Dec 9. PMID: 19066312; PMCID: PMC2646050.
Fatty acid incubation of myotubes from humans with type 2 diabetes leads to enhanced release of beta-oxidation products because of impaired fatty acid oxidation: effects of tetradecylthioacetic acid and eicosapentaenoic acid
Tetradecylthioacetic acid (TTA) enhances fatty acid oxidation and energy production in the hearts of Atlantic salmon, as shown through in vivo and in vitro experiments that revealed increased cardiac fatty acid uptake, upregulation of genes for peroxisomal oxidation and lipid metabolism, and improved cardiac performance indicators during early sea transfer.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC2646050/.Lindquist C, Bjørndal B, Bakke HG, Slettom G, Karoliussen M, Rustan AC, Thoresen GH, Skorve J, Nygård OK, Berge RK. A mitochondria-targeted fatty acid analogue influences hepatic glucose metabolism and reduces the plasma insulin/glucose ratio in male Wistar rats. PLoS One. 2019 Sep 24;14(9):e0222558. doi: 10.1371/journal.pone.0222558. PMID: 31550253; PMCID: PMC6759202.
A mitochondria-targeted fatty acid analogue influences hepatic glucose metabolism and reduces the plasma insulin/glucose ratio in male Wistar rats
The mitochondrially targeted fatty acid analogue 1-triple TTA improves glucose tolerance in rats by lowering plasma insulin levels and reducing hepatic glucose and glycogen content, primarily through inhibition of gluconeogenesis and glucose oxidation, while promoting fatty acid oxidation and enhancing pathways like the pentose phosphate cycle, highlighting its potential to shift liver energy metabolism from carbohydrates to lipids.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC6759202/.Burri L, Bjørndal B, Wergedahl H, Berge K, Bohov P, Svardal A, Berge RK. Tetradecylthioacetic acid increases hepatic mitochondrial β-oxidation and alters fatty acid composition in a mouse model of chronic inflammation. Lipids. 2011 Aug;46(8):679-89. doi: 10.1007/s11745-011-3536-2. Epub 2011 Apr 9. PMID: 21479675; PMCID: PMC3131506.
Tetradecylthioacetic acid increases hepatic mitochondrial β-oxidation and alters fatty acid composition in a mouse model of chronic inflammation
Tetradecylthioacetic acid (TTA) improved lipid metabolism in human TNFα transgenic mice by enhancing mitochondrial β-oxidation, lowering plasma triacylglycerol levels, and shifting hepatic fatty acid composition toward a more anti-inflammatory profile, suggesting its potential in treating inflammation-related lipid disorders, though the observed increase in hepatic TAG levels requires further investigation.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3131506/.Grammes F, Takle H. Anti-inflammatory effects of tetradecylthioacetic acid (TTA) in macrophage-like cells from Atlantic salmon (Salmosalar L.). BMC Immunol. 2011 Jul 20;12:41. doi: 10.1186/1471-2172-12-41. PMID: 21774812; PMCID: PMC3161001.
Anti-inflammatory effects of tetradecylthioacetic acid (TTA) in macrophage-like cells from Atlantic salmon (Salmosalar L.)
Tetradecylthioacetic acid (TTA), a synthetic fatty acid used in Atlantic salmon feed, enhances mitochondrial β-oxidation and modulates immune responses by promoting lipid metabolism and inducing anti-inflammatory gene expression in macrophage-like cells, suggesting its potential as a beneficial feed additive to improve fish health and resistance to viral infections.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3161001/.Morken T, Bohov P, Skorve J, Ulvik R, Aukrust P, Berge RK, Livden JK. Anti-inflammatory and hypolipidemic effects of the modified fatty acid tetradecylthioacetic acid in psoriasis–a pilot study. Scand J Clin Lab Invest. 2011 Jul;71(4):269-73. doi: 10.3109/00365513.2011.559552. Epub 2011 Feb 21. PMID: 21338276.
Anti-inflammatory and hypolipidemic effects of the modified fatty acid tetradecylthioacetic acid in psoriasis–a pilot study
Tetradecylthioacetic acid (TTA), a synthetic fatty acid used in Atlantic salmon feed, enhances mitochondrial β-oxidation and modulates immune responses by promoting lipid metabolism and inducing anti-inflammatory gene expression in macrophage-like cells, suggesting its potential as a beneficial feed additive to improve fish health and resistance to viral infections.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3161001/.Bivol LM, Berge RK, Iversen BM. Tetradecylthioacetic acid prevents the inflammatory response in two-kidney, one-clip hypertension. Am J PhysiolRegulIntegr Comp Physiol. 2008 Feb;294(2):R438-47. doi: 10.1152/ajpregu.00590.2007. Epub 2007 Nov 21. PMID: 18032469.
Tetradecylthioacetic acid prevents the inflammatory response in two-kidney, one-clip hypertension
Tetradecylthioacetic acid (TTA) significantly lowers blood pressure and exhibits anti-inflammatory effects in a rat model of two-kidney, one-clip (2K1C) hypertension by inhibiting NF-kappaB activation, reducing proinflammatory cytokines and reactive oxygen species (ROS), and restoring nitric oxide (NO) production to normal levels.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajpregu.00590.2007?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.orgKuiper KK, Salem M, Gudbrandsen OA, Muna ZA, Berge RK, Nordrehaug JE. Dose-dependent coronary artery intimal thickening after local delivery of the anti-oxidant tetradecylthioacetic acid from stents. Atherosclerosis. 2007 Nov;195(1):e39-47. doi: 10.1016/j.atherosclerosis.2007.02.018. Epub 2007 Mar 30. PMID: 17399716.
