Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Health Benefits of Phentermine
- What is Phentermine?
- How Phentermine Works
- Chemical Structure of Phentermine
- Research on Phentermine
- Associated Side Effects of Phentermine
- Phentermine Pills
- Phentermine Tablets
- Phentermine and Depression
- Phentermine Efficacy
- Phentermine Liquid
- Phentermine Diet Pills
- Phentermine Interactions
- Phentermine Dosage
- FAQ
- Reference
Table of Contents
- Potential Health Benefits of Phentermine
- What is Phentermine?
- How Phentermine Works
- Chemical Structure of Phentermine
- Research on Phentermine
- Associated Side Effects of Phentermine
- Phentermine Pills
- Phentermine Tablets
- Phentermine and Depression
- Phentermine Efficacy
- Phentermine Liquid
- Phentermine Diet Pills
- Phentermine Interactions
- Phentermine Dosage
- FAQ
- Reference
Potential Health Benefits of Phentermine
- Promotes healthy weight loss [2-38]
- Lowers blood pressure [39-45]
- Improves heart health [46-50]
- Treats sleep problems [51-52]
- Lowers cholesterol levels [53-55]
What is Phentermine?
Phentermine is used for a limited period of time (3 to 6 weeks) to significantly reduce weight in overweight and obese individuals. This FDA-approved weight loss medication belongs to a class of drugs known as sympathomimetic amines. It works by suppressing your appetite while increasing your energy expenditure. By stimulating the release of brain chemicals that will manipulate your mind to feel full with lesser food intake, phentermine helps achieve your weight loss goals without any adverse side effects. [1] This drug comes as tablets and extended-release capsules and is sold under the names Adipex or Suprenza.
How does Phentermine Work?
Phentermine is an anorectic drug, which means it is an appetite suppressant. The drug stimulates the release of chemicals, or neurotransmitters, in the brain. The neurotransmitters whose levels are increased by this drug are norepinephrine, dopamine, and serotonin.
Chemical Structure of Phentermine
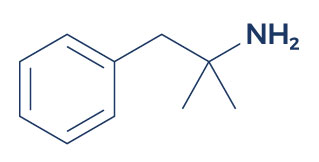
Research on Phentermine
Promotes Healthy Weight Loss
Phentermine is a prescription medication that promotes healthy weight loss by suppressing appetite and increasing energy levels. It stimulates the central nervous system, helping reduce hunger and support calorie restriction, making it easier for individuals to adopt healthier eating habits and exercise routines. Often used in short-term weight management plans, phentermine is most effective when combined with lifestyle changes such as a balanced diet and regular physical activity.
-
- An analysis of several clinical trials found that phentermine can result in a weight loss of 3.6 kg at six months compared with placebo treatment. [2]
- In obese subjects, phentermine administration initially at 15 mg daily and slowly up-titrated to 37.5 mg daily resulted in a 13% reduction in baseline peak weight. [3]
- In obese or overweight patients with weight-related comorbidities, treatment with phentermine consistently demonstrated statistically significant weight loss after 56 weeks compared with placebo. [4-5]
- When combined with other weight loss drugs, phentermine can produce greater weight loss. [6-14]
- In one-hundred-eight obese women, continuous daily phentermine administration resulted in a mean weight loss of 27 pounds (12.2 kg) while intermittent phentermine (4 weeks on therapy and 4 weeks off of therapy) resulted in a mean weight loss of 28.7 pounds (13 kg). [15]
- Several studies also found that phentermine only reduces weight during the time that it is being taken. [16-22]
- When combined with topiramate, an anti-seizure drug, phentermine produced significant weight loss without any adverse side effects. [23-29]
- Studies suggest that phentermine promotes weight loss by inducing changes in eating behavior. [30-34]
- Studies found that overweight and obese patients as young as 3 years and as old as 88 years can be safely treated with phentermine. [35-36]
- An analysis of 6 studies found that patients using 15-30 mg of phentermine daily for 2 to 24 weeks had a mean total weight loss of 6.3 kg. [37]
- In healthy obese people, short-term phentermine administration significantly reduced weight and waist circumference without clinically problematic adverse events. [38]
Lowers Blood Pressure
While phentermine is primarily used as an appetite suppressant for weight loss, its indirect effect of promoting fat reduction can lead to lowered blood pressure in some individuals. As excess weight is a significant contributor to hypertension, the weight loss achieved with phentermine may help reduce strain on the cardiovascular system and improve overall heart health. However, phentermine itself can increase heart rate and blood pressure in some users, so its effects should be monitored by a healthcare professional.
-
- In overweight hypertensive patients, phentermine administration reduced blood pressure and was well tolerated. [39]
- In obese patients, phentermine treatment improved blood pressure and prevented the progression to hypertension. [40]
- The Swedish Obese Subjects (SOS) study found that phentermine reduced the incidence of hypertension by 2.6 times. [41]
- In overweight and obese individuals with type 2 diabetes, phentermine treatment was associated with a 5-mmHg decrease in diastolic blood pressure. [42]
- A recent phentermine clinical trial conducted in healthy obese patients reported a slight decrease in blood pressure. [38]
- In obese patients, administration of a 30 mg capsule of phentermine daily over a period of 12 weeks significantly reduced blood pressure. [43]
- In hypertensive patients, phentermine reduced systolic blood pressure by −7.5 to −11.8 mmHg. [44]
- In both lean and diet-induced obese mice, phentermine significantly reduced blood pressure. [45]
Improves Heart Health
Phentermine may contribute to improved heart health indirectly by promoting weight loss, which can reduce risk factors such as high blood pressure, high cholesterol, and insulin resistance. As excess body weight is a major contributor to cardiovascular disease, shedding pounds with the help of phentermine can ease the workload on the heart and improve overall circulation. However, because phentermine is a stimulant, it must be used under medical supervision to ensure it does not elevate heart rate or blood pressure to unsafe levels.
-
- Patients using phentermine had lower rates of hospitalization for acute myocardial infarction. [46]
- Phentermine increases heart rate due to its amphetamine-like side effect. [47]
- In overweight and obese adults with metabolic abnormalities, phentermine was associated with a slight increase in heart rate (0.1 to 1.3 beats per minute). [48-49]
- Since phentermine promotes weight loss, it can significantly reduce cardiovascular mortality for the following 4-5 years even in patients with pre-existing heart disease. [50]
Treats Sleep Problems
Phentermine is not typically used to treat sleep problems and may actually cause insomnia as a side effect due to its stimulant properties. As a central nervous system stimulant, taking phentermine increases alertness and energy, which can interfere with normal sleep patterns if taken too late in the day. While it aids in weight loss by suppressing appetite and boosting metabolism, individuals taking phentermine should be cautious about its potential impact on sleep and discuss any sleep-related issues with their healthcare provider.
-
- In obese subjects with moderate to severe obstructive sleep apnea who were unable or unwilling to comply with standard treatment, administration of phentermine 15 mg plus extended-release topiramate 92 mg significantly reduced symptoms. [51]
- In obese adults, phentermine treatment reduced the incidence of apnea. [52]
Lowers Cholesterol Levels
Phentermine may help lower cholesterol levels indirectly by promoting weight loss, which is often associated with improvements in lipid profiles. As individuals lose weight, levels of LDL (bad cholesterol) and triglycerides tend to decrease, while HDL (good cholesterol) may increase. By suppressing appetite and supporting calorie reduction, phentermine aids in reducing overall body fat, which plays a crucial role in improving cholesterol balance and supporting cardiovascular health.
