Health Library
Cardarine (GW-501516)
Author: Dr. George Shanlikian, M.D. | Last Updated: May 22nd, 2025
- Home
- >
- Health Library
- >
- Cardarine (GW-501516)
Peptides
- 5-amino-1MQ
- Aminophylline
- Aniracetam
- ARA 290
- Argireline + Leuphasyl
- BPC-157
- Bremelanotide
- Cerebrolysin
- CJC-1295
- Delta Sleep-Inducing Peptide
- Dihexa
- Elampretide (SS-31)
- Epithalon
- FG Loop Peptide (FGL)
- GHK-Cu
- Ginsenoside Rg3
- Glycyrrhetinic Acid
- Ipamorelin
- Kisspeptin
- KPV
- LL-37
- Melanotan 1
- Melanotan 2
- Mitochondrial ORF of the twelve S c (MOTS-c)
- MK-677 (IBUTAMOREN)
- Nicotinamide Adenine Dinucleotide (NAD+)
- Nicotinamide Riboside
- NMN (Nicotinamide Mononucleotide)
- Noopept
- Pegylated Mechano Growth Factor
- Selank
- Semax
- Sermorelin
- SRT2104
- Tesamorelin
- Thymosin Alpha 1
- Thymosin Beta 4
- Tiger 17
- Valproic Acid
- Valproic acid + PTD-DBM
- Vasoactive Intestinal Peptide
- Zinc-Thymulin
- Potential Health Benefits of Cardarine
- What is Cardarine (GW-501516)?
- How does Cardarine work?
- Chemical Structure of Cardarine
- Research on Cardarine
- Cardarine Side Effects
- Cardarine vs Ostarine
- Cardarine Cycle Length
- Cardarine Capsules
- Cardarine and Alcohol
- Cardarine Blood Work
- Cardarine Rad140 Stack
- How Long does Cardarine Take to Work
- Cardarine Supplement
- Cardarine for Women
- Cardarine Dosage for Males
- When to Take Cardarine
- Cardarine Dosage
- Ostarine and Cardarine Stack
- Ostarine and Cardarine
- What does Cardarine Do
- Stenabolic vs Cardarine
- FAQ
- References
Table of Contents
- Potential Health Benefits of Cardarine
- What is Cardarine (GW-501516)?
- How does Cardarine work?
- Chemical Structure of Cardarine
- Research on Cardarine
- Cardarine Side Effects
- Cardarine vs Ostarine
- Cardarine Cycle Length
- Cardarine Capsules
- Cardarine and Alcohol
- Cardarine Blood Work
- Cardarine Rad140 Stack
- How Long does Cardarine Take to Work
- Cardarine Supplement
- Cardarine for Women
- Cardarine Dosage for Males
- When to Take Cardarine
- Cardarine Dosage
- Ostarine and Cardarine Stack
- Ostarine and Cardarine
- What does Cardarine Do
- Stenabolic vs Cardarine
- FAQ
- References
Potential Health Benefits of Cardarine
Cardarine benefits include enhanced endurance, improved fat metabolism, and increased energy efficiency by activating the PPAR-delta pathway. It may also support cardiovascular health and aid in reducing inflammation, making it popular among athletes and individuals seeking metabolic improvements.
- Promotes fat loss [1-9]
- Increases muscle mass and strength [10-16]
- Improves exercise endurance [14, 17-18]
- Lowers cholesterol levels [4, 19-24]
- Improves brain health [25-29]
- Lowers risk of heart disease [1, 30-34]
- Improves blood sugar levels [14, 35-39]
- Fights kidney disease [40-43]
- Improves liver health [39, 44-51]
- Prevents and Treats Cancer [52-54]
Key Takeaways
- Enhances Endurance – Cardarine activates the PPAR-delta pathway, improving stamina and overall physical performance.
- Boosts Fat Metabolism – It promotes fat oxidation, helping the body burn stored fat more efficiently.
- Supports Cardiovascular Health – Research suggests it may improve heart health by reducing inflammation and enhancing blood vessel function.
- Increases Energy Efficiency – By optimizing mitochondrial activity, Cardarine helps the body use energy more effectively.
- Not a Steroid – Unlike anabolic steroids, Cardarine does not affect hormone levels, making it a distinct performance-enhancing compound.
What is Cardarine (GW-501516)?
Cardarine, also known by GW501516, was initially prescribed for the treatment of various disorders related to elevated cholesterol levels such as atherosclerosis, myocardial infarction, stroke, and other blood vessel diseases. Today, this drug has gained popularity among athletes and bodybuilders due to its ability to improve muscle strength and exercise endurance. Researchers believe that cardarine exerts its beneficial effects by activating the peroxisome proliferator-activated receptor-delta (PPAR-delta) pathway. Activation of the PPAR-delta pathway is associated with increased energy levels, fat reduction, muscle building, increased endurance, and decreased blood levels of cholesterol.
How does Cardarine work?
Cardarine works by increasing muscle cell metabolism and decreasing fat deposits by stimulating lipolysis or fat breakdown. Its active ingredient can significantly increase muscle growth and endurance. Cardarine also helps regulate cholesterol levels and maintain liver health. All of these beneficial effects can be attributed to cardarine’s ability to activate the peroxisome proliferator-activated receptor-delta (PPAR-delta) pathway.
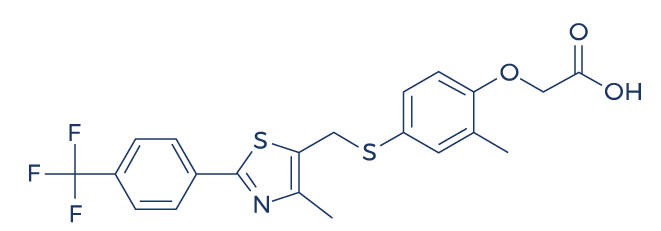
Chemical Structure of Cardarine
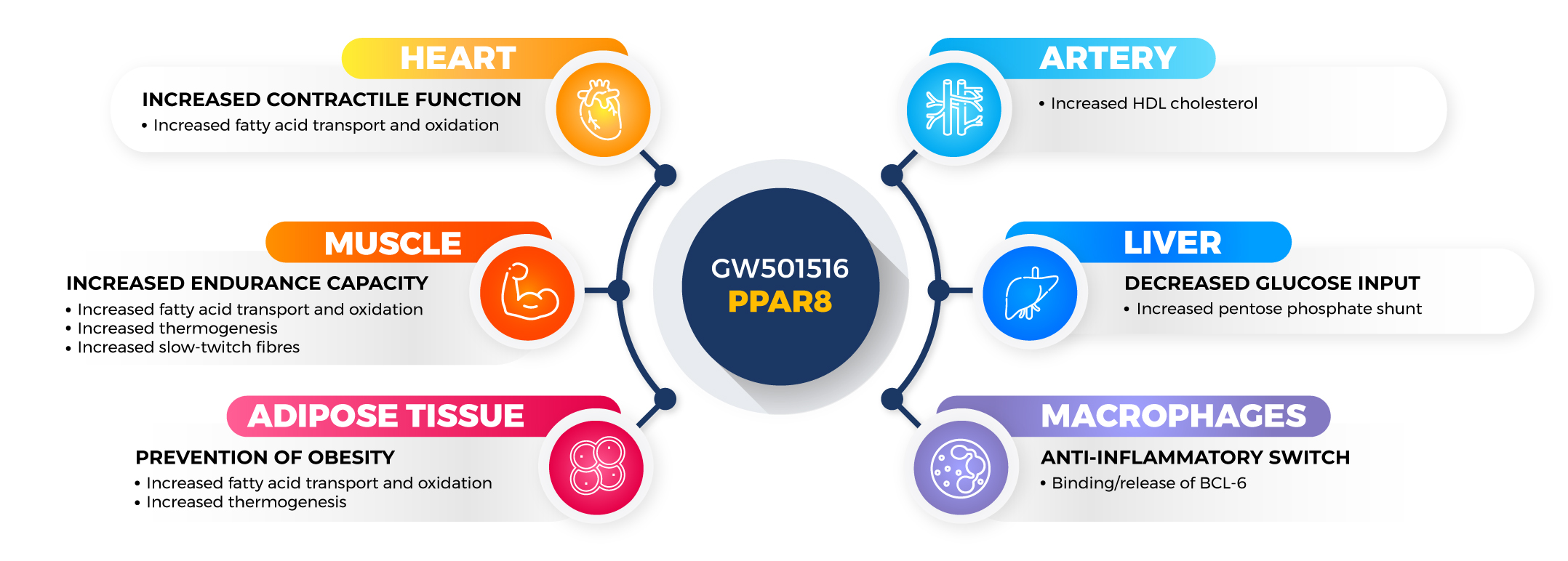
Research on Cardarine
Promotes Fat Loss

Cardarine (GW501516) is a compound that has gained popularity for its potential to promote fat loss, primarily by enhancing fat oxidation and increasing endurance during physical activity. It works by activating the peroxisome proliferator-activated receptor delta (PPARδ), which leads to increased mitochondrial activity, boosting the body’s ability to burn fat for energy rather than storing it. This can result in improved exercise performance, greater energy expenditure, and more efficient fat utilization, making it a potential aid in weight management and body composition improvement. However, it’s important to note that Cardarine’s use is controversial due to safety concerns and its ban by major sporting organizations.
- In men with high belly fat, administration of 2.5 mg of cardarine daily for 6 weeks resulted in weight reduction. [1]
- In inactive volunteers, subjects treated with cardarine burned more fats (20%) compared to untreated subjects. [2]
- In mice, cardarine protected against diet-induced obesity. [3]
- In moderately obese men, cardarine reduced weight by increasing fatty acid oxidation. [4]
- In overweight and obese men and women, cardarine reduced body weight by improving cholesterol profile. [5]
- A study found that cardarine can significantly reduce weight by correcting the causes of insulin resistance and abnormal cholesterol profile. [6]
- A study also found that cardarine helps reduce weight by increasing energy expenditure in muscles. [7]
- In overweight and obese healthy volunteers, cardarine treatment at a dose of 10 mg for 12 weeks reduced body fat levels. [8]
- A cell study found that cardarine activates pathways involved in fat metabolism. [9]
Increases Muscle Mass and Strength

Cardarine, also known as GW-501516, is often touted for its potential to enhance endurance and fat burning, but some claim it can indirectly promote muscle mass and strength by improving exercise performance. By enhancing the body’s ability to burn fat for fuel, Cardarine can help athletes maintain lean body mass during training, allowing them to focus more on strength-building exercises. Its role in improving cardiovascular endurance and stamina enables individuals to engage in longer, more intense workouts, which, over time, can lead to increased muscle mass and enhanced strength. However, its direct effects on muscle growth are not as pronounced as those of anabolic agents or other performance enhancers.
- Studies show that cardarine improves muscle health by regulating many different biological activities such as skeletal reprogramming, mitochondrial respiration, lipid and lipoprotein metabolism, body heat production, and muscle regeneration. [10-12]
- Studies show that cardarine improves exercise-induced reprogramming of muscle fibers by regulating the formation of genes associated with contractile proteins. [13]
- A study found that trained mice treated with cardarine had 113% more muscle fibers than untrained sedentary mice. [14]
- In mice with muscle abnormalities, cardarine treatment restored the integrity of skeletal muscle fibers. [15]
- A study found that PPAR-delta activator like cardarine regulates muscle fiber contraction and metabolism. [16]
Improves Exercise Endurance
Cardarine (GW501516) is a popular compound known for its potential to enhance exercise endurance. It works by activating the PPAR-δ receptor, which in turn boosts fat metabolism and increases the body’s ability to utilize stored fat for energy, sparing glycogen during prolonged physical activity. This mechanism can lead to improved stamina, allowing for longer and more intense workouts. Many users report experiencing enhanced endurance, quicker recovery times, and greater overall performance in endurance-based activities like running, cycling, and swimming. However, it’s important to note that the long-term safety and efficacy of Cardarine are still subjects of ongoing research.