Dose-dependent coronary artery intimal thickening after local delivery of the anti-oxidant tetradecylthioacetic acid from stents. Atherosclerosis
Tetradecylthioacetic acid (TTA) can be successfully loaded onto phosphorylcholine-coated stents, showing prolonged retention in the coronary vessel wall for up to four weeks; however, it is associated with increased arterial wall inflammation, higher intimal thickness, and reduced anti-inflammatory fatty acid index, indicating a potential pro-inflammatory effect when used in stents.
You can read the abstract of the article at https://www.atherosclerosis-journal.com/article/S0021-9150(07)00123-2/abstract.Stunes AK, Westbroek I, Fossmark R, Berge RK, Reseland JE, Syversen U. Skeletal effects of the saturated 3-thia Fatty Acid tetradecylthioacetic Acid in rats. PPAR Res. 2011;2011:436358. doi: 10.1155/2011/436358. Epub 2011 Dec 5. PMID: 22190907; PMCID: PMC3236357.
Skeletal effects of the saturated 3-thia Fatty Acid tetradecylthioacetic Acid in rats
Tetradecylthioacetic acid (TTA) increases femoral bone mineral density (BMD) and cortical area in normal rats and partially prevents estrogen-related bone loss in ovariectomized rats by maintaining femoral bone mineral content (BMC), trabecular thickness, and cortical volume, suggesting potential skeletal benefits.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3236357/.Tronstad KJ, Bruserud Ø, Berge K, Berge RK. Antiproliferative effects of a non-beta-oxidizable fatty acid, tetradecylthioacetic acid, in native human acute myelogenous leukemia blast cultures. Leukemia. 2002 Nov;16(11):2292-301. doi: 10.1038/sj.leu.2402698. PMID: 12399975.
Antiproliferative effects of a non-beta-oxidizable fatty acid, tetradecylthioacetic acid, in native human acute myelogenous leukemia blast cultures
Tetradecylthioacetic acid (TTA) inhibits the proliferation of acute myeloid leukemia (AML) blasts more effectively than palmitic acid (PA), without affecting normal blood cell counts in healthy volunteers, suggesting TTA’s antiproliferative effects are independent of cytokine secretion and oxidative status.
You can read the abstract of the article at https://www.nature.com/articles/2402698.Lundemo AG, Pettersen CH, Berge K, Berge RK, Schønberg SA. Tetradecylthioacetic acid inhibits proliferation of human SW620 colon cancer cells–gene expression profiling implies endoplasmic reticulum stress. Lipids Health Dis. 2011;10:190. Published 2011 Oct 25. doi:10.1186/1476-511X-10-190.
Tetradecylthioacetic acid inhibits proliferation of human SW620 colon cancer cells–gene expression profiling implies endoplasmic reticulum stress
Tetradecylthioacetic acid (TTA) inhibits the growth of SW620 colon cancer cells by inducing endoplasmic reticulum (ER) stress and activating the unfolded protein response (UPR), leading to changes in gene and protein expression associated with cell cycle inhibition and potential cell death.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3235040/.Jensen LR, Berge K, Bathen TF, Wergedahl H, Schønberg SA, Bofin A, Berge RK, Gribbestad IS. Effect of dietary tetradecylthioacetic acid on colon cancer growth studied by dynamic contrast enhanced MRI. Cancer BiolTher. 2007 Nov;6(11):1810-6. doi: 10.4161/cbt.6.11.4887. Epub 2007 Aug 16. PMID: 18287814.
Effect of dietary tetradecylthioacetic acid on colon cancer growth studied by dynamic contrast enhanced MRI
Tetradecylthioacetic acid (TTA) inhibits the growth of SW620 colon cancer cells in vitro and in vivo, with dynamic contrast-enhanced MRI revealing that TTA also alters tumor vascular properties, indicating its potential for both direct anti-cancer effects and modulation of tumor vasculature.
You can read the abstract of the article at https://www.tandfonline.com/doi/10.4161/cbt.6.11.4887?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.Muna ZA, Bolann BJ, Chen X, Songstad J, Berge RK. Tetradecylthioacetic acid and tetradecylselenoacetic acid inhibit lipid peroxidation and interact with superoxide radical. Free Radic Biol Med. 2000 Apr 1;28(7):1068-78. doi: 10.1016/s0891-5849(00)00196-9. PMID: 10832068.
Tetradecylthioacetic acid and tetradecylselenoacetic acid inhibit lipid peroxidation and interact with superoxide radical
Tetradecylselenoacetic acid (TSA), a selenium-containing analogue of tetradecylthioacetic acid (TTA), shows superior antioxidant effects by increasing LDL oxidation lag time, reducing lipid peroxidation, and interacting with metal ions (iron and copper), suggesting its enhanced potential for preventing oxidative damage.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0891584900001969?via%3Dihub.Bjørndal B, Grimstad T, Cacabelos D, Nylund K, Aasprong OG, Omdal R, Portero-Otin M, Pamplona R, Lied GA, Hausken T, Berge RK. Tetradecylthioacetic acid attenuates inflammation and has antioxidative potential during experimental colitis in rats. Dig Dis Sci. 2013 Jan;58(1):97-106. doi: 10.1007/s10620-012-2321-2. Epub 2012 Aug 2. PMID: 22855292.
Tetradecylthioacetic acid attenuates inflammation and has antioxidative potential during experimental colitis in rats
Tetradecylthioacetic acid (TTA), a pan-PPAR agonist, reduced colonic oxidative damage and pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) in a rat model of dextran sulfate sodium (DSS)-induced colitis, despite not improving overall disease activity, suggesting its potential as an anti-inflammatory agent for colitis.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s10620-012-2321-2.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