-
- In obese patients, phentermine administration resulted in lower total cholesterol and low-density lipoprotein (bad cholesterol) concentrations. [53]
- A review of the metabolic effects of phentermine found that this drug exerted favorable effects on lipid profile, especially on high-density lipoprotein (good cholesterol) and triglycerides. [54]
- In patients with abnormal lipid profiles, phentermine treatment was associated with significant improvements in blood levels of high-density lipoprotein (good cholesterol) and triglyceride as well as a net reduction in lipid-lowering medication use. [55]
Associated Side Effects of Phentermine
Phentermine side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on phentermine. However, these side effects weren’t confirmed to be associated with the treatment and could have been a coincidence and not related to the use of phentermine. Despite this, it was listed as a side effect associated with phentermine even though these associated side effects are very uncommon.
Side effects associated with phentermine may include the following:
- Constipation
- Dry mouth
- Erectile dysfunction
- High blood pressure
- Increased heart rate
- Insomnia
- Nervousness
- Sleeplessness
- Tingling or prickling feeling in the hands and feet
- Tremors
Phentermine Pills
Phentermine is a prescription medication primarily used as an appetite suppressant to aid weight loss in individuals who are overweight or have weight-related medical conditions. It belongs to a class of drugs known as sympathomimetic amines and works by stimulating the central nervous system, which increases heart rate and reduces appetite. Often prescribed as a short-term treatment alongside a low-calorie diet and regular exercise, phentermine helps individuals jumpstart their weight loss journey by decreasing hunger and food cravings.
Phentermine pills come in various forms, including tablets and capsules, and are typically taken once a day, either before breakfast or one to two hours after. The dosage may vary depending on the individual’s medical condition, response to treatment, and body weight. Common brand names include Adipex-P, Lomaira, and Suprenza. Patients using phentermine should be closely monitored by healthcare providers due to the potential for side effects such as increased blood pressure, dizziness, dry mouth, insomnia, or nervousness.
While phentermine can be highly effective in promoting short-term weight loss, it is not a long-term solution and should be used as part of a comprehensive weight management plan. Its use is typically limited to a few weeks, and it’s essential to adopt healthy lifestyle changes for sustainable results. Phentermine is also not suitable for everyone, particularly individuals with a history of cardiovascular disease, hyperthyroidism, or glaucoma. Consulting with a healthcare provider before starting phentermine ensures safe and effective use tailored to individual health needs.
Phentermine Tablets
Phentermine tablets are a widely prescribed weight loss aid designed to help individuals struggling with obesity or weight-related health issues. As an appetite suppressant, phentermine works by stimulating the central nervous system, which helps reduce hunger and increase energy levels. This makes it easier for users to follow a calorie-restricted diet and adopt healthier eating habits. Phentermine tablets are typically used short-term and are most effective when combined with lifestyle changes like improved diet and increased physical activity.
These tablets are available in various strengths, commonly ranging from 8 mg to 37.5 mg, and are usually taken once a day before breakfast or 1 to 2 hours after. The dosage depends on individual factors such as weight, response to the medication, and the presence of any other medical conditions. Brand names for phentermine tablets include Adipex-P and Lomaira. While effective, users may experience side effects such as dry mouth, insomnia, dizziness, or elevated heart rate, so regular monitoring by a healthcare provider is important.
Phentermine tablets are not intended for long-term use due to the risk of dependence and potential cardiovascular side effects. They serve as a jumpstart to weight loss, helping users build momentum toward healthier habits. For best results, phentermine should be part of a comprehensive weight management plan that includes regular exercise, nutritious meals, and behavioral support. As with any medication, it’s crucial to use phentermine under medical supervision to ensure safety and maximize its benefits.
Phentermine and Depression
Phentermine is a prescription medication commonly used as an appetite suppressant to aid weight loss in individuals with obesity. It works by stimulating the central nervous system, which increases heart rate and energy levels while decreasing appetite. While it can be effective for short-term weight management, phentermine is a stimulant similar to amphetamines and may influence mood and mental health in some users.
There have been reports of phentermine both improving and worsening symptoms of depression. For some individuals, the weight loss and increased energy brought on by the medication may lead to an improved mood and better self-esteem. However, for others, especially those with a history of mental health issues, phentermine may exacerbate symptoms of anxiety, irritability, or depression due to its stimulating effects and potential to disrupt sleep, particularly if not paired with a balanced diet and structured exercise program.
Because phentermine can affect neurotransmitter levels, it’s important for users with existing mood disorders to approach it with caution and consult their healthcare provider. Combining phentermine with certain antidepressants, particularly MAO inhibitors or SSRIs, can also lead to serious interactions. Monitoring mental health during phentermine use is essential, and any changes in mood or behavior should be reported to a medical professional promptly.
Phentermine Efficacy
Phentermine has proven to be an effective short-term aid in weight loss, particularly when combined with a reduced-calorie diet, regular physical activity, and behavioral changes. As a sympathomimetic amine, it works by suppressing appetite through the central nervous system, helping users reduce caloric intake. Clinical studies have shown that individuals using phentermine often experience greater weight loss over a few weeks compared to those relying on lifestyle changes alone.
The efficacy of phentermine is typically most noticeable during the initial 12-week treatment period. During this time, users may lose an average of 5% to 10% of their starting body weight, depending on dosage, adherence to dietary recommendations, and individual metabolic differences. The rapid weight reduction can also help improve obesity-related conditions like type 2 diabetes, hypertension, and high cholesterol, making phentermine a valuable tool in medical weight management.
However, phentermine’s long-term efficacy is limited due to its potential for tolerance and side effects. Over time, the appetite-suppressing effects may diminish, and prolonged use increases the risk of dependency or cardiovascular issues. Therefore, healthcare providers typically recommend using phentermine only for short durations, emphasizing the importance of long-term lifestyle changes to sustain weight loss after the medication is discontinued.
Phentermine Liquid
Phentermine liquid is an alternative form of the popular weight loss medication, designed for individuals who may have difficulty swallowing pills or prefer a liquid formulation. Like its tablet and capsule counterparts, liquid phentermine acts as an appetite suppressant by stimulating the central nervous system. This stimulation increases heart rate and energy expenditure while reducing hunger, which can help users maintain a calorie deficit for effective weight loss; however, some users may experience side effects such as feet trouble due to circulation changes or increased physical activity.
The liquid form is usually administered once daily, often in the morning to minimize the risk of insomnia, a common side effect. Dosage can be more easily adjusted with liquid phentermine, offering flexibility for those who may need lower or more precise amounts. It is typically prescribed for short-term use, as part of a broader weight management plan that includes dietary modifications, exercise, and behavioral strategies to support long-term results.
As with other forms of phentermine, the liquid version may cause side effects such as dry mouth, insomnia, increased heart rate, or nervousness. It should be used under the supervision of a healthcare provider, especially in individuals with pre-existing conditions like heart disease or high blood pressure. Although some weight loss aids are available over the counter, phentermine is a prescription medication and should not be confused with over the counter alternatives. While effective for jumpstarting weight loss, liquid phentermine is most successful when used alongside sustainable lifestyle changes rather than as a standalone solution.