- In both trained and untrained mice, cardarine treatment enhanced running endurance. [14]
- In adult mice, cardarine administration along with exercise training improved running endurance. [17]
- In sedentary mice, cardarine administration improved running endurance by preserving blood sugar. [18]
Lowers Cholesterol Levels
Cardarine (GW-501516) is often associated with benefits in improving cardiovascular health, particularly by lowering cholesterol levels. Studies suggest that Cardarine may help reduce LDL (bad) cholesterol while increasing HDL (good) cholesterol, which contributes to better lipid profiles and a reduced risk of heart disease. This compound works by activating the PPAR-δ receptor, which regulates fat metabolism and enhances the oxidation of fatty acids. As a result, it can support better cholesterol regulation and improve overall cardiovascular health, although it should be used with caution and under proper supervision due to potential side effects.
- In moderately obese men, treatment with cardarine reduced low density lipoprotein cholesterol by 23%. [4]
- In subjects with abnormally low high-density lipoprotein cholesterol levels, cardarine increased the levels of good cholesterol in just 12 weeks of treatment. [19]
- In patients with abnormal cholesterol profile, cardarine treatment reduced low density lipoprotein and increased high density lipoprotein cholesterol levels. [20-21]
- In healthy volunteers, cardarine enhanced the levels of high-density lipoprotein. [22]
- Administration of cardarine in patients with abnormal cholesterol profile for 12 weeks significantly reduced low-density lipoprotein. [23]
- In insulin-resistant middle-aged obese rhesus monkeys, cardarine administration resulted in a dramatic dose-dependent rise in blood levels of high-density lipoprotein cholesterol while lowering the levels of small-dense low-density lipoprotein and fasting triglycerides. [24]
Improves Brain Health
Cardarine (GW-501516) is primarily known for its effects on endurance and fat metabolism, but there is some evidence suggesting it may also have potential benefits for brain health. It activates the PPARδ receptor, which plays a role in improving mitochondrial function and enhancing neuroprotection. This could help reduce oxidative stress and inflammation in the brain, potentially supporting cognitive function and reducing the risk of neurodegenerative conditions. However, while studies in animals have shown promise, further research is needed to fully understand its impact on human brain health and its long-term safety.
- In mice, cardarine improved cognitive function by increasing blood flow to the brain. [25]
- In rat brain cells, treatment with cardarine reduced brain inflammation. [26]
- A cell study found that cardarine can lower the risk for central nervous system disorders by modulating inflammatory signaling network in the cells of the immune system. [27]
- In mice, cardarine improved spatial memory by promoting formation of new neurons (neurogenesis). [28]
- In a model of brain inflammation, cardarine protected against nerve cell damage by reducing inflammatory processes. [29]
Lowers Risk of Heart Disease
Cardarine (GW501516) is a synthetic compound that has been shown to potentially lower the risk of heart disease by improving lipid profiles and enhancing cardiovascular health. It works by activating the peroxisome proliferator-activated receptor delta (PPARδ), which plays a crucial role in fat metabolism and energy expenditure. By increasing the oxidation of fatty acids, Cardarine helps reduce triglyceride levels and raises high-density lipoprotein (HDL) cholesterol, often referred to as “good” cholesterol. Additionally, it may reduce the accumulation of visceral fat and improve overall blood vessel function, which can contribute to a reduced risk of atherosclerosis and other heart-related issues. However, its long-term safety and efficacy require further research to fully understand its impact on heart health.
- Cardarine may reduce the risk of plaque build-up in the arteries of the heart (atherosclerosis) by relaxing/widening the blood vessels via nitric oxide production. [1]
- Cardarine prevents atherosclerosis by antagonizing multiple proinflammatory pathways. [30]
- Cardarine also has the ability to prevent the formation of lesions. [31-32]
- Cardarine stimulates the growth of new blood vessels in the heart by boosting the levels of vascular endothelial growth factor (VEGF). [33]
- Cardarine improves overall heart health by regulating cardiomyocyte (heart cell) proliferation and cardiac repair. [34]
Improves Blood Sugar Levels
Cardarine (GW501516) has been shown to improve blood sugar levels by enhancing insulin sensitivity and promoting glucose uptake in muscles. This can lead to more efficient use of glucose, preventing excess buildup in the bloodstream. It works by activating the peroxisome proliferator-activated receptor delta (PPAR-δ), which increases fatty acid oxidation and helps regulate energy metabolism. As a result, Cardarine may support better blood sugar control, particularly in individuals with insulin resistance or metabolic syndrome, although its use should be approached with caution due to concerns over long-term safety.
- In mice, cardarine enhanced specific consumption of fatty acids and reduced blood sugar utilization. [14]
- In high fat-fed rats and mice, cardarine improved insulin response which in turn reduced blood sugar levels. [35]
- In a mouse model of metabolic syndrome, cardarine administration ameliorated insulin resistance. [36]
- In mice fed with a high-fat diet, cardarine improved blood sugar levels by enhancing insulin signaling. [37]
- Cardarine also improved blood sugar in mice with obesity-related disorders by regulating blood sugar metabolism and insulin sensitivity. [38]
- In mice, cardarine prevented cytokine-induced insulin resistance in liver cells. [39]
Fights Kidney Disease
Cardarine (GW501516) is a compound often linked to performance enhancement, but some studies suggest it may also offer benefits in fighting kidney disease. It is believed to work by activating the PPARδ (peroxisome proliferator-activated receptor delta), which helps regulate fat metabolism, reduce inflammation, and improve mitochondrial function. These actions may support kidney health by reducing oxidative stress, lowering inflammatory markers, and improving overall cellular function within kidney tissues. However, while these potential benefits are promising, more clinical research is needed to conclusively determine its effectiveness in combating kidney disease and its long-term safety.
- Cardarine protects against kidney disease by reducing the activity of MCP-1, a gene related to kidney disorders. [40]
- In high-fructose fed mouse model, cardarine improved inflammatory pathways in the kidneys. [41-42]
- A study found that cardarine has the potential to reduce kidney inflammation, thus, preventing kidney disease progression. [43]
Improves Liver Health
Cardarine (GW501516) has been suggested to improve liver health by promoting fat metabolism and reducing inflammation in the liver. It works by activating the peroxisome proliferator-activated receptor delta (PPARδ), which enhances the body’s ability to burn fat for energy, potentially reducing the accumulation of fat in liver cells, a key contributor to fatty liver disease. Additionally, Cardarine may help reduce oxidative stress, which can damage liver cells, leading to better overall liver function. However, it’s important to note that while some animal studies show promising results, further research is needed to fully understand its long-term effects on human liver health.
- A study found that cardarine has the potential to treat kidney diseases associated with metabolic syndrome. [44]
- In animal models of nonalcoholic fatty liver disease, cardarine treatment reduced liver inflammation. [45]
- In mice, cardarine treatment significantly reduced the prevalence of liver damage from a high-fructose diet and the development of nonalcoholic fatty liver disease. [39, 46]
- In animal models of non-alcholic steatohepatitis (fat build-up in the liver), cardarine ameliorated symptoms by enhancing fatty acid β-oxidation. [47-50]
- A study found that cardarine and other peroxisome proliferator-activated receptors have the ability to stimulate liver regeneration by modulating Akt and E2f Signaling. [51]
Prevents and Treats Cancer
Cardarine (GW501516) is a synthetic compound that has shown promise in research for its potential to prevent and treat cancer. It works by activating the PPARδ (peroxisome proliferator-activated receptor delta) pathway, which plays a key role in regulating metabolism, inflammation, and cellular growth. Studies suggest that Cardarine can help inhibit the proliferation of cancer cells and reduce tumor growth, particularly in cancers like breast, colon, and liver. Its anti-inflammatory effects, combined with its ability to enhance fat metabolism, may contribute to its potential as a cancer prevention and treatment agent. However, it’s important to note that more clinical trials are needed to fully understand its efficacy and safety in humans.
- A study showed the anti-inflammatory properties of cardarine in human pancreatic cancer. [52]
- In mice, cardarine inhibited tumor growth in nasopharyngeal carcinoma (NPC). [53]
- A study showed that cardarine induced apoptosis in invasive bladder cancer cells. [54]
Cardarine Side Effects
Cardarine side effects are very uncommon. There have been some side effects associated with the use of this drug wherein the patient had one of the issues listed below at some point while being on cardarine. However, the issue wasn’t’ confirmed to be caused by the treatment and could have been a coincidence and not related to the use of cardarine. Despite this, it was listed as a side effect associated with cardarine even these associated side effects are very uncommon.
Side effects associated with cardarine may include the following:
- Dizziness
- Flushing
- Headache
- Lightheadedness
- Swelling of the ankles/feet
Cardarine vs ostarine
Cardarine, Ostarine, and MK-677 are all popular compounds in the fitness and bodybuilding community, but they serve different purposes. Cardarine (GW-501516) is a PPAR-delta agonist designed to enhance endurance, boost fat metabolism, and improve cardiovascular health. It does not affect muscle growth directly but helps users sustain longer workouts and burn fat more efficiently.
Ostarine (MK-2866), on the other hand, is a selective androgen receptor modulator (SARM) primarily used for muscle preservation and growth. It helps prevent muscle loss during cutting phases and supports lean muscle gains without the androgenic side effects of steroids. Unlike Cardarine, Ostarine directly influences muscle tissue by binding to androgen receptors, promoting muscle repair and growth. MK-677, also known for its growth hormone-releasing effects, can be stacked with these compounds to promote further muscle recovery and enhance muscle mass.
While both Cardarine and Ostarine can be used together in a cutting cycle, and MK-677 can support the process by improving muscle repair, they have distinct mechanisms of action. Cardarine focuses on endurance and fat oxidation, while Ostarine helps maintain muscle mass, and MK-677 supports muscle growth and recovery. Choosing between them depends on individual fitness goals—Cardarine is ideal for enhancing stamina and fat loss, while Ostarine is better suited for muscle retention and recovery, with MK-677 complementing these effects for overall muscle development.
Cardarine cycle length
Cardarine, Ostarine, and MK 677 are all popular compounds in the fitness and bodybuilding community, but they serve different purposes. Cardarine (GW-501516) is a PPAR-delta agonist designed to enhance endurance, boost fat metabolism, and improve cardiovascular health. It does not affect muscle growth directly but helps users sustain longer workouts and burn fat more efficiently.
Ostarine (MK-2866), on the other hand, is a selective androgen receptor modulator (SARM) primarily used for muscle preservation and growth. It helps prevent muscle loss during cutting phases and supports lean muscle gains without the androgenic side effects of steroids. MK 677, another compound used for muscle growth, promotes the secretion of growth hormone, further enhancing the potential for muscle recovery and growth. Unlike Cardarine, Ostarine and MK 677 directly influence muscle tissue by binding to androgen receptors and stimulating growth hormone release, respectively.
While all three compounds can be used together in a cutting cycle, they have distinct mechanisms of action. Cardarine focuses on endurance and fat oxidation, while Ostarine and MK 677 help maintain muscle mass and promote recovery. Choosing between them depends on individual fitness goals—Cardarine is ideal for enhancing stamina and fat loss, while Ostarine and MK 677 are better suited for muscle retention and growth.