Phentermine Diet Pills
Phentermine diet pills are a widely prescribed medication for short-term weight loss, primarily used in individuals with obesity or weight-related health conditions. As an appetite suppressant, phentermine stimulates the central nervous system, increasing energy levels and decreasing hunger signals. However, in rare cases, its stimulating effects may increase the risk of serious cardiovascular events such as a heart attack. This helps users reduce their calorie intake more effectively, making it easier to stick to a low-calorie diet and initiate weight loss.
These diet pills are most commonly prescribed as part of a medically supervised weight loss program that includes a healthy diet, regular physical activity, and behavior modification strategies. While phentermine can significantly aid in reducing body weight, it is not a miracle pill. Its effectiveness relies heavily on the user’s commitment to adopting healthier habits and maintaining them even after the medication course ends.
Although phentermine diet pills are generally considered safe when taken as directed, they can cause side effects such as dry mouth, insomnia, elevated blood pressure, and increased heart rate. They are typically prescribed for a limited duration—usually a few weeks—to jumpstart weight loss. For long-term success, healthcare providers often emphasize the importance of using phentermine as a tool to build lasting lifestyle changes rather than relying on it as a permanent solution, unless a doctor tells you otherwise.
Phentermine Interactions
Phentermine, while effective for short-term weight loss, can interact with various medications and substances, potentially altering its effects or increasing the risk of adverse reactions. One of the most critical interactions is with monoamine oxidase inhibitors (MAOIs); combining these can lead to dangerously high blood pressure and other serious cardiovascular issues, including a rapid spike in high blood pressure that can be life-threatening. In some cases, such interactions may also contribute to decreased interest in daily activities or overall well-being. For this reason, phentermine should not be taken within 14 days of using an MAOI.
Additionally, phentermine may interact with other medications used for weight loss or depression, such as SSRIs, SNRIs, or bupropion. These combinations can increase the risk of serotonin syndrome, a potentially life-threatening condition characterized by confusion, rapid heart rate, and muscle rigidity. It may also amplify stimulant effects when taken with other drugs that affect the central nervous system, leading to jitteriness, anxiety, or heart palpitations.
Patients with chronic conditions like hypertension, diabetes, or thyroid disorders must be especially cautious, as phentermine can affect blood pressure, blood sugar levels, metabolism, and may contribute to symptoms such as walking weakness. It’s essential to inform healthcare providers about all current medications, supplements, and underlying health issues before starting phentermine to ensure safe use and avoid harmful drug interactions that could compromise blood sugar control or the effectiveness of treatment.
Phentermine Dosage
Phentermine dosage varies depending on the specific formulation, patient needs, and medical guidance. It is typically prescribed in strengths ranging from 8 mg to 37.5 mg, with options available as tablets, capsules, or orally disintegrating tablets. The most common dosage is 37.5 mg once daily, usually taken in the morning to avoid sleep disturbances. Some formulations may be divided into smaller doses throughout the day, especially in patients sensitive to stimulants.
The appropriate dosage of phentermine is determined by the patient’s weight loss goals, health status, and how well they tolerate the medication. Physicians often start with a lower dose to assess side effects and gradually adjust based on the patient’s response. It’s important to follow dosing instructions closely, as exceeding the recommended amount can increase the risk of side effects such as insomnia, elevated blood pressure, or dependence, and may also result in changes in performance increased interest.
Because phentermine is intended for short-term use, usually for a few weeks, dosage should not be continued long-term without careful medical supervision. Abrupt discontinuation after prolonged use may lead to withdrawal symptoms, so dosage tapering might be necessary to avoid the following symptoms. Regular follow-ups with a healthcare provider help monitor effectiveness and ensure that the prescribed dosage remains safe and suitable throughout the treatment period.
FAQ
Can I take a laxative with phentermine?
You should consult your doctor before combining phentermine, a controlled substance, with a laxative, as it may increase side effects and risks associated with using a controlled substance.
Is phentermine prescription needed?
Yes, phentermine requires a doctor’s prescription, especially because it can be dangerous for individuals with uncontrolled high blood pressure, and doctors must carefully evaluate patients for conditions like uncontrolled high blood pressure before prescribing it.
What does the drug phentermine do?
Phentermine suppresses appetite to help with weight loss, and understanding how the medication affects the body is important for safe and effective use.
What is the strongest weight loss prescription pill?
Phentermine-topiramate (Qsymia) is considered one of the most effective prescription weight loss pills, but it’s important to follow the prescription label carefully for safe use.
What does phentermine do to your system?
It stimulates the central nervous system to reduce hunger and increase energy expenditure, but if you have a missed dose, follow your doctor’s instructions on how to proceed.
How much weight can you lose with phentermine?
Average weight loss is around 5% to 10% of initial body weight over 12 weeks, but it’s important to stop use and seek medical help if signs of an allergic reaction occur. Symptoms of an allergic reaction may include rash, itching, swelling, severe dizziness, or trouble breathing.
Do doctors still prescribe phentermine for weight loss?
Yes, doctors still prescribe it short-term for weight loss, and it’s important to note that phentermine may also affect blood sugar levels, particularly in people with diabetes.
Is phentermine a safe weight loss drug?
It can be safe for short-term use under medical supervision but has potential risks and side effects, especially for individuals with an overactive thyroid. People with an overactive thyroid should avoid using phentermine due to the increased risk of serious cardiovascular complications.
What are the major side effects of phentermine?
Major side effects include increased heart rate, insomnia, dry mouth, anxiety, and chest pain. If you experience chest pain while taking phentermine, you should seek immediate medical attention. Persistent chest pain could indicate a serious cardiovascular issue that requires prompt evaluation by a healthcare provider.
What do phentermine pills do to your body?
They increase energy and reduce appetite, helping with calorie reduction and weight loss, and belong to a class of medications called anorectics.
How fast will I lose weight on phentermine?
Weight loss can begin within the first week, depending on diet and lifestyle; if you experience any unusual symptoms, contact your doctor immediately.
What kind of narcotic is phentermine?
Phentermine is not a narcotic but a stimulant similar to amphetamines. It may interact with other medicines, so it’s important to inform your doctor about any other medicines you’re taking. Additionally, combining phentermine with other medicines that affect the central nervous system could increase the risk of side effects. Always consult your healthcare provider before using phentermine alongside other medicines.
What do phentermine pills do?
They help suppress appetite and increase energy to support weight loss, but individuals with coronary artery disease should avoid using these medications without medical supervision.
What happens after 3 months of phentermine?
Effects may lessen due to tolerance, and doctors may recommend stopping or switching treatments, especially if there are concerns about conditions like heart valve disease.
How much weight do you lose with phentermine?
Most people lose about 10 to 20 pounds in 12 weeks with proper diet and exercise, but drinking alcohol can interfere with weight loss efforts. Additionally, drinking alcohol while using weight loss medications may increase the risk of side effects.
How safe is phentermine for weight loss?
It is generally safe short-term when prescribed by a healthcare provider, but long-term use is not recommended due to potential side effects and decreased ability to maintain weight loss without medication.
Will a doctor prescribe phentermine?
Yes, if you meet the criteria for weight loss treatment, but it’s important to discuss with your doctor how phentermine might interact with birth control, as some medications can affect the effectiveness of birth control.
How much weight did you lose in 3 months on phentermine?
Weight loss varies but can range from 10 to 20 pounds or more in 3 months, though individuals with kidney disease should consult their doctor before using phentermine.
Is phentermine 37.5 a prescription?
Yes, phentermine 37.5 mg is a prescription-only medication, and some individuals may experience sexual intercourse difficulty as a potential side effect.
Will phentermine cause depression?
In some people, phentermine may contribute to mood changes or depressive symptoms, and those with a history of valvular heart disease should use it with caution under medical supervision.
Is phentermine a stimulant or depressant?
Phentermine is a central nervous system stimulant, and how the medicine affects each individual can vary depending on their overall health and concurrent medications.
Does phentermine affect serotonin levels?
Phentermine primarily affects norepinephrine but may have minor effects on serotonin, and it can lead to trouble sleeping, especially if taken late in the day; trouble sleeping is a common side effect reported by users.
Does phentermine get you in a good mood?
It can initially improve mood and energy, though some may experience irritability or anxiety, and in some cases, it may cause an irregular heartbeat.
What is the success rate of phentermine?
Success rates vary, but many users experience meaningful weight loss within a few months when using this prescription medicine.
What are three common side effects?
Phentermine is a stimulant that suppresses appetite and boosts energy; to answer the question “how does phentermine work”—it primarily stimulates the release of norepinephrine in the brain, which reduces hunger signals.
What to avoid when taking phentermine?
Avoid alcohol, other stimulants, and high-fat or high-sugar foods, as combining these with phentermine may increase the risk of severe mental changes.
Is phentermine hard on your heart?
It can increase heart rate and blood pressure, so it’s not recommended for people with heart conditions, especially since phentermine interact with certain medications can further raise cardiovascular risk.
Can phentermine tablets be crushed?
No, crushing phentermine tablets is not recommended unless directed by a doctor, as the medication is habit forming and altering its form can increase the risk of misuse or side effects.
What can I combine with phentermine to lose weight?
A balanced diet, regular exercise, and behavioral changes enhance phentermine’s effects, but it should not be used during pregnancy due to potential risks to the unborn baby.
Do you swallow phentermine or let it dissolve?
It depends on the formulation; tablets are usually swallowed, while orally disintegrating versions dissolve, both designed to help speed weight loss when used with diet and exercise.
Is phentermine 37.5 extended-release?
No, phentermine 37.5 mg is typically an immediate-release tablet and should not be taken with certain following medications without consulting a healthcare provider.
What does phentermine do for your body?
It reduces hunger and boosts energy to support weight loss, but it should only be used under the supervision of a health care professional. Always consult a health care professional before starting or adjusting phentermine treatment.
Is phentermine the same as Adderall?
No, but both are stimulants; phentermine is for weight loss, Adderall is for ADHD, and phentermine may sometimes cause an unpleasant taste as a side effect.
Is phentermine used for anything else besides weight loss?
It is primarily used for weight loss, with few off-label uses, and it’s important to store phentermine at room temperature away from moisture and heat.
How much weight can you lose taking phentermine?
Users can typically lose 5%–10% of body weight over 12 weeks, depending on the dosage forms prescribed and adherence to lifestyle changes; various dosage forms are available to suit individual treatment needs.
What should you not mix with phentermine?
Avoid MAO inhibitors, alcohol, and other stimulants, as combining these with phentermine may increase the risk of side effects such as skin rash.
What people should not take phentermine?
People with heart disease, hyperthyroidism, glaucoma, or a history of drug abuse should avoid it, as it may also lead to side effects such as legs swelling in some individuals.
Can you drink caffeine while on phentermine?
Caffeine should be limited, as it can worsen side effects like jitteriness and insomnia, and may also affect your bowel movement patterns.
What disqualifies you from taking phentermine?
Conditions like uncontrolled hypertension, cardiovascular disease, or drug abuse history may disqualify you, and you may also experience an unusual sense of discomfort or side effects when using phentermine.
How does phentermine make you lose weight so fast?
It suppresses appetite and increases energy expenditure, leading to reduced calorie intake and potentially lowering the risk of conditions like kidney stones.
What not to do while on phentermine?
Avoid skipping meals, overexerting yourself, or mixing with alcohol or stimulants, as these can interfere with appetite suppression.
Why can you only take phentermine for 3 months?
It’s approved only for short-term use due to risk of dependence and tolerance, and some users may experience side effects such as lower legs trembling.
How quickly does phentermine start working?
Phentermine usually begins working within a few hours of the first dose and may, in some cases, affect sexual ability.
Reference
Chugh PK, Sharma S. Recent advances in the pathophysiology and pharmacological treatment of obesity. J Clin Pharm Ther. 2012;37(5):525-35.
Recent advances in the pathophysiology and pharmacological treatment of obesity
This review explores the growing need for effective obesity treatments, highlighting new weight loss drugs in development that target appetite, fat absorption, energy use, and fat storage, with promising combinations like topiramate-phentermine and bupropion-naltrexone showing improved efficacy and safety, suggesting a hopeful future for obesity pharmacotherapy despite past drug withdrawals.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1111/j.1365-2710.2012.01347.x,Li Z, Maglione M, Tu W, et al. Meta-analysis: pharmacologic treatment of obesity. Ann Intern Med. 2005;142(7):532-46.
Meta-analysis: pharmacologic treatment of obesity
This review evaluates the efficacy and safety of various weight loss medications, finding that FDA-approved drugs like sibutramine, orlistat, phentermine, and others—including bupropion, fluoxetine, and topiramate—can lead to modest weight loss when combined with dietary recommendations, though long-term health outcome data is limited and side effects vary across treatments.
You can read the full article at https://www.acpjournals.org/doi/full/10.7326/0003-4819-142-7-200504050-00012?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.Uwaifo GI, Melcescu E, Mcdonald A, Koch CA. A case of profound weight loss secondary to use of phentermine. J Miss State Med Assoc. 2009;50(12):407-15.
A case of profound weight loss secondary to use of phentermine
This case study highlights the significant weight loss and health improvements in a morbidly obese 34-year-old Choctaw woman following phentermine treatment, demonstrating its potential as an effective, affordable pharmacologic option in managing obesity—especially in high-prevalence areas like Mississippi—while emphasizing the need for further research to clarify its role in obesity care.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/20806811/.Smith SM, Meyer M, Trinkley KE. Phentermine/topiramate for the treatment of obesity. Ann Pharmacother. 2013;47(3):340-9.
Phentermine/topiramate for the treatment of obesity
Phentermine/topiramate (PHEN/TPM) is a once-daily combination drug approved for chronic weight management that has demonstrated significant and sustained weight loss in obese patients across multiple Phase 3 trials, along with improvements in metabolic parameters, though its long-term safety still requires further study.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/23482732/.Cosentino G, Conrad AO, Uwaifo GI. Phentermine and topiramate for the management of obesity: a review. Drug Des DevelTher. 2011;7:267–278. Published 2011 Apr 5. doi:10.2147/DDDT.S31443.
Phentermine and topiramate for the management of obesity: a review
Obesity is a growing global health issue with serious complications and high healthcare costs, requiring long-term, multifaceted treatment strategies; the FDA-approved combination of phentermine and extended-release topiramate (Qsymia™) has shown promising results in clinical trials, offering effective and sustained weight loss and marking a shift toward multi-agent pharmacotherapy for chronic obesity management.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3623549/.Atkinson RL, Blank RC, Schumacher D, Dhurandhar NV, Ritch DL. Long-term drug treatment of obesity in a private practice setting. Obes Res. 1997 Nov;5(6):578–586.