Cardarine capsules
Cardarine capsules are a popular oral form of the PPAR-delta agonist, designed for convenient dosing and optimal absorption. These capsules are commonly used by athletes and fitness enthusiasts seeking to enhance endurance, boost fat metabolism, and improve energy efficiency without affecting hormone levels. Their standardized dosage makes it easier to track intake and maintain consistent results.
When taken as directed, Cardarine capsules work by stimulating the PPAR-delta pathway, increasing the body’s ability to burn fat while preserving muscle mass. This makes them especially appealing for individuals looking to improve body composition and athletic performance. Additionally, some research suggests potential cardiovascular benefits, such as reducing inflammation and improving lipid profiles.
Despite their potential advantages, users should be aware of ongoing debates regarding the long-term safety of Cardarine. While animal studies have raised concerns about prolonged use, human research is still limited. As with any supplement, it’s essential to source Cardarine capsules from reputable suppliers and consult a healthcare professional before use.
Cardarine and alcohol
Cardarine blood work is essential for monitoring its effects on lipid profiles, liver function, and overall metabolic health. Studies suggest that Cardarine can significantly improve cholesterol levels by increasing HDL (good cholesterol) and reducing LDL (bad cholesterol), which may contribute to cardiovascular benefits. Regular blood tests can help track these changes and ensure that lipid levels remain within a healthy range.
Liver function tests are also important when using Cardarine, as any compound affecting metabolism may influence liver enzymes. While research has not shown direct liver toxicity, routine blood work can help detect any potential abnormalities early. Checking markers such as ALT and AST levels can provide insights into liver health and ensure safe use over time.
Additionally, blood work can assess inflammation markers and glucose metabolism, as Cardarine is known to enhance insulin sensitivity. Monitoring fasting blood sugar and C-reactive protein (CRP) levels can help determine whether Cardarine is positively influencing metabolic health. Regular testing ensures that any unexpected side effects are detected early, allowing for adjustments if needed.
Cardarine blood work
Cardarine blood work is essential for monitoring its effects on lipid profiles, liver function, and overall metabolic health. Studies suggest that Cardarine can significantly improve cholesterol levels by increasing HDL (good cholesterol) and reducing LDL (bad cholesterol), which may contribute to cardiovascular benefits. Regular blood tests can help track these changes and ensure that lipid levels remain within a healthy range.
Liver function tests are also important when using Cardarine, as any compound affecting metabolism may influence liver enzymes. While research has not shown direct liver toxicity, routine blood work can help detect any potential abnormalities early. Checking markers such as ALT and AST levels can provide insights into liver health and ensure safe use over time.
Additionally, blood work can assess inflammation markers and glucose metabolism, as Cardarine is known to enhance insulin sensitivity. Monitoring fasting blood sugar and C-reactive protein (CRP) levels can help determine whether Cardarine is positively influencing metabolic health. Regular testing ensures that any unexpected side effects are detected early, allowing for adjustments if needed.
Cardarine rad140 stack
Stacking Cardarine (GW-501516) and RAD-140 (Testolone) is popular among athletes and bodybuilders for its potential synergy in enhancing endurance, fat loss, and muscle growth. Cardarine is known for improving stamina and metabolic efficiency by activating the PPAR-delta pathway, while RAD-140, a selective androgen receptor modulator (SARM), promotes lean muscle gains and strength. Together, they offer a combination of endurance enhancement and muscle-building effects.
This stack is often used during cutting cycles, as Cardarine helps with fat oxidation while RAD-140 preserves muscle mass and boosts recovery. Users report increased workout intensity, improved vascularity, and a leaner physique. Additionally, since Cardarine does not suppress natural testosterone production, it may help counterbalance some of the hormonal suppression caused by RAD-140.
Despite the benefits, potential side effects should be considered. RAD-140 can suppress testosterone, requiring post-cycle therapy (PCT), while Cardarine’s long-term safety remains debated due to animal studies linking it to cancer risk. Careful dosing, cycle length management, and regular health monitoring are essential for minimizing risks and maximizing results from this stack.
How long does cardarine take to work
Cardarine typically begins to take effect within a few days to a week, with users reporting increased endurance and energy levels early on. Since it activates the PPAR-delta pathway, which enhances fat metabolism and muscle efficiency, some benefits may be noticeable relatively quickly, especially during physical activity. However, the full effects on fat loss and endurance improvement may take a few weeks to become more pronounced.
Most users experience significant results within 4 to 6 weeks of consistent use, particularly in terms of stamina and fat oxidation. As the body adapts to the increased mitochondrial activity and improved metabolic efficiency, endurance continues to improve over time. Individual response varies based on dosage, activity level, and overall lifestyle factors like diet and exercise.
By the 8 to 12-week mark, users often see peak benefits, including noticeable improvements in cardiovascular performance and body composition. However, as with any performance-enhancing compound, results depend on sustained use, and cycling protocols are often recommended to optimize long-term effectiveness while minimizing potential risks.
Cardarine supplement
Cardarine is a research chemical often marketed as a supplement for its potential to enhance endurance, boost fat metabolism, and improve energy efficiency. Originally developed for treating metabolic and cardiovascular disorders, it gained popularity among athletes and fitness enthusiasts due to its ability to activate the PPAR-delta pathway, which promotes fat oxidation and muscle endurance. Unlike traditional stimulants, Cardarine does not act on the central nervous system, making it appealing for those looking to improve performance without jittery side effects.
Despite its promising benefits, Cardarine remains an experimental compound, and its long-term safety profile is not fully understood. Some studies in animal models raised concerns about potential cancer risks with prolonged use, leading to its discontinuation in pharmaceutical research. As a result, it is not approved for human consumption by regulatory bodies like the FDA and is primarily sold as a research chemical rather than a dietary supplement.
Individuals considering Cardarine should be cautious and aware of potential risks, as well as the legal status in their region. While some users report positive effects on endurance and fat loss, the lack of clinical trials in humans means its safety and efficacy remain uncertain. Consulting a healthcare professional before use is essential, especially for those with underlying health conditions or those subject to drug testing regulations.
Cardarine for women
Cardarine is often used by women seeking to improve endurance, boost fat metabolism, and enhance overall athletic performance. Unlike anabolic steroids, it does not interfere with hormone levels, making it an appealing option for women looking to increase stamina without the risk of masculinizing side effects. Its ability to promote fat oxidation can also help with body composition goals, making it a popular choice among female athletes and fitness enthusiasts.
For women, the typical Cardarine dosage is lower than that used by men, often ranging from 5 to 10 mg per day. This helps minimize any potential side effects while still providing significant benefits in endurance and fat burning. Since Cardarine does not directly stimulate muscle growth like steroids, it is commonly stacked with other supplements or training programs focused on lean muscle development.
While Cardarine is not known to cause hormonal imbalances, long-term safety remains a concern due to limited human research. Women considering its use should be cautious, monitor their response closely, and consult with a healthcare professional before starting. Additionally, sourcing high-quality products is essential to avoid contamination or counterfeit substances, as Cardarine is often sold in the research chemical market.
Cardarine dosage for males
The optimal Cardarine dosage for males typically ranges from 10-20 mg per day, depending on individual goals and experience with the compound. Beginners often start with 10 mg daily to assess tolerance, while more experienced users may increase to 20 mg per day for enhanced endurance and fat metabolism. It is usually taken once daily due to its long half-life of around 24 hours.
A common cycle length for Cardarine is 6-12 weeks, followed by a break to allow the body to reset. While it does not suppress natural testosterone production, taking periodic breaks can help minimize potential risks. Some users choose to stack Cardarine with other compounds like SARMs for synergistic benefits, but caution is advised when combining substances.
Since Cardarine is not liver-toxic, it does not require post-cycle therapy (PCT). However, maintaining a healthy diet and exercise routine is essential to maximize its effects. As with any research compound, consulting a healthcare professional before use is recommended to ensure safety and proper dosing.
When to take cardarine
The timing of taking Cardarine largely depends on your personal fitness goals and routine. For those using Cardarine to enhance endurance and performance, it’s commonly recommended to take it about 30 to 60 minutes before a workout. This timing allows the compound to activate the PPAR-delta pathway, optimizing fat metabolism and energy utilization during exercise, leading to better performance and stamina.
If you’re using Cardarine for fat loss or metabolic support, it can be taken at any time during the day, as it works by improving overall fat oxidation and energy efficiency. Some prefer to take it in the morning with their first meal to start the day with an energy boost, while others take it before a workout for maximum benefit during physical activity.
As with any supplement, consistency is key. To maintain stable levels in your system, taking Cardarine at the same time each day, whether before exercise or at a time that suits your routine, can help you achieve the best results over time. However, it’s essential to follow the recommended dosage and consult with a healthcare provider, particularly if combining it with other supplements or medications.
Cardarine dosage
Cardarine dosage typically starts at a lower range to assess individual tolerance and gradually increases if necessary. A common starting dose for athletes and individuals using Cardarine for performance enhancement is around 10 mg per day. This dosage is often taken once daily, although some users may split the dose into two smaller servings to reduce potential side effects. It’s important to note that the optimal dosage can vary based on factors like body weight, goals, and response to the compound.
For those seeking to maximize endurance or fat-burning effects, higher doses of up to 20 mg per day may be used, but it is essential to stay within safe limits to minimize any risks. Doses exceeding 20 mg per day are generally not recommended, as the long-term safety of higher doses is not well-established. Consistent use over extended periods is not typically advised, as there is limited data on the long-term effects of Cardarine.
As with any supplement or performance-enhancing compound, it is crucial to consult with a healthcare professional before starting Cardarine, especially if there are underlying health conditions. Monitoring for any adverse effects or unusual symptoms is recommended, and users should cycle off the compound after a set period to give the body a break and reduce the risk of potential side effects.
Ostarine and cardarine stack
The combination of Ostarine (MK-2866) and Cardarine (GW-501516), often referred to as a “stack,” is popular among athletes and fitness enthusiasts looking to improve performance, enhance fat loss, and increase endurance. Ostarine is a selective androgen receptor modulator (SARM) known for promoting lean muscle mass and strength without the side effects commonly associated with anabolic steroids. Cardarine, on the other hand, is a PPAR-delta activator that enhances endurance, fat metabolism, and cardiovascular health. Together, they create a synergy that can accelerate fat loss while maintaining or even increasing lean muscle mass.
Ostarine works by binding to androgen receptors in muscle tissue, stimulating muscle growth and repair, while Cardarine helps to improve the body’s ability to burn fat by boosting metabolic efficiency and increasing endurance. This combination makes the stack appealing for individuals aiming to get leaner, more toned, and stronger, as it helps maximize fat loss while preserving muscle. Moreover, Cardarine’s endurance-enhancing effects allow users to push harder in training, potentially leading to more effective workouts and greater overall progress.
However, while the Ostarine and Cardarine stack can offer performance benefits, it is important to approach it with caution, as both substances are not approved for long-term use outside of research settings. Side effects such as hormone imbalances with Ostarine or potential liver strain with Cardarine are concerns to monitor. Always consider consulting with a healthcare professional before using such compounds, especially when combining them for enhanced performance.
Ostarine and cardarine
Ostarine and Cardarine are both popular compounds in the fitness and bodybuilding communities, though they serve different purposes. Ostarine, also known as MK-2866, is a selective androgen receptor modulator (SARM) that promotes muscle growth, strength, and fat loss by binding to androgen receptors in the body. It is often used by athletes looking to enhance muscle mass without the negative side effects of anabolic steroids. Ostarine can help improve recovery, increase lean muscle, and support fat reduction during cutting phases.