Long-term drug treatment of obesity in a private practice setting
This study found that long-term treatment with phentermine and fenfluramine, combined with lifestyle changes, led to significant and sustained weight loss, improved blood pressure and lipid levels, and was generally safe over an average of 15.9 months in a private practice setting, though dropout rates increased over time and further research is needed beyond three years.
You can read the abstract of the article at https://onlinelibrary.wiley.com/doi/abs/10.1002/j.1550-8528.1997.tb00579.x?sid=nlm%3Apubmed.Spitz AF, Schumacher D, Blank RC, Dhurandhar NV, Atkinson RL. Long-term pharmacologic treatment of morbid obesity in a community practice. EndocrPract. 1997 Sep-Oct;3(5):269–275.
Long-term pharmacologic treatment of morbid obesity in a community practice
This open-label study showed that combining d,l-fenfluramine and phentermine with diet, exercise, and behavioral therapy safely led to significant, sustained weight loss and metabolic improvements in morbidly obese patients over 12–24 months, though long-term compliance remains a challenge.
You can read the abstract of the article at https://www.endocrinepractice.org/article/S1530-891X(20)43441-X/abstract.Astrup A, Breum L, Toubro S, Hein P, Quaade F. The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy restricted diet. A double blind trial. Int J ObesRelatMetabDisord. 1992 Apr;16(4):269–277.
The effect and safety of an ephedrine/caffeine compound compared to ephedrine, caffeine and placebo in obese subjects on an energy restricted diet. A double blind trial
This study found that a combination of ephedrine and caffeine significantly enhanced weight loss in obese patients over 24 weeks compared to placebo, while ephedrine or caffeine alone had no effect, and side effects were mild and transient.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/1318281/.Weintraub M, Sundaresan PR, Schuster B, et al. Long-term weight control study. II (weeks 34 to 104). An open-label study of continuous fenfluramine plus phentermine versus targeted intermittent medication as adjuncts to behavior modification, caloric restriction, and exercise. ClinPharmacolTher. 1992 May;51(5):595–601.
Long-term weight control study. II (weeks 34 to 104). An open-label study of continuous fenfluramine plus phentermine versus targeted intermittent medication as adjuncts to behavior modification, caloric restriction, and exercise
This open-label study compared continuous versus intermittent use of fenfluramine plus phentermine over weeks 34 to 104 as adjuncts to lifestyle changes in long-term weight management, finding both strategies supported sustained weight loss when combined with behavior modification, calorie restriction, and exercise.
You can read the abstract of the article at https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1038/clpt.1992.70?sid=nlm%3Apubmed.Weintraub M, Sundaresan PR, Madan M, et al. Long-term weight control study. I (weeks 0 to 34). The enhancement of behavior modification, caloric restriction, and exercise by fenfluramine plus phentermine versus placebo. ClinPharmacolTher. 1992 May;51(5):586–594.
Long-term weight control study. I (weeks 0 to 34). The enhancement of behavior modification, caloric restriction, and exercise by fenfluramine plus phentermine versus placebo
In a 34-week double-blind trial, combining extended-release fenfluramine and phentermine with behavior modification, calorie restriction, and exercise led to significantly greater weight loss (15.9% vs. 4.9%) than placebo, with mild, transient side effects and high participant satisfaction, supporting the effectiveness of this combination as an adjunctive obesity treatment.
You can read the full article at https://ascpt.onlinelibrary.wiley.com/doi/abs/10.1038/clpt.1992.69?sid=nlm%3Apubmed.Astrup A, Toubro S, Cannon S, Hein P, Madsen J. Thermogenic synergism between ephedrine and caffeine in healthy volunteers: a double-blind, placebo-controlled study. Metabolism. 1991 Mar;40(3):323–329.
Thermogenic synergism between ephedrine and caffeine in healthy volunteers: a double-blind, placebo-controlled study
This study found that combining ephedrine and caffeine, particularly at 20 mg/200 mg, produced a thermogenic effect greater than either alone or in lower combinations, demonstrating a supra-additive synergism in healthy lean subjects, with modest increases in blood pressure and heart rate, supporting its potential use in obesity treatment through enhanced metabolic stimulation.
You can read the abstract of the article at https://www.metabolismjournal.com/article/0026-0495(91)90117-F/abstract.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011 Apr 16;377(9774):1341–1352.
Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial
In this 56-week phase 3 trial, phentermine/topiramate significantly outperformed placebo in promoting weight loss and reducing metabolic risk in overweight and obese adults with comorbidities, with patients on higher doses achieving up to 9.8% weight loss; adverse events were generally mild to moderate and included dry mouth, paraesthesia, and constipation, suggesting the combination is an effective adjunct to lifestyle interventions for obesity management.
You can read the abstract of the article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60205-5/abstract.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J ClinNutr. 2012 Feb;95(2):297–308.
Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study
This 108-week extension study found that phentermine/topiramate controlled-release (PHEN/TPM CR), combined with lifestyle changes, led to significant and sustained weight loss in overweight and obese individuals with cardiometabolic disease, along with improvements in metabolic health and a reduced risk of developing diabetes, while maintaining a favorable safety and tolerability profile over the long term.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3260065/.Allison DB, Gadde KM, Garvey WT, et al. Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP) Obesity (Silver Spring) 2012 Feb;20(2):330–342.
Controlled-release phentermine/topiramate in severely obese adults: a randomized controlled trial (EQUIP)
In this 56-week randomized trial involving individuals with class II and III obesity, phentermine/topiramate controlled-release (PHEN/TPM CR) significantly improved weight loss and metabolic health in a dose-dependent manner, with the higher dose (15/92 mg) leading to an average 10.9% weight reduction and notable improvements in waist circumference, blood pressure, glucose, and lipid levels, while being generally well tolerated with no serious adverse events reported.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3270297/.Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968 Feb 10;1(5588):352–354.
Bray GA. Drug insight: appetite suppressants. Nat ClinPractGastroenterolHepatol. 2005 Feb;2(2):89–95.
Drug insight: appetite suppressants
Appetite suppressants are drugs that primarily act on central nervous system neurotransmitters to reduce food intake, including those that affect norepinephrine, serotonin, GABA, and cannabinoid pathways; while traditional sympathomimetic drugs like phentermine are approved only for short-term use in some countries, newer agents like sibutramine allow for long-term use, and emerging mechanisms are being explored to enhance their role as adjuncts to diet and exercise in weight management.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/16265126/.Makoundou V, Golay A. Drug control of appetite. Rev Med Suisse. 2011 Jan 12;7(277):57–60.
Drug control of appetite
Appetite control is key to obesity treatment, but many older drugs were withdrawn due to serious side effects; newer agents with improved receptor specificity (like lorcaserin) or novel mechanisms (such as GLP-1, leptin, or anti-ghrelin) show promise, and combination therapies (e.g., naltrexone-bupropion, phentermine-topiramate) may enhance effectiveness, though a deeper understanding of appetite dysregulation is still needed to minimize the risk of serious side effects.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/21309176/.Munro JF, MacCuish AC, Wilson EM, Duncan LJ. Comparison of continuous and intermittent anorectic therapy in obesity. Br Med J. 1968 Feb 10;1(5588):352–354.