Cardarine, on the other hand, is not a SARM but a PPAR-delta agonist. It works by activating the PPAR-delta pathway, which helps increase endurance, improve fat metabolism, and enhance cardiovascular performance. Athletes often use Cardarine to boost stamina and energy efficiency, allowing them to train harder and longer. Its fat-burning properties also make it a popular choice for those looking to improve body composition and overall metabolic health.
While both Ostarine and Cardarine are used to enhance athletic performance, they work through different mechanisms in the body. Ostarine primarily supports muscle growth and recovery, while Cardarine is more focused on increasing endurance and fat oxidation. Combining the two can provide synergistic benefits, as users may experience muscle gain and improved endurance, making them a popular pairing in training regimens. However, it is important to note that the long-term safety and regulatory status of these compounds remain uncertain, and they are not approved by major sports organizations for competition.
What does cardarine do
Cardarine, also known as GW-501516, is a synthetic compound that activates the PPAR-delta pathway, a receptor involved in regulating metabolism. By activating this pathway, Cardarine increases the body’s ability to oxidize fat, improving fat-burning processes and enhancing endurance. It is often used by athletes and bodybuilders to boost stamina during prolonged physical activity, as it helps the body use fat as a primary energy source rather than carbohydrates.
In addition to boosting endurance and fat metabolism, Cardarine is believed to support cardiovascular health. Research suggests that it can reduce inflammation and improve blood vessel function, contributing to better overall heart health. This makes it potentially beneficial for individuals looking to improve their cardiovascular fitness and reduce the risk of metabolic diseases such as obesity or diabetes.
While Cardarine does not influence hormones like anabolic steroids, it is valued for its ability to enhance energy efficiency and physical performance. Its effects on fat metabolism and endurance make it a popular choice for those seeking to improve exercise output and body composition, though long-term safety and regulatory status require further study.
Stenabolic vs cardarine
Stenabolic and Cardarine are both performance-enhancing compounds that target similar pathways in the body, but they differ in their mechanisms and uses. Stenabolic, also known as SR9009, is a Rev-Erb agonist that regulates circadian rhythms and boosts metabolism, leading to improved endurance, fat loss, and energy expenditure. It is often favored by athletes looking for enhanced physical performance and fat-burning effects without the need for stimulants.
Cardarine, also known as GW501516, works by activating the PPAR-delta pathway, which increases fat oxidation and improves endurance. It is primarily used by individuals aiming to improve cardiovascular health, increase stamina, and enhance fat metabolism. Cardarine is well-known for its ability to help burn fat while maintaining muscle mass, making it a popular choice for those focused on weight management and athletic performance.
While both compounds offer similar benefits, such as improved endurance and fat loss, their safety profiles and potential long-term effects differ. Stenabolic has not been extensively studied in humans, and there are concerns about its safety, particularly related to potential liver toxicity. On the other hand, Cardarine was linked to cancer development in animal studies, leading to concerns about its long-term use. Both substances should be approached with caution, and users should carefully consider the risks before incorporating them into their regimen.
FAQ
What is Cardarine best for?
Cardarine is best for improving endurance, fat metabolism, and cardiovascular health. It is commonly used by athletes and bodybuilders to enhance stamina and aid in fat cutting without affecting muscle and bone tissue. Many users also report that Cardarine helps preserve muscle and bone tissue during intense cutting cycles. Its ability to support fat loss while maintaining muscle and bone tissue makes it a popular choice for performance enhancement.
Does Cardarine show up on drug tests?
Yes, Cardarine can show up on drug tests, particularly in sports testing, as it is listed as a banned substance by organizations like the World Anti-Doping Agency (WADA). Athletes using substances like Cardarine often include post cycle therapy to help restore natural hormone levels, including insulin-like growth factor. However, even with post cycle therapy, traces of Cardarine can still be detected, depending on the testing methods. It’s important to note that post cycle therapy does not eliminate the substance itself, but supports recovery after its use.
What is the function of GW501516?
GW501516 (Cardarine) functions by activating the PPAR-delta pathway, which increases body fat oxidation, cardarine enhances endurance, and promotes cardiovascular health. This mechanism makes it popular among athletes aiming to reduce body fat while improving performance. Additionally, GW501516 may support long-term cardiovascular benefits by optimizing metabolism and helping regulate body fat distribution.
How long is GW501516 detectable in urine?
GW501516 can remain detectable in urine for several weeks to months, depending on the dosage and individual metabolism. For those using it as part of a performance-enhancing stack to promote weight loss, incorporating post cycle therapy (PCT) is essential to help restore natural hormone balance. Additionally, understanding the detection window is crucial when planning post cycle therapy (PCT), especially for athletes subject to drug testing. Even after discontinuation, traces of GW501516 may linger, making it important to align your post cycle therapy (PCT) schedule accordingly.
Does Cardarine reduce inflammation?
Yes, Cardarine has anti-inflammatory effects, which can support cardiovascular health and reduce inflammation in various tissues, including skeletal muscle tissue. These effects may help enhance recovery and performance by protecting skeletal muscle tissue from damage caused by oxidative stress. Additionally, reducing inflammation in skeletal muscle tissue can contribute to improved endurance and overall muscular function, allowing the body to develop rapidly.
Does Cardarine affect insulin?
Cardarine may improve insulin sensitivity, which could help regulate blood sugar levels and support metabolic health. This effect can be particularly beneficial for individuals suffering from muscle wasting diseases, where metabolic dysfunction is often a concern. By enhancing insulin sensitivity, Cardarine may offer supportive therapy in the management of muscle wasting diseases, helping to preserve muscle tissue. Its role in promoting better energy utilization also makes it a potential adjunct in treating muscle wasting diseases during a SARMs cycle.
How much Cardarine to take with Ostarine?
When combining Cardarine (GW501516) with Ostarine (MK-2866) in a SARMs cycle, typical dosages for Cardarine are 10-20 mg per day, while Ostarine is often dosed at 10-25 mg per day. This combination is popular for promoting lean muscle growth while enhancing endurance and recovery. However, it’s crucial to adjust based on personal goals and tolerance to maximize lean muscle growth benefits. Always monitor your body’s response to optimize for performance and lean muscle growth.
How long does it take for Ostarine to kick in
Ostarine typically takes about 2-3 weeks to show noticeable effects, such as lean mass gain and fat loss. Users often report an increase in lean mass along with improved muscle definition. Over time, Ostarine helps preserve lean mass even during a caloric deficit, contributing to boosting endurance and can be considered as part of a strategy for treating obesity.
Does Ostarine make you leaner?
Yes, Ostarine helps reduce fat while preserving or increasing lean muscle mass, leading to a leaner physique. It’s a popular choice for those aiming for rapid fat loss without sacrificing muscle. Users often report that rapid fat loss is achievable when Ostarine is combined with proper diet and training. Additionally, Ostarine may support protein synthesis, which is essential for muscle growth and repair. Overall, it’s considered effective for promoting rapid fat loss while supporting muscle maintenance.
Does Ostarine mess up testosterone?
Ostarine has a mild impact on testosterone levels, and while it doesn’t significantly suppress them, there may be a slight reduction during prolonged use. Many users incorporate Ostarine into their routines to support faster fat burning while preserving muscle mass. This makes it a popular option during cutting phases where faster fat burning is a primary goal. Additionally, even with its mild hormonal impact, the compound is often chosen for its ability to promote lean muscle retention alongside faster fat burning.
How long does GW501516 stay in your system?
GW501516 can stay in your system for several weeks to months, depending on various factors such as dosage, duration of use, and individual metabolism. This extended presence in the body can affect muscle tone, as the compound influences fat metabolism and energy utilization. It’s important to monitor changes in muscle tone during usage, especially if you’re aiming for specific fitness goals or adjustments in muscle composition.
Can you take Cardarine before bed?
Yes, Cardarine can be taken before bed, although its stimulating effects may make it better suited for use earlier in the day. For those focusing on weight loss, it’s important to understand how the timing of Cardarine can impact its effectiveness. Some users find that taking it earlier enhances their weight loss goals, while others may prefer the evening if weight loss is not the primary concern. Always consult with a healthcare professional to ensure it aligns with your weight loss plan.
Does GW501516 affect the liver?
While there is no direct evidence from initial research that GW501516 causes liver damage at typical dosages, long-term use, especially at higher doses, could potentially have liver-related risks. Initial research suggests that monitoring liver function is important. Further studies are needed to confirm the findings of initial research and fully assess any potential risks.
Does Cardarine increase mitochondria?
Yes, Cardarine, as a metabolic modulator, has been shown to promote mitochondrial biogenesis, which can increase the number of mitochondria in cells, enhancing energy production and endurance. As a metabolic modulator, Cardarine helps in optimizing cellular functions, improving overall metabolic efficiency. Its role as a metabolic modulator contributes significantly to enhanced physical performance and endurance.
Does alcohol interfere with SARMs?
Yes, alcohol can interfere with SARMs, as both can stress the liver, potentially increasing the risk of liver damage when taken together. This is especially concerning for individuals with muscle wasting conditions, as both alcohol and SARMs can have an impact on overall muscle health. For those dealing with muscle wasting conditions, the combination of alcohol and SARMs may exacerbate the damage and further hinder recovery or muscle preservation.
Is it okay to drink alcohol while taking steroids?
It is not recommended to drink alcohol while taking steroids, as both substances can stress the liver, leading to potential liver damage. Additionally, alcohol can exacerbate muscle pain, making it harder to manage conditions associated with muscle pain. If you are already experiencing muscle pain, combining alcohol with steroids may worsen the situation and increase the risk of further complications.
How long can you test positive for Cardarine?
Cardarine can be detectable in urine or blood tests for several weeks to months after use, especially at high dosages or prolonged use, as it may target androgen receptors in the body. The ability of Cardarine to target androgen receptors can lead to its prolonged presence in the system. This is particularly true when users have been taking high doses or using it for extended periods, allowing it to effectively target androgen receptors over time.
Does Cardarine show up in blood tests?
Yes, Cardarine can be detected in blood tests, particularly in the context of doping tests or specific drug screenings. The detection of substances like Cardarine in such tests can be influenced by various factors, including the metabolic rate of the individual and the time elapsed since the substance was last used. While Cardarine itself may not directly affect testosterone levels, it is important to consider how the use of performance-enhancing substances could indirectly influence testosterone levels in the body. Additionally, drug screenings may sometimes look for alterations in hormone levels, including testosterone levels, as part of a broader assessment for substances that could affect physical performance or health.
Does Cardarine affect blood sugar?
Cardarine in a sarms stack may improve insulin sensitivity and regulate blood sugar levels, which can benefit individuals with metabolic conditions like type 2 diabetes. When combined with other compounds in a sarms stack, Cardarine’s effects on metabolic function could be enhanced. Including Cardarine as part of a sarms stack may provide additional support for those looking to improve their insulin sensitivity and blood sugar regulation.
What effects does Cardarine have on cholesterol?
Cardarine may improve cholesterol profiles by increasing HDL (good cholesterol) and reducing LDL (bad cholesterol), supporting overall heart health. When included in a sarms stack, Cardarine is often praised for its cardiovascular benefits. Many users add it to their sarms stack specifically for its positive effects on lipid levels. Incorporating Cardarine into a sarms stack can be a strategic choice for those aiming to enhance both performance and heart health.
How much does Cardarine help?
Cardarine helps significantly with increasing endurance, fat loss, and improving cardiovascular health, especially when combined with regular exercise. Many users choose to stack sarms like Cardarine with other compounds to enhance results. When you stack sarms, you can often experience improved stamina and quicker fat-burning effects. It’s important to research properly before you stack sarms to ensure safety and effectiveness.