Phentermine: an appetite-suppressant amphetamine classified as a narcotic in France. Is a combination with topiramate on the horizon? Prescrire Int. Sep;21(130):209.
Phentermine: an appetite-suppressant amphetamine classified as a narcotic in France.Is a combination with topiramate on the horizon?
In France, phentermine faces extra restrictions due to its adverse effects and unproven benefits in preventing obesity-related complications, though it remains widely used in many other countries.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/23016252/.Bray GA. Drug insight: appetite suppressants. Nat ClinPractGastroenterolHepatol. 2005 Feb;2(2):89–95.
Drug insight: appetite suppressants
Appetite suppressants act on central nervous system neurotransmitters to reduce food intake, including drugs that mimic norepinephrine, inhibit neurotransmitter reuptake, or affect specific receptors; while some, like phentermine, are approved for short-term use, others like sibutramine are used long-term, and emerging therapies aim for improved safety and effectiveness as adjuncts to lifestyle changes for weight loss.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/16265126/.Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 2009 Sep;17(9):1730–1735.
How physician obesity specialists use drugs to treat obesity
A survey of obesity specialists in the American Society of Bariatric Physicians revealed widespread use of phentermine, often at doses higher than FDA recommendations, and frequent use of combinations like phentermine with 5-HTP/carbidopa—an unreported regimen—highlighting prescribing practices that diverge from NIH guidelines and suggesting the need for controlled trials to assess the safety and efficacy of such combinations in obesity treatment.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1038/oby.2009.69.Spitz AF, Schumacher D, Blank RC, Dhurandhar NV, Atkinson RL. Long-term pharmacologic treatment of morbid obesity in a community practice. EndocrPract. 1997 Sep-Oct;3(5):269–275.
Long-term pharmacologic treatment of morbid obesity in a community practice
In a 24-month open-label trial involving 298 morbidly obese patients, daily treatment with d,l-fenfluramine and phentermine, combined with diet, exercise, and behavioral support, led to significant and sustained weight loss, along with improvements in metabolic markers such as blood glucose, lipids, and blood pressure, with no serious adverse effects observed; however, high dropout rates highlight the need for strategies to improve long-term adherence.
You can read the abstract of the article at https://www.endocrinepractice.org/article/S1530-891X(20)43441-X/abstract.Cosentino G, Conrad AO, Uwaifo GI. Phentermine and topiramate for the management of obesity: a review. Drug Des DevelTher. 2011;7:267–278. Published 2011 Apr 5. doi:10.2147/DDDT.S31443.
Phentermine and topiramate for the management of obesity: a review
Obesity, a growing global health concern with significant complications and costs, requires a multifaceted treatment approach; while bariatric surgery offers the most robust results, pharmacotherapy has been limited until recently. The FDA-approved combination of phentermine and extended-release topiramate (Qsymia™) represents a promising new option, showing sustained weight loss of ≥10% in over half of patients for up to two years, and marks a shift toward more effective multi-agent therapies for long-term obesity management.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3623549.Singh J, Kumar R. Phentermine-topiramate: First combination drug for obesity. Int J Appl Basic Med Res. 2015;5(2):157–158. doi:10.4103/2229-516X.157177.
Phentermine-topiramate: First combination drug for obesity
Obesity is rapidly increasing worldwide, and while lifestyle modification remains the foundation of treatment, pharmacological options have been limited. In 2012, the FDA approved a fixed-dose combination of immediate-release phentermine and extended-release topiramate for long-term use in obese or overweight patients with comorbidities, showing significant weight loss sustained for up to two years compared to placebo.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4456896/.Malgarini RB, Pimpinella G. Phentermine plus topiramate in the treatment of obesity. Lancet. 2011;378(9786):125-6.
Cameron F, Whiteside G, Mckeage K. Phentermine and topiramate extended release (Qsymia™): first global approval. Drugs. 2012;72(15):2033-42.
Phentermine and topiramate extended release (Qsymia™): first global approval
Vivus’ once-daily oral capsule, Qsymia™—a combination of appetite suppressant phentermine and extended-release topiramate—has been approved in the US for obesity treatment, aiming to reduce appetite and increase satiety; it is also being explored for sleep apnea and type 2 diabetes, with this article outlining its development milestones.
You can read the abstract of the article at https://link.springer.com/article/10.2165/11640860-000000000-00000Bays H. Phentermine, topiramate and their combination for the treatment of adiposopathy (‘sick fat’) and metabolic disease. Expert Rev CardiovascTher. 2010;8(12):1777-801.
Phentermine, topiramate and their combination for the treatment of adiposopathy (‘sick fat’) and metabolic disease
Adiposopathy, or “sick fat,” results from positive caloric balance and contributes to metabolic disease, but weight loss can help reverse these effects; since non-surgical therapies are often limited, combining drugs with complementary actions—like phentermine and topiramate—offers a promising strategy. Phentermine/topiramate controlled-release (PHEN/TPM CR) has shown effectiveness in promoting weight loss and improving metabolic disease linked to adiposopathy, with clinical trials supporting its efficacy and tolerability.
You can read the full article at https://www.tandfonline.com/doi/10.1586/erc.10.125?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.Bays HE, Gadde KM. Phentermine/topiramate for weight reduction and treatment of adverse metabolic consequences in obesity. Drugs Today. 2011;47(12):903-14.
Phentermine/topiramate for weight reduction and treatment of adverse metabolic consequences in obesity
Phentermine, a noradrenergic agent for short-term obesity treatment, and topiramate, approved for seizures and migraines, both promote weight loss and have no reported adverse interactions when combined. Their controlled-release formulation in varying doses has demonstrated effective weight reduction and improvements in metabolic conditions related to adiposopathy, with side effects consistent with those of the individual drugs.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/22348915/.Bays HE, Gadde KM. Phentermine/topiramate for weight reduction and treatment of adverse metabolic consequences in obesity. Drugs Today. 2011;47(12):903-14.
Phentermine/topiramate for weight reduction and treatment of adverse metabolic consequences in obesity
Phentermine, a noradrenergic agent for short-term obesity treatment, and topiramate, approved for seizures and migraines, both promote weight loss and have no reported adverse interactions when combined. Their controlled-release formulation in varying doses has demonstrated effective weight reduction and improvements in metabolic conditions related to adiposopathy, with side effects consistent with those of the individual drugs.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/22348915/.Halford JC, Boyland EJ, Blundell JE, Kirkham TC, Harrold JA. Pharmacological management of appetite expression in obesity. Nat Rev Endocrinol. 2010;6(5):255–269.
Pharmacological management of appetite expression in obesity
Traditional obesity drugs often overlook psychological drivers of appetite, focusing narrowly on intake and weight, but newer therapies—such as GLP-1 analogs, serotonin receptor agonists, and combination treatments like phentermine-topiramate—aim to target appetite expression more effectively; however, their behavioral impacts remain underexplored, and future success may lie in tailoring pharmacotherapy to modulate eating desire, enjoyment, satiation, and satiety.
You can read the abstract of the article at https://www.nature.com/articles/nrendo.2010.19.Greenway FL, Fujioka K, Plodkowski RA, et al. Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2010;376(9741):595–605.