How does Cardarine affect fat loss?
Cardarine promotes fat oxidation and increases energy expenditure, which can support a calorie deficit, leading to more efficient fat loss and improved body composition. When combined with a consistent calorie deficit, its effects on metabolism can be even more pronounced. By helping the body utilize fat for fuel, Cardarine makes it easier to maintain a calorie deficit without significant drops in energy levels.
How many mL of Cardarine should I take a day?
Cardarine is typically dosed at 10-20 mg per day, depending on individual goals and whether it’s being used in conjunction with a calorie deficit. This equates to roughly 0.5-1 mL of a 20 mg/mL solution, which many users find effective when aiming for fat loss through a calorie deficit. For optimal results, combining Cardarine with regular exercise and a calorie deficit can enhance endurance and support body composition goals.
What are the side effects of Cardarine for women?
Cardarine is generally well-tolerated by women, but possible side effects include headaches, nausea, and digestive issues. It is often used to support muscle recovery, making it popular among athletes and fitness enthusiasts. While many report enhanced endurance and quicker muscle recovery, long-term safety data is still limited. As with any supplement, it’s important to monitor for adverse effects and evaluate how it supports your overall muscle recovery goals.
Does Cardarine change muscle fibers?
Cardarine does not directly change muscle fibers, but it can improve endurance and fat loss, indirectly supporting muscle preservation and performance. Through ongoing research collaboration, scientists are exploring these effects in greater depth. Research collaboration also helps validate Cardarine’s potential benefits in athletic contexts. Continued research collaboration may uncover new applications and safety profiles for long-term use.
What is Cardarine best for?
Cardarine is best for enhancing endurance, aiding fat loss, and improving cardiovascular health through its activation of the PPAR-delta pathway. While not a growth hormone secretagogue itself, it is often stacked with a growth hormone secretagogue to amplify fat-burning and recovery benefits. Many users report that combining Cardarine with a growth hormone secretagogue creates a synergistic effect, boosting overall performance and metabolic health.
Can you take Cardarine before bed?
Yes, you can take Cardarine before bed, though some individuals may experience heightened energy levels, making it more beneficial to take earlier in the day. Cardarine is often used to help build muscle by enhancing endurance and promoting fat loss. If your primary goal is to build muscle, timing your dose to align with your workouts may be more effective. Regardless of when you take it, consistency is key when using supplements to build muscle.
How often should I take Cardarine?
Cardarine is typically taken once per day, with doses ranging from 10-20 mg depending on individual needs and goals. Many users turn to Cardarine to increase fat burning capacity, especially during cutting phases. It’s widely recognized for its ability to increase fat burning capacity without stimulating the central nervous system. By enhancing endurance and promoting metabolic efficiency, Cardarine helps users increase fat burning capacity while supporting overall performance.
Reference
Ooi EM, Watts GF, Sprecher DL, Chan DC, Barrett PH. Mechanism of action of a peroxisome proliferator-activated receptor (PPAR)-delta agonist on lipoprotein metabolism in dyslipidemic subjects with central obesity. J ClinEndocrinolMetab. 2011;96(10):E1568-76.
Mechanism of action of a peroxisome proliferator-activated receptor (PPAR)-delta agonist on lipoprotein metabolism in dyslipidemic subjects with central obesity
In a randomized, double-blind, crossover trial involving 13 dyslipidemic men with central obesity, the study aimed to investigate the impact of GW501516, a peroxisome proliferator-activated receptor (PPAR)-δ agonist, on lipoprotein metabolism. The results revealed that GW501516 effectively lowered plasma triglycerides, fatty acid, and various apolipoprotein concentrations, while increasing high-density lipoprotein (HDL) cholesterol levels. This was achieved by enhancing the clearance of very low-density lipoprotein (VLDL) particles and increasing the production of beneficial HDL particles. Additionally, GW501516 reduced cholesteryl ester transfer protein activity, leading to favorable changes in lipid content within lipoprotein particles. These findings highlight GW501516’s potential as a treatment option for dyslipidemia in individuals with obesity.
You can read the full article at https://academic.oup.com/jcem/article/96/10/E1568/2834755?login=false.
Sprecher DL, Massien C, Pearce G, et al. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. ArteriosclerThrombVasc Biol. 2007;27(2):359-65.
Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist
The study aimed to investigate the impact of the PPARdelta agonist GW501516 on lipid etabolism and its potential effects on high-density lipoprotein cholesterol (HDLc) and triglycerides (TG). Healthy volunteers received placebo or varying doses of GW501516 for two weeks, with lipid and lipoprotein measurements conducted alongside in vivo fatfeeding studies. The results revealed a trend towards reduced TG levels and improved TG clearance with the drug, while HDLc was significantly increased in both treatment groups. In vitro cell culture experiments supported these findings by showing increased fatty acid oxidation and upregulated expression of lipid-related genes. These results suggest that GW501516 may positively affect HDLc and TG levels through enhanced pripheral fat utilization and lipidation.
You can read the full article at https://www.ahajournals.org/doi/10.1161/01.ATV.0000252790.70572.0c?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Doktorova M, Zwarts I, Zutphen TV, et al. Intestinal PPARδ protects against diet-induced besity, insulin resistance and dyslipidemia. Sci Rep. 2017;7(1):846. Published 2017 Apr 12. doi:10.1038/s41598-017-00889-z.
Intestinal PPARδ protects against diet-induced obesity, insulin resistance and dyslipidemia
Peroxisome proliferator-activated receptor δ (PPARδ) is a transcription factor crucial for lipid metabolism. Activating PPARδ promotes fatty acid oxidation in adipose tissue and muscle, benefiting lipid profiles. PPARδ is highly expressed in the gut, yet its gut-specific role remains unclear. Using mice with intestine-specific PPARδ deletion, this study reveals that intestinal PPARδ guards against diet-induced obesity, insulin resistance, and dyslipidemia. Moreover, the absence of intestinal PPARδ blocks the ability of PPARδ agonist GW501516 to elevate HDL-cholesterol levels. These findings underscore the importance of intestinal PPARδ in metabolic balance, suggesting gut-specific PPARδ activation as a potential therapeutic strategy for metabolic syndrome and dyslipidemia with reduced systemic side effects.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5429805/.
Risérus U, Sprecher D, Johnson T, et al. Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men. Diabetes. 2008;57(2):332-9.
Activation of peroxisome proliferator-activated receptor (PPAR)delta promotes reversal of multiple metabolic abnormalities, reduces oxidative stress, and increases fatty acid oxidation in moderately obese men
The study aimed to investigate the metabolic effects of peroxisome proliferator-activated receptor (PPAR)delta agonists in humans. In a double-blind, randomized trial involving moderately overweight individuals, GW501516, a PPARdelta agonist, was compared to a PPARalpha agonist and a placebo. GW501516 led to significant reductions in fasting triglycerides, apolipoprotein B, LDL cholesterol, and insulin levels, along with a decrease in liver fat content and oxidative stress. These changes were not observed with the PPARalpha agonist. GW501516 increased fat oxidation in skeletal muscle and the expression of carnitine palmitoyl-transferase 1b (CPT1b). These findings suggest that GW501516 can mitigate metabolic syndrome-related abnormalities through enhanced fat oxidation in muscles without increasing oxidative stress.
You can read the full article at https://diabetesjournals.org/diabetes/article/57/2/332/13123/Activation-of-Peroxisome-Proliferator-Activated.
Greene NP, Fluckey JD, Lambert BS, Greene ES, Riechman SE, Crouse SF. Regulators of blood lipids and lipoproteins? PPARδ and AMPK, induced by exercise, are correlated with lipids and lipoproteins in overweight/obese men and women. Am J PhysiolEndocrinolMetab. 2012;303(10):E1212-21.
Regulators of blood lipids and lipoproteins? PPARδ and AMPK, induced by exercise, are correlated with lipids and lipoproteins in overweight/obese men and women
The study aimed to investigate the association between skeletal muscle PPARδ content and blood lipids and lipoproteins before and after exercise in overweight and obese individuals. Following 12 weeks of endurance exercise training, PPARδ, PGC-1α, FAT/CD36, and LPL content increased after acute exercise, while PPARα, AMPKα, CPT I, and COX-IV content increased only after training. PPARδ content negatively correlated
with total and LDL cholesterol levels, particularly in the untrained condition, while AMPKα was positively correlated with HDL cholesterol levels irrespective of exercise. These findings suggest that exercise-induced changes in skeletal muscle PPARs and their target proteins are associated with improved blood lipid profiles in obese adults.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajpendo.00309.2012?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
MylènePerreault, David V. Erbe, and James F. Tobin, “PPAR Agonism for the Treatment of Obesity and Associated Disorders: Challenges and Opportunities,” PPAR Research, vol. 2008, Article ID 125387, 9 pages, 2008. https://doi.org/10.1155/2008/125387.
PPAR Agonism for the Treatment of Obesity and Associated Disorders: Challenges and Opportunities
Obesity has become a global epidemic, with existing anti-obesity drugs offering limited efficacy and unwanted side effects. Peroxisome proliferator-activated receptor (PPAR) modulators hold promise for obesity treatment, but their development faces various challenges. Despite these obstacles, PPAR modulators present an appealing option for addressing obesity and its complications, potentially serving as an alternative for individuals who struggle to lose weight through diet and exercise alone. Moreover, these modulators could provide additional benefits by improving associated health conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2577153/.
Dressel U, Allen TL, Pippal JB, Rohde PR, Lau P, Muscat GE. The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. MolEndocrinol. 2003;17(12):2477-93.
The peroxisome proliferator-activated receptor beta/delta agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells
Lipid homeostasis is primarily regulated by peroxisome proliferator-activated receptors (PPARs), including PPARalpha, -beta/delta, and -gamma. While PPARalpha is involved in lipid catabolism and PPARgamma in lipid storage, the role of PPARbeta/delta, particularly in skeletal muscle, remains largely unexplored. This study investigates the f
unction of PPARbeta/delta in skeletal muscle cells, demonstrating its activation leads to the expression of genes related to lipid utilization, beta-oxidation, cholesterol efflux, and energy uncoupling. These findings suggest that PPARbeta/delta agonists may have therapeutic potential in addressing hyperlipidemia, atherosclerosis, and obesity by enhancing fatty acid catabolism, cholesterol removal, and energy expenditure in muscle cells.
You can read the full article at https://academic.oup.com/mend/article/17/12/2477/2747399?login=false.
Available from https://www.clinicaltrials.gov/ct2/show/NCT00388180?term=GW501516&rank=3.
An Exploratory Study To Look At The Effect Of Two Investigational Drugs On Body Fat And Inflammation
The experimental drugs used in this study activate PPARs (peroxisome proliferator-activated receptors). Existing scientific research, along with animal and preliminary clinical data from GSK, indicate that PPAR activation may promote the utilization of fatty acids for energy, potentially resulting in reduced body fat. PPARs also appear to play a role in regulating lipid levels, such as cholesterol, and controlling inflammation. This study aims to delve deeper into these various functions of PPARs through clinical investigation.
You can read the abstract of the article at https://www.clinicaltrials.gov/ct2/show/NCT00388180?term=GW501516&rank=3.
Wang YX, Lee CH, Tiep S, et al. Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity. Cell. 2003;113(2):159-70.