Effect of naltrexone plus bupropion on weight loss in overweight and obese adults (COR-I): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial
The COR-I study found that a sustained-release combination of naltrexone and bupropion significantly reduced body weight in overweight and obese individuals compared to placebo, with nearly half of participants on the higher dose achieving at least 5% weight loss over 56 weeks; while common side effects included nausea and headache, the treatment was generally well tolerated and not linked to increased depression or suicidality.
You can read the abstract of the article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(10)60888-4/abstractRebello CJ, Greenway FL. Reward-induced eating: therapeutic approaches to addressing food cravings. AdvTher. 2016;33(11):1853–1866.
Reward-induced eating: therapeutic approaches to addressing food cravings
Appetite regulation is a complex interaction between internal metabolic needs and external food-related cues, with cravings—driven by brain reward pathways—playing a key role in overeating in today’s food-rich environment; understanding this neurocircuitry has led to treatments like naltrexone-bupropion, lorcaserin, phentermine, and liraglutide, which may help control cravings and support long-term weight management.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC5083777/.Thomas EA, Mcnair B, Bechtell JL, Ferland A, Cornier MA, Eckel RH. Greater hunger and less restraint predict weight loss success with phentermine treatment. Obesity (Silver Spring) 2016;24(1):37–43.
Greater hunger and less restraint predict weight loss success with phentermine treatment
This study found that individuals who reported higher baseline hunger and lower cognitive restraint were more likely to achieve significant weight loss with phentermine over 8 weeks, suggesting that baseline appetite-related behaviors may help predict who will benefit most from phentermine treatment.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4688167/.Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring) 2011;19(12):2351–2360.
Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity
This study found that phentermine treatment in obese patients did not increase blood pressure or heart rate and was associated with greater weight loss compared to those not treated with phentermine; moreover, it contributed to favorable blood pressure changes and potentially slowed the progression to hypertension.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1038/oby.2011.94.Lorber J. Obesity in childhood. A controlled trial of anorectic drugs. Arch Dis Child. 1966;41(217):309–312.
Hendricks EJ, Srisurapanont M, Schmidt SL, et al. Addiction potential of phentermine prescribed during long-term treatment of obesity. Int J Obes (Lond) 2014;38(2):292–298.
Addiction potential of phentermine prescribed during long-term treatment of obesity
This study found no evidence that phentermine causes abuse, psychological dependence, or drug craving in obese patients, even after long-term use of up to 21 years, and abrupt discontinuation did not result in amphetamine-like withdrawal symptoms, suggesting phentermine has low addiction potential.
You can read the abstract of the article at https://www.nature.com/articles/ijo201374.Haddock CK, Poston WS, Dill PL, Foreyt JP, Ericsson M. Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials. Int J ObesRelatMetabDisord. 2002;26(2):262–273.
Pharmacotherapy for obesity: a quantitative analysis of four decades of published randomized clinical trials
This meta-analysis of 108 randomized clinical trials found that medications for obesity generally produced medium effect sizes, with four drugs (amphetamine, benzphetamine, fenfluramine, and sibutramine) showing large effects, though no single drug or class stood out as clearly superior, and placebo-subtracted weight loss for individual drugs never exceeded 4.0 kg.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/11850760/.Kim KK, Cho HJ, Kang HC, Youn BB, Lee KR. Effects on weight reduction and safety of short-term phentermine administration in Korean obese people. Yonsei Med J. 2006;47(5):614–625. doi:10.3349/ymj.2006.47.5.614.
Effects on weight reduction and safety of short-term phentermine administration in Korean obese people
In a randomized, double-blind, placebo-controlled study in Korea, short-term treatment with phentermine 37.5 mg significantly reduced body weight and waist circumference in obese adults without causing clinically concerning side effects; dry mouth and insomnia were the only notable adverse events, and overall, the drug was well tolerated.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC2687747/.-
Dramatic weight loss benefits with combination phentermine, topiramate
A phase 3 study of 2,500 overweight patients with metabolic comorbidities showed that combination therapy with phentermine and topiramate resulted in significantly greater weight loss and cardiometabolic improvements compared to placebo, with the high-dose group achieving nearly 10% weight loss and notable improvements in lipids, glycemic control, and inflammation markers; both doses were well tolerated with only mild side effects.
You can read the abstract of the article at https://www.healio.com/news/endocrinology/20120331/dramatic-weight-loss-benefits-with-combination-phentermine-topiramate. Hendricks EJ, Greenway FL, Westman EC, Gupta AK. Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity. Obesity (Silver Spring). 2011;19(12):2351-60.
Blood pressure and heart rate effects, weight loss and maintenance during long-term phentermine pharmacotherapy for obesity
This study found that phentermine treatment for obesity, combined with a low-carb ketogenic diet, did not increase blood pressure or heart rate and was associated with greater weight loss and favorable shifts in blood pressure categories, suggesting it may help slow the progression to hypertension without adding cardiovascular risk.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1038/oby.2011.94.Torgerson JS, Sjöström L. The Swedish Obese Subjects (SOS) study–rationale and results. Int J ObesRelatMetabDisord. 2001;25Suppl 1:S2-4.
The Swedish Obese Subjects (SOS) study–rationale and results
The Swedish Obese Subjects (SOS) study showed that significant weight loss from obesity surgery led to major reductions in diabetes and hypertension incidence over two years, with a lasting fivefold lower diabetes risk after eight years, though hypertension incidence eventually equaled that of controls; long-term effects on mortality remain uncertain.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/11466577/.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34(7):1481-6.
Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes
In the Look AHEAD study, overweight and obese individuals with type 2 diabetes who lost 5–10% of their body weight in one year saw significant improvements in cardiovascular risk factors such as blood pressure, triglycerides, HDL cholesterol, and HbA1c, with even greater benefits observed in those who lost 10–15%, supporting the recommendation for modest weight loss to improve health outcomes.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3120182/.Vallé-jones JC, Brodie NH, O’hara H, O’hara J, Mcghie RL. A comparative study of phentermine and diethylpropion in the treatment of obese patients in general practice. Pharmatherapeutica. 1983;3(5):300-4.
A comparative study of phentermine and diethylpropion in the treatment of obese patients in general practice
In a 12-week general practice study comparing phentermine and diethylpropion for weight loss, patients on phentermine experienced significantly greater weight loss—especially in the final 4 weeks—along with reductions in blood pressure and heart rate likely due to weight loss, while both medications had similar and generally mild side effects.
You can read the full article at https://pubmed.ncbi.nlm.nih.gov/6844367.Davidson MH, Tonstad S, Oparil S, Schwiers M, Day WW, Bowden CH. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m(2). Am J Cardiol. 2013;111(8):1131-8.
Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m(2)
In the 56-week CONQUER trial, treatment with phentermine/topiramate extended-release led to dose-dependent weight loss and significant improvements in cardiovascular risk factors—such as reduced triglycerides, non-HDL cholesterol, and blood pressure—in obese patients with dyslipidemia or hypertension, especially among those who lost at least 5% of their baseline weight.
You can read the full article at https://www.ajconline.org/article/S0002-9149(12)02641-0/fulltext.Simonds SE, Pryor JT, Koegler FH, et al. Determining the Effects of Combined Liraglutide and Phentermine on Metabolic Parameters, Blood Pressure, and Heart Rate in Lean and Obese Male Mice. Diabetes. 2019;68(4):683-695.
Determining the Effects of Combined Liraglutide and Phentermine on Metabolic Parameters, Blood Pressure, and Heart Rate in Lean and Obese Male Mice
In a 21-day mouse study, combining liraglutide and phentermine led to the greatest weight loss in both lean and obese mice compared to either drug alone, but while it caused modest cardiovascular improvements in obese mice, it raised heart rate and reduced blood pressure in lean mice, highlighting the need to carefully assess the cardiovascular effects of this combination therapy.
You can read the full article at https://diabetesjournals.org/diabetes/article/68/4/683/39785/Determining-the-Effects-of-Combined-Liraglutide.Ritchey ME, Harding A, Hunter S, et al. Cardiovascular Safety During and After Use of Phentermine and Topiramate. J ClinEndocrinolMetab. 2019;104(2):513-522.
Cardiovascular Safety During and After Use of Phentermine and Topiramate
A retrospective study found no increased risk of major adverse cardiovascular events (MACE) in current users of phentermine/topiramate (PHEN/TPM), including fixed-dose combinations, compared to unexposed periods, although confidence intervals were wide due to the rarity of events; interestingly, topiramate alone was associated with a higher MACE risk.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC6318778/.Jordan J, Astrup A, Engeli S, Narkiewicz K, Day WW, Finer N. Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity. J Hypertens. 2014;32(6):1178–1188. doi:10.1097/HJH.0000000000000145.
Cardiovascular effects of phentermine and topiramate: a new drug combination for the treatment of obesity
Phentermine and topiramate, individually and in combination, have shown effectiveness in weight loss, and long-term data suggest their extended-release combination may be a safe and effective treatment for overweight or obese adults with low-to-intermediate cardiovascular risk, despite past concerns about heart safety with weight loss drugs.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC4011567/.Davidson MH, Tonstad S, Oparil S, Schwiers M, Day WW, Bowden CH. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m2. Am J Cardiol 2013; 111:1131–1138.
Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m(2)
In the 56-week CONQUER trial, treatment with phentermine/topiramate extended-release led to dose-dependent weight loss and significant improvements in cardiovascular risk factors—such as reduced triglycerides, non-HDL cholesterol, and blood pressure—in obese patients with dyslipidemia or hypertension, especially among those who lost at least 5% of their baseline weight.
You can read the full article at https://www.ajconline.org/article/S0002-9149(12)02641-0/fulltext.Gadde KM, Allison DB, Ryan DH, Peterson CA, Troupin B, Schwiers ML, Day WW. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet 2011; 377:1341–1352.
Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial
In a 56-week phase 3 trial, phentermine/topiramate controlled-release significantly enhanced weight loss and metabolic risk reduction in overweight and obese adults with comorbidities, with patients receiving the higher dose losing an average of 10.2 kg and 70% achieving at least 5% weight loss—compared to 1.4 kg and 21% with placebo—though the treatment was associated with dose-related increases in side effects such as dry mouth, paraesthesia, and insomnia.
You can read the abstract of the article at https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(11)60205-5/abstract.Caterson ID, Finer N, Coutinho W, et al. Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial. Diabetes ObesMetab. 2012;14(6):523-30.
Maintained intentional weight loss reduces cardiovascular outcomes: results from the Sibutramine Cardiovascular OUTcomes (SCOUT) trial
In the SCOUT trial, modest weight loss of 3–10 kg over 6 to 12 months was associated with reduced cardiovascular mortality over 4–5 years in overweight or obese individuals with existing cardiovascular disease or type 2 diabetes, despite the sibutramine group experiencing more cardiovascular events overall; greater weight loss appeared to offset this increased risk.
You can read the abstract of the article at https://dom-pubs.pericles-prod.literatumonline.com/doi/10.1111/j.1463-1326.2011.01554.x.Winslow DH, Bowden CH, Didonato KP, Mccullough PA. A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults. Sleep. 2012;35(11):1529-39.
A randomized, double-blind, placebo-controlled study of an oral, extended-release formulation of phentermine/topiramate for the treatment of obstructive sleep apnea in obese adults
In a 28-week randomized trial, phentermine 15 mg plus extended-release topiramate 92 mg significantly reduced apnea-hypopnea index, body weight, blood pressure, and improved oxygen saturation in obese adults with moderate to severe obstructive sleep apnea not using PAP therapy, suggesting it may be an effective alternative for managing OSA through weight loss.
You can read the full article at https://pmc.ncbi.nlm.nih.gov/articles/PMC3466800/.-
Study Finds Significant Improvements In Patients With Obstructive Sleep Apnea Treated With Phentermine And Topiramate Extended-Release Capsules
You can read the full article atA 28-week randomized, placebo-controlled study published in SLEEP found that phentermine and topiramate extended-release capsules significantly improved obstructive sleep apnea (OSA) severity and cardiovascular risk factors in obese adults not using positive airway pressure (PAP) therapy. Participants receiving the drug saw their apnea-hypopnea index drop from severe to mild levels, lost an average of 10.3% of their body weight, and showed improvements in blood pressure and oxygen saturation compared to placebo. These findings suggest that the combination treatment, though not currently approved for OSA, may be a promising alternative for patients unable to tolerate PAP therapy.
You can read the full article at https://journals.lww.com/ebp/Abstract/2018/04000/Does_phentermine_improve_cardiovascular_outcomes.24.aspx.
You can read the full article at 53. Available from https://journals.lww.com/ebp/Abstract/2018/04000/Does_phentermine_improve_cardiovascular_outcomes.24.aspx.
Does phentermine improve cardiovascular outcomes in obese patients who lose weight taking this medication?
The impact of phentermine on patient-oriented cardiovascular outcomes in obese individuals remains unclear, though it does not lower blood pressure or heart rate more than diet alone; however, it may reduce total and LDL cholesterol levels.
You can read the full article at https://journals.lww.com/ebp/Abstract/2018/04000/Does_phentermine_improve_cardiovascular_outcomes.24.aspx.Kiortsis DN. A review of the metabolic effects of controlled-release Phentermine/Topiramate. Hormones (Athens). 2013;12(4):507-16.
A review of the metabolic effects of controlled-release Phentermine/Topiramate
Phentermine/topiramate controlled-release (PHEN/TPM CR), recently FDA-approved for chronic weight management, has been shown in clinical trials to produce significant weight loss, improve metabolic markers like blood pressure, lipid profile, and insulin sensitivity, and reduce the risk of type 2 diabetes, though it may slightly increase heart rate and cause mild side effects; its long-term safety and efficacy remain to be fully established.
You can read the abstract of the article at https://link.springer.com/article/10.14310/horm.2002.1438.Davidson MH, Tonstad S, Oparil S, Schwiers M, Day WW, Bowden CH. Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m2. Am J Cardiol 2013; 111:1131–1138.
Changes in cardiovascular risk associated with phentermine and topiramate extended-release in participants with comorbidities and a body mass index ≥27 kg/m2
In the 56-week CONQUER trial, treatment with phentermine/topiramate extended-release (PHEN/TPM ER) led to significant, dose-related weight loss and improvements in cardiovascular risk factors—such as reductions in triglycerides, non-HDL cholesterol, and systolic blood pressure—in obese patients with dyslipidemia or hypertension, highlighting the potential of combining pharmacotherapy with lifestyle changes to reduce cardiovascular risk in this population.
You can read the full article at https://www.ajconline.org/article/S0002-9149(12)02641-0/fulltext.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