Peroxisome-proliferator-activated receptor delta activates fat metabolism to prevent obesity
In contrast to the well-established roles of PPARgamma and PPARalpha in lipid metabolism, there is limited understanding of PPARdelta’s involvement in this process. This study demonstrates that targeted activation of PPARdelta in adipose tissue induces the expression of genes essential for fatty acid oxidation and energy dissipation, leading to improved lipid profiles and reduced adiposity. Remarkably, these animals exhibit complete resistance to both diet-induced and genetically predisposed obesity. As anticipated, acute treatment of obese mice with a PPARdelta agonist reduces lipid accumulation. In parallel, PPARdelta-deficient mice challenged with a high-fat diet display reduced energy uncoupling and a predisposition to obesity. In vitro, PPARdelta activation in adipocytes and skeletal muscle cells promotes fatty acid oxidation and utilization. These findings highlight PPARdelta as a key regulator of fat burning and a potential target for treating obesity and its related disorders.
You can read the full article at https://www.cell.com/cell/fulltext/S0092-8674(03)00269-1?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS0092867403002691%3Fshowall%3Dtrue.
Ehrenborg E. &Krook A. Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta. Pharmacol. Rev. 61, 373–393 (2009).
Regulation of skeletal muscle physiology and metabolism by peroxisome proliferator-activated receptor delta
Agonists targeting the alpha and gamma isoforms of peroxisome proliferator-activated receptors (PPARs) are pivotal in treating hypertriglyceridemia and insulin resistance linked to metabolic disorders. PPARdelta, the least understood isoform, is increasingly recognized as a crucial regulator of skeletal muscle metabolism, especially in terms of muscle lipid oxidation, making it a promising drug target. Additionally, PPARdelta appears to play a central role in determining skeletal muscle fiber type and may mediate adaptations observed in response to exercise. This review provides an overview of the current knowledge on the regulation and metabolic effects of PPARdelta in skeletal muscle.
You can read the abstract of the article at https://pharmrev.aspetjournals.org/content/61/3/373.long.
Varga T., Czimmerer Z., & Nagy L. PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation. Biochim. Biophys. Acta. 1812, 1007–1022 (2011).
PPARs are a unique set of fatty acid regulated transcription factors controlling both lipid metabolism and inflammation
Cells are continually exposed to a diverse array of lipids, traditionally viewed as energy storage molecules. However, it’s now recognized that lipids also serve as signaling molecules that profoundly impact development, cellular differentiation, metabolism, and related functions by regulating gene expression. Multicellular organisms have evolved a substantial family of nuclear receptors dedicated to these roles. These unique proteins combine characteristics of transcription factors and receptors, allowing them to bind lipid signaling molecules and transmit these signals to regulate gene expression. Among these nuclear receptors, the peroxisome proliferator-activated receptors (PPARs) stand out for their ability to sense and interpret fatty acid signals from dietary lipids, pathogenic lipoproteins, or essential fatty acid metabolites. Initially identified as key regulators of lipid and carbohydrate metabolism, PPARs have also been found to modulate inflammatory responses. This review outlines how these transcription factors/receptors bridge lipid metabolism and inflammation, shedding light on their novel regulatory mechanisms in both homeostasis and certain pathological conditions.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3117990/.
Billin A. N. PPAR-beta/delta agonists for Type 2 diabetes and dyslipidemia: an adopted orphan still looking for a home. Expert. Opin. Investig. Drugs 17, 1465–1471 (2008).
PPAR-beta/delta agonists for Type 2 diabetes and dyslipidemia: an adopted orphan still looking for a home
The discovery of small molecule agonists for the nuclear receptor peroxisome proliferator-activated receptor beta/delta (PPAR-beta/delta, NR1C2) has paved the way for investigating this receptor’s functions in preclinical models, leading to several PPAR-beta/delta agonists entering clinical trials. This review assesses key preclinical findings supporting the potential benefits of PPAR-beta/delta agonists in addressing dyslipidemia and Type 2 diabetes, alongside emerging clinical data involving various PPAR-beta/delta agonists. Current clinical results broadly align with preclinical models, yet it remains uncertain whether these agonists will offer significant advantages over existing treatments for dyslipidemia or Type 2 diabetes. Developing PPAR-beta/delta agonists faces substantial challenges, and their ultimate role in therapy is still uncertain.
You can read the abstract of the article at https://www.tandfonline.com/doi/abs/10.1517/13543784.17.10.1465.
Luquet S. et al. Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability. FASEB J. 17, 2299–2301 (2003).
Peroxisome proliferator-activated receptor delta controls muscle development and oxidative capability
Peroxisome proliferator-activated receptors (PPARs) are nuclear receptors with various roles in development and metabolism, and the precise functions of PPARdelta have remained unclear. Using a CRE-Lox recombination method, we created a muscle-specific PPARdelta overexpression model to investigate its role in muscle tissue. This overexpression led to significant changes in muscle fiber composition, promoting oxidative fibers and enhancing enzymatic activities and genes involved in oxidative metabolism. Additionally, it resulted in reduced body fat mass primarily due to smaller adipose cells. Moreover, we found that endurance exercise increased PPARdelta protein levels in muscle of wild-type animals. These findings suggest that PPARdelta plays a crucial role in muscle development and adaptive responses to factors like exercise, highlighting its potential in preventing metabolic disorders such as obesity and type 2 diabetes.
You can read the full article at https://faseb.onlinelibrary.wiley.com/doi/epdf/10.1096/fj.03-0269fje.
Chen W, Gao R, Xie X, et al. A metabolomic study of the PPARδ agonist GW501516 for enhancing running endurance in Kunming mice. Sci Rep. 2015;5:9884.
A metabolomic study of the PPARδ agonist GW501516 for enhancing running endurance in Kunming mice
Exercise has been found to increase the expression of peroxisome proliferator-activated receptor-δ (PPARδ) in skeletal muscle, influencing muscle metabolism and fiber types to improve endurance. This study used metabolomic profiling to assess the impact of GW501516, a PPARδ agonist, on running endurance in mice. While exercise training alone improved running performance and metabolic profiles, GW501516 treatment further enhanced endurance and the proportion of succinate dehydrogenase (SDH)-positive muscle fibers, along with increased levels of intermediate metabolites and fatty acid oxidation enzymes. Interestingly, GW501516 alone and in combination with training influenced serum metabolites differently, suggesting distinct mechanisms behind their effects on running capacity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4421799/.
Grimaldi P. A. Roles of PPARdelta in the control of muscle development and metabolism. Biochem. Soc. Trans. 31, 1130–1132 (2003).
Roles of PPARdelta in the control of muscle development and metabolism
PPARdelta-specific agonists have been shown to reduce plasma lipids and insulin levels in obese animals. To understand the role of PPARdelta in muscle development and lipid metabolism, two approaches were employed. In cultured C(2)C(12) myotubes, PPARdelta agonists increased the expression of genes related to fatty acid catabolism and enhanced fatty acid oxidation, with stronger effects in C(2)C(12)-PPARdelta cells and reduced effects in C(2)C(12)-PPARdeltaDN cells. Additionally, mouse models with muscle-specific PPARdelta expression revealed PPARdelta’s importance in muscle fiber typing and oxidative capacity regulation, while muscle-specific PPARdelta overexpression reduced adipocyte size and body fat mass. These findings suggest that PPARdelta plays a key role in muscle fatty acid catabolism and its activation by synthetic agonists may have implications for obesity and type 2 diabetes management.
You can read the abtract of the article at https://portlandpress.com/biochemsoctrans/article-abstract/31/6/1130/64500/Roles-of-PPAR-amp-Delta-in-the-control-of-muscle?redirectedFrom=fulltext.
Wang, Y. X., Zhang, C. L., Yu, R. T., Cho, H. K., Nelson, M. C., Bayuga-Ocampo, C. R., Ham, J., Kang, H., and Evans, R. M. (2004). Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2, e294. doi: 10.1371/journal.pbio.0020294.
Regulation of muscle fiber type and running endurance by PPARδ
Endurance exercise training can lead to a transformation of muscle fibers and increased mitochondrial biogenesis, but the transcription factors responsible for this process were unknown. Researchers engineered mice capable of running longer distances by expressing an activated form of peroxisome proliferator-activated receptor delta (PPARdelta) in their skeletal muscles, resulting in an increased number of type I muscle fibers. Treating normal mice with a PPARdelta agonist produced a similar effect, offering resistance to obesity and improved metabolic profiles even without exercise. This study demonstrates that molecular manipulation can affect complex physiological traits like endurance and running capacity.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC509410/.
Narkar VA, Downes M, Yu RT, et al. AMPK and PPARdelta agonists are exercise mimetics. Cell. 2008;134(3):405–415. doi:10.1016/j.cell.2008.06.051.
AMPK and PPARdelta agonists are exercise mimetics
Endurance exercise provides significant health benefits, and there’s interest in finding drugs that can mimic these effects to treat metabolic disorders. Researchers tested various drugs on mice’s endurance capacity during treadmill running and discovered that a PPARbeta/delta agonist, when combined with exercise training, increased oxidative muscle fibers and running endurance. Additionally, the orally active AMPK agonist AICAR, even without exercise, induced metabolic genes and enhanced running endurance by 44% in sedentary mice. These findings highlight the potential of targeting the AMPK-PPARdelta pathway with drugs to enhance endurance or improve metabolic outcomes without exercise.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2706130/.
Fan W, Waizenegger W, Lin CS, et al. PPARδ Promotes Running Endurance by Preserving Glucose. Cell Metab. 2017;25(5):1186–1193.e4. doi:10.1016/j.cmet.2017.04.006.
PPARδ Promotes Running Endurance by Preserving Glucose
Efficient energy management is crucial during endurance exercise, and a shift from glucose to fat utilization is a characteristic adaptation in trained muscles. This metabolic shift is dependent on muscle PPARδ and can be stimulated by PPARδ ligands. Additionally, higher muscle PPARδ expression is positively associated with endurance performance. PPARδ activation not only enhances fatty acid metabolism but also suppresses glucose catabolism, without affecting muscle fiber type or mitochondrial content. This preservation of systemic glucose levels by PPARδ delays the onset of hypoglycemia, extending running time by approximately 100 minutes in treated mice. These findings suggest the potential of PPARδ-targeted compounds as exercise mimetics for metabolic disorders, muscular dystrophies, and athletic performance enhancement.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5492977/.
Available from https://clinicaltrials.gov/ct2/show/NCT00158899.
GW501516 In Subjects Who Have Low Level Of High-Density Lipoprotein Cholesterol
This clinical research study aims to assess the safety, tolerability, and effectiveness of up to three doses of an investigational drug, GW501516, in comparison to a placebo (an inert pill resembling GW501516). The primary objective is to determine whether GW501516 can enhance low levels of high-density lipoprotein cholesterol (HDLc), often referred to as “good cholesterol.
You can read the abstract of the article at https://classic.clinicaltrials.gov/ct2/show/NCT00158899.
Available from https://www.clinicaltrials.gov/ct2/show/NCT00841217?term=GW501516&rank=4.
Regulation of Lipoprotein Transport in Metabolic Syndrome
The metabolic syndrome (MetS) is associated with an increased risk of diabetes and cardiovascular disease (CVD), often characterized by dyslipoproteinaemia, including elevated triglyceride levels and reduced high-density lipoprotein (HDL) concentrations. These lipid abnormalities are independent predictors of CVD and are therapeutic targets for risk reduction. GW5015156, a novel PPAR-delta agonist, holds promise for treating dyslipidemia in individuals with insulin resistance and obesity. However, the precise mechanisms by which this agent affects lipoprotein metabolism in individuals with MetS require further investigation. Therefore, this study aims to examine the impact of GW5015156 on lipoprotein transport in MetS subjects.
You can read the abstract of the article at https://classic.clinicaltrials.gov/ct2/show/NCT00841217?term=GW501516.
Olson EJ, Pearce GL, Jones NP, Sprecher DL. Lipid effects of peroxisome proliferator-activated receptor-d agonist GW501516 in subjects with low high-density lipoprotein cholesterol: characteristics of metabolic syndrome. ArteriosclerThrombVascBiol 2012; 32: 2289-2294.
Lipid effects of peroxisome proliferator-activated receptor-d agonist GW501516 in subjects with low high-density lipoprotein cholesterol: characteristics of metabolic syndrome
The objective of this study was to investigate the impact of GW501516, a peroxisome proliferator-activated receptor-δ agonist, on lipid and lipoprotein profiles. In a 12-week trial involving 268 patients with low high-density lipoprotein (HDL) cholesterol, GW501516 demonstrated significant increases in HDL cholesterol (up to 16.9%) and
apoA-I (up to 6.6%), along with reductions in low-density lipoprotein (LDL) cholesterol (-7.3%), triglycerides (-16.9%), apoB (-14.9%), and free fatty acids (-19.4%). An exploratory study with 5 and 10 mg doses of GW501516 showed substantial decreases in very low-density lipoprotein (VLDL), intermediate-density lipoprotein (IDL), and LDL concentrations, while the number of HDL particles increased. These findings suggest that GW501516 has the potential to improve cardiovascular protection in patients with metabolic syndrome-like conditions by modifying lipoprotein profiles.
You can read the full article at https://www.ahajournals.org/doi/10.1161/ATVBAHA.112.247890?url_ver=Z39.88-2003\\\&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Sprecher DL, Massien C, Pearce G, Billin AN, Perlstein I, Willson TM, Hassall DG, Ancellin N, Patterson SD, Lobe DC, Johnson TG. Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist. ArteriosclerThrombVasc Biol. 2007;27:359–365.
Triglyceride:high-density lipoprotein cholesterol effects in healthy subjects administered a peroxisome proliferator activated receptor delta agonist
The study aimed to assess the effects of GW501516, a PPARdelta agonist, on lipid metabolism and high-density lipoprotein cholesterol (HDLc) in sedentary healthy volunteers. Participants received either placebo or GW501516 (2.5 mg or 10 mg) for two weeks. The results showed a trend of reduced serum triglycerides (TG) with the 10 mg dose and improved TG clearance after fat feeding. Both GW501516 doses led to increased HDLc levels. In vitro experiments also demonstrated GW501516-induced fatty acid oxidation and upregulated genes related to lipid metabolism. This study suggests that GW501516 may influence peripheral fat utilization and lipidation, contributing to its effects on HDL and TG levels.
You can read the full article at https://www.ahajournals.org/doi/10.1161/01.ATV.0000252790.70572.0c?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Olson EJ, Pearce GL, Jones NP, Sprecher DL. Lipid effects of peroxisome proliferator-activated receptor-δ agonist GW501516 in subjects with low high-density lipoprotein cholesterol: characteristics of metabolic syndrome. ArteriosclerThrombVasc Biol. 2012;32(9):2289-2294. doi:10.1161/ATVBAHA.112.247890.
Lipid effects of peroxisome proliferator-activated receptor-δ agonist GW501516 in subjects with low high-density lipoprotein cholesterol: characteristics of metabolic syndrome
The objective of this study was to investigate the effects of GW501516, a peroxisome proliferator-activated receptor-delta (PPARδ) agonist, on lipid and lipoprotein profiles in patients with low high-density lipoprotein (HDL) cholesterol levels. Over a 12-week period, different doses of GW501516 were administered to 268 patients, and various lipid-related parameters were measured. The results demonstrated significant increases in HDL cholesterol and apoA-I levels, along with reductions in low-density lipoprotein (LDL) cholesterol, triglycerides, apoB, and free fatty acids. A smaller exploratory study further supported these findings, showing reductions in very low-density lipoprotein (VLDL) and LDL concentrations, as well as an increase in the number of HDL particles. These results suggest that targeting PPARδ with GW501516 could have potential cardiovascular benefits for individuals with metabolic syndrome-like profiles.
You can read the abstract of the article at https://pubmed.ncbi.nlm.nih.gov/22814748/.
Oliver WR Jr, Shenk JL, Snaith MR, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. Proc Natl Acad Sci U S A. 2001;98(9):5306-5311. doi:10.1073/pnas.091021198.
A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport
The peroxisome proliferator-activated receptors (PPARs) are sensors for dietary lipids, playing crucial roles in regulating fatty acid and carbohydrate metabolism. While fibrate drugs and glitazone drugs target the alpha and gamma subtypes of PPARs, respectively, little was known about the therapeutic potential of the delta subtype. Through innovative drug design, GW501516, a potent and subtype-selective PPARdelta agonist, was developed. In various cell types, including macrophages and intestinal cells, GW501516 demonstrated the ability to increase the expression of reverse cholesterol transporter ATP-binding cassette A1 and promote apolipoprotein A1-specific cholesterol efflux. In obese rhesus monkeys with insulin resistance, GW501516 effectively raised high-density lipoprotein cholesterol levels and lowered small-dense low-density lipoprotein, fasting triglycerides, and fasting insulin levels, suggesting its potential in enhancing reverse cholesterol transport and reducing cardiovascular risks associated with metabolic syndrome X.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC33205/.
Santhanam AV, d’Uscio LV, He T, Katusic ZS. PPARδ agonist GW501516 prevents uncoupling of endothelial nitric oxide synthase in cerebral microvessels of hph-1 mice. Brain Res. 2012;1483:89–95. doi:10.1016/j.brainres.2012.09.012.
PPARδ agonist GW501516 prevents uncoupling of endothelial nitric oxide synthase in cerebral microvessels of hph-1 mice
Peroxisome proliferator-activated receptor delta (PPARδ) is widely expressed in the vascular system, including cerebral blood vessels. However, its role in regulating the metabolism of tetrahydrobiopterin (BH₄) in the cerebral microvasculature hasn’t been explored. In this study, the impact of the PPARδ agonist GW501516 on endothelial nitric oxide synthase (eNOS) uncoupling was investigated in BH₄-deficient hph-1 mice. Treatment with GW501516 in hph-1 mice resulted in a significant reduction in BH₄ oxidation and an increased ratio of BH₄ to 7,8-BH₂. This treatment also demonstrated a potential to prevent eNOS uncoupling, as evidenced by a decrease in L-NAME-inhibitable superoxide anion levels. Additionally, GW501516 selectively increased the expression of antioxidant enzymes, CuZn superoxide dismutase, and catalase in endothelial cells. These findings suggest that PPARδ activation can help maintain endothelial function and NO bioavailability in the BH₄-deficient cerebral microvasculature.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3474319/.
Defaux A, Zurich MG, Braissant O, Honegger P, Monnet-tschudi F. Effects of the PPAR-beta agonist GW501516 in an in vitro model of brain inflammation and antibody-induced demyelination. J Neuroinflammation. 2009;6:15.
Effects of the PPAR-beta agonist GW501516 in an in vitro model of brain inflammation and antibody-induced demyelination
In various brain pathologies, including multiple sclerosis (MS), brain inflammation is a central factor. Microglial cells and astrocytes are key players in neuroinflammation and can be triggered by agents like interferon-gamma (IFN-gamma) and lipopolysaccharide (LPS). Peroxisome proliferator-associated receptor (PPAR) pathways are known to regulate inflammatory processes, with PPAR-beta playing a significant role in central inflammation control. Additionally, PPAR-beta agonists have shown potential trophic effects on oligodendrocytes in vitro and partial protection in experimental autoimmune encephalomyelitis (EAE), an MS animal model. In this study, a three-dimensional brain cell culture system served as an in vitro model to investigate antibody-induced demyelination and inflammatory responses. The specific PPAR-beta agonist GW 501516 was evaluated for its ability to protect against antibody-mediated demyelination and mitigate inflammatory responses induced by IFN-gamma and LPS.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2687435/.
John J. Bright, SaravananKanakasabai, WanidaChearwae, and SharmisthaChakraborty, “PPAR Regulation of Inflammatory Signaling in CNS Diseases,” PPAR Research, vol. 2008, Article ID 658520, 12 pages, 2008. https://doi.org/10.1155/2008/658520.
PPAR Regulation of Inflammatory Signaling in CNS Diseases
Despite being an immune privileged site, the central nervous system (CNS) often exhibits inflammation in various CNS diseases. Peroxisome proliferator-activated receptors (PPARs), a family of nuclear hormone receptors known for their regulation of immune and inflammatory responses, have shown promise in animal models of conditions such as multiple sclerosis (MS), Alzheimer’s disease, Parkinson’s disease, and trauma/stroke, suggesting their potential use in treating neuroinflammatory diseases. These receptors influence key pathways like NF-kappaB and Jak-Stat signaling, as well as the secretion of inflammatory cytokines, which play pivotal roles in the pathogenesis of CNS diseases. Notably, PPAR agonists have been found to alleviate CNS diseases by modulating the inflammatory signaling network in immune cells. This manuscript provides an overview of the current understanding of how PPARs regulate neuroinflammatory signaling networks in CNS diseases.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2490815/.
Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011;18(2):103–107. Published 2011 Jan 18. doi:10.1101/lm.2001611.
Endurance factors improve hippocampal neurogenesis and spatial memory in mice
The impact of compounds that enhance muscle endurance on cognitive function remains unclear, despite the known benefits of physical activity on learning and hippocampal neurogenesis. To address this, we explored the effects of endurance-boosting factors, specifically the peroxisome proliferator-activated receptor δ agonist GW501516 and AICAR, an activator of AMP-activated protein kinase, on memory and neurogenesis in mice. Mice received injections of GW for 7 days or AICAR for 7 or 14 days, followed by Morris water maze testing two weeks later. The results showed that both AICAR (7 days) and GW improved spatial memory, with AICAR leading to a significant increase and GW causing a modest rise in dentate gyrus neurogenesis. These findings suggest that pharmacological activation of skeletal muscle may play a role in mediating cognitive effects.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3032576/.
Defaux A, Zurich MG, Braissant O, Honegger P, Monnet-Tschudi F. Effects of the PPAR-beta agonist GW501516 in an in vitro model of brain inflammation and antibody-induced demyelination. J Neuroinflammation. 2009;6:15. Published 2009 May 7. doi:10.1186/1742-2094-6-15.
Effects of the PPAR-beta agonist GW501516 in an in vitro model of brain inflammation and antibody-induced demyelination
In various brain disorders characterized by brain inflammation, including multiple sclerosis (MS), microglial cells and astrocytes are key players in neuroinflammation and can be triggered by agents like interferon-gamma (IFN-gamma) and lipopolysaccharide (LPS). Peroxisome proliferator-associated receptor (PPAR) pathways, particularly PPAR-beta, are implicated in controlling these inflammatory processes. PPAR-beta agonists have demonstrated potential benefits in experimental autoimmune encephalomyelitis (EAE), an MS animal model, and exhibit anti-inflammatory effects. This study utilized a three-dimensional brain cell culture system to investigate their impact on antibody-induced demyelination and inflammation induced by IFN-gamma and LPS. While the PPAR-beta agonist GW 501516 reduced certain inflammatory responses, it did not protect against antibody-induced demyelination, suggesting that the protective effects of PPAR-beta agonists observed in vivo are primarily due to their anti-inflammatory properties rather than direct trophic effects on oligodendrocytes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2687435/.
Barish GD, Atkins AR, Downes M, et al. PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis. ProcNatlAcadSci U S A. 2008;105(11):4271–4276. doi:10.1073/pnas.0711875105.
PPARdelta regulates multiple proinflammatory pathways to suppress atherosclerosis
Maintaining proper lipid levels and managing inflammation are critical factors in the development of atherosclerosis, a condition characterized by the formation of lipid-laden foam cell macrophages in arterial lesions. The nuclear receptor PPARdelta has been linked to both systemic lipid regulation and macrophage inflammation, but its potential as a therapeutic target for vascular disease has remained uncertain. This study demonstrates that orally active PPARdelta agonists significantly reduce atherosclerosis in apoE(-/-) mice. Through a combination of metabolic and gene expression analyses, it is revealed that PPARdelta mitigates lesion progression by elevating HDL levels and exerting anti-inflammatory effects within the vessel wall. These effects include the suppression of chemokine signaling, down-regulation of chemokines, and induction of regulator of G protein signaling (RGS) genes, which can inhibit chemokine receptor signal transduction. Consequently, PPARdelta ligands inhibit monocyte transmigration and macrophage inflammatory responses triggered by atherogenic cytokines. These findings indicate that PPARdelta acts as an antagonist to multiple proinflammatory pathways, suggesting that PPARdelta-selective drugs have the potential to serve as therapeutics for atherosclerosis.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2393796/.
Oliver WR, Jr, et al. A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport. ProcNatlAcadSci USA. 2001;98:5306–5311.
A selective peroxisome proliferator-activated receptor delta agonist promotes reverse cholesterol transport
The peroxisome proliferator-activated receptors (PPARs) are crucial sensors of dietary lipids, regulating both fatty acid and carbohydrate metabolism. While fibrate drugs activate the alpha subtype and glitazone drugs affect the gamma subtype, the therapeutic potential of the delta subtype has remained uncertain due to a lack of specific ligands. However, a potent and subtype-selective PPARdelta agonist called GW501516 has been developed using combinatorial chemistry and structure-based drug design. In various cell types, GW501516 enhances the expression of ATP-binding cassette A1, a reverse cholesterol transporter, and promotes apolipoprotein A1-specific cholesterol efflux. When administered to insulin-resistant, middle-aged obese rhesus monkeys, GW501516 leads to a substantial dose-dependent increase in serum high-density lipoprotein cholesterol, alongside reductions in small-dense low-density lipoprotein, fasting triglycerides, and fasting insulin levels. These findings suggest that PPARdelta agonists could be promising drugs for enhancing reverse cholesterol transport and mitigating cardiovascular disease linked to metabolic syndrome X.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC33205/.
Sznaidman ML, et al. Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)—synthesis and biological activity. Bioorg MedChemLett. 2003;13:1517–1521.
Novel selective small molecule agonists for peroxisome proliferator-activated receptor delta (PPARdelta)—synthesis and biological activity
We describe the development of a novel class of small molecule agonists targeting the human Peroxisome Proliferator-Activated Receptor delta (PPARdelta). Starting from a library of lipophilic carboxylic acids, we identified promising compounds. Through a process of structure-guided design, we optimized these compounds, resulting in 7k (GW501516) and 7l (GW0742), both exhibiting exceptional potency with an EC(50) of 1.1 nM against PPARdelta and a remarkable 1000-fold selectivity over other human PPAR subtypes.
You can read the full article at https://www.sciencedirect.com/science/article/abs/pii/S0960894X03002075?via%3Dihub.
Piqueras L, Reynolds AR, Hodivala-dilke KM, et al. Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis. ArteriosclerThrombVasc Biol. 2007;27(1):63-9.
Activation of PPARbeta/delta induces endothelial cell proliferation and angiogenesis
The study aimed to investigate the role of the nuclear receptor peroxisome-proliferator activated receptor (PPAR)-beta/delta in endothelial cells and its potential impact on angiogenesis. Using the selective PPARbeta/delta ligand GW501516, the researchers found that PPARbeta/delta was expressed in various types of endothelial cells. Treatment with GW501516 stimulated endothelial cell proliferation, morphogenesis, and angiogenesis both in vitro and in vivo. This effect was associated with increased vascular endothelial growth factor (VEGF) expression and adipose differentiation-related protein (ADRP), a PPARbeta/delta target gene. When dominant-negative PPARbeta/delta was introduced into endothelial cells, the GW501516-induced effects were abolished. Additionally, inhibiting VEGF receptors counteracted the GW501516-induced endothelial cell responses, suggesting that PPARbeta/delta plays a role in angiogenesis through VEGF regulation. These findings suggest that caution may be needed when using GW501516 to treat dyslipidemia in patients susceptible to angiogenic disorders.
You can read the full article at https://www.ahajournals.org/doi/10.1161/01.ATV.0000250972.83623.61?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed.
Magadum A, Ding Y, He L, et al. Live cell screening platform identifies PPARδ as a regulator of cardiomyocyte proliferation and cardiac repair. Cell Res. 2017;27(8):1002-1019.
Live cell screening platform identifies PPARδ as a regulator of cardiomyocyte proliferation and cardiac repair
Zebrafish have a remarkable ability to regenerate their hearts through cardiomyocyte proliferation, a capacity that is limited in postnatal mammals. To enhance the potential for cardiomyocyte proliferation in postnatal mammals, a study systematically screened chemical compounds and identified carbacyclin as a promoter of postnatal cardiomyocyte proliferation. Carbacyclin activated a signaling pathway involving peroxisome proliferator-activated receptor δ (PPARδ), PDK1, p308Akt, GSK3β, and β-catenin, leading to increased cardiomyocyte proliferation. Inhibition of PPARδ reduced cardiomyocyte proliferation during zebrafish heart regeneration. Furthermore, experiments involving mice showed that activating PPARδ induced cell cycle progression in cardiomyocytes, reduced scarring, and improved cardiac function after myocardial infarction. These findings suggest a potential therapeutic target for cardiac conditions involving cardiomyocyte loss.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5539351/.
Ye JM, Tid-Ang J, Turner N, et al. PPARδ agonists have opposing effects on insulin resistance in high fat-fed rats and mice due to different metabolic responses in muscle. Br J Pharmacol. 2011;163(3):556–566. doi:10.1111/j.1476-5381.2011.01240.x.
PPARδ agonists have opposing effects on insulin resistance in high fat-fed rats and mice due to different metabolic responses in muscle
The study aimed to investigate the effects of PPARδ agonists NNC61-5920 and GW501516 in the context of insulin resistance in the whole body, muscle, and liver. In rats fed a high-fat diet, NNC61-5920 treatment for 3 weeks worsened insulin resistance, leading to reduced glucose infusion rates and glucose disposal into muscle. Despite upregulating genes related to fatty acid metabolism in muscle, it increased plasma and muscle triglyceride levels. Similar metabolic effects were observed with extended treatment. However, in high-fat diet mice, NNC61-5920 improved their plasma lipid profile, glucose tolerance, and muscle insulin sensitivity. Interestingly, NNC61-5920 treatment also attenuated hepatic insulin resistance and reduced the expression of certain liver proteins associated with lipid metabolism. These results highlight the differing effects of PPARδ agonists on insulin resistance in rats and mice, primarily due to their impact on lipid metabolism and insulin sensitivity in skeletal muscle.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3101618/.
Chen W, Wang LL, Liu HY, Long L, Li S. Peroxisome proliferator-activated receptor delta-agonist, GW501516, ameliorates insulin resistance, improves dyslipidaemia in monosodium L-glutamate metabolic syndrome mice. Basic ClinPharmacolToxicol. 2008;103(3):240-6.
Peroxisome proliferator-activated receptor delta-agonist, GW501516, ameliorates insulin resistance, improves dyslipidaemia in monosodium L-glutamate metabolic syndrome mice
We examined the effects of GW501516, a specific agonist for peroxisome proliferator-activated receptor beta/delta (PPARdelta), in mice with metabolic syndrome induced by perinatal monosodium L-glutamate injection. Our study found that GW501516 treatment for 14 days effectively improved glucose intolerance, normalized fasting blood glucose levels, and increased serum high-density lipoprotein cholesterol (HDL-C) levels. It also reduced postprandial blood glucose, serum insulin, leptin, free fatty acids (FFA), and the total cholesterol/HDL-C ratio. Additionally, gene expression analysis suggested that these improvements were linked to enhanced fatty acid oxidation in muscle, adipose tissue, and the liver, improved insulin-stimulated glucose transport in skeletal muscle and adipose tissue, and reduced local glucocorticoid synthesis. These findings suggest that GW501516 could be a promising approach for addressing dyslipidemia and insulin resistance associated with metabolic syndrome, highlighting the potential of PPARdelta activation as a therapeutic strategy for metabolic syndrome and related conditions.
You can read the full article at https://onlinelibrary.wiley.com/doi/10.1111/j.1742-7843.2008.00268.x.
Yoo T, Ham SA, Lee WJ, et al. Ligand-Dependent Interaction of PPARδ With T-Cell Protein Tyrosine Phosphatase 45 Enhances Insulin Signaling. Diabetes. 2018;67(3):360-371.
Ligand-Dependent Interaction of PPARδ With T-Cell Protein Tyrosine Phosphatase 45 Enhances Insulin Signaling. Diabetes
We reveal a novel mechanism by which peroxisome proliferator-activated receptor (PPAR) δ impacts insulin signaling. Upon short-term activation, PPARδ forms a stable complex with nuclear T-cell protein tyrosine phosphatase 45 (TCPTP45), preventing TCPTP45 from translocating to the cytoplasm and inhibiting its interaction with the insulin receptor. This interaction with TCPTP45 also mitigates interleukin 6-induced insulin resistance by retaining TCPTP45 in the nucleus, facilitating the deactivation of the signal transducer and activator of transcription 3 (STAT3)-suppressor of cytokine signaling 3 (SOCS3) signal. Activation of PPARδ by GW501516 in mice on a high-fat diet improves insulin signaling and glucose tolerance. This newly identified interaction of PPARδ represents a crucial component in regulating insulin signaling.
You can read the full article at https://diabetesjournals.org/diabetes/article/67/3/360/40018/Ligand-Dependent-Interaction-of-PPAR-With-T-Cell.
Lee CH, Olson P, Hevener A, et al. PPARdelta regulates glucose metabolism and insulin sensitivity. ProcNatlAcadSci U S A. 2006;103(9):3444–3449. doi:10.1073/pnas.0511253103.
PPARdelta regulates glucose metabolism and insulin sensitivity
The metabolic syndrome, associated with obesity-related disorders, is of growing concern. Peroxisome proliferator-activated receptors (PPARs) are potential therapeutic targets in this context. PPARdelta (NR1C2) knockout mice display reduced metabolic activity and glucose intolerance, while receptor activation in db/db mice enhances insulin sensitivity. Further experiments using euglycemic-hyperinsulinemic-clamp show that a PPARdelta-specific agonist suppresses hepatic glucose output, increases glucose utilization, and inhibits adipocyte free fatty acid release. Unexpectedly, gene array and functional analyses reveal that PPARdelta improves hyperglycemia by boosting glucose flow through the pentose phosphate pathway and enhancing fatty acid synthesis. This dual role in hepatic carbohydrate catabolism and muscle beta-oxidation positions PPARdelta as a regulator of metabolic balance and insulin action in different tissues, offering potential therapeutic strategies for type II diabetes.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1413918/.
Serrano-marco L, Barroso E, El kochairi I, et al. The peroxisome proliferator-activated receptor (PPAR) β/δ agonist GW501516 inhibits IL-6-induced signal transducer and activator of transcription 3 (STAT3) activation and insulin resistance in human liver cells. Diabetologia. 2012;55(3):743-51.
The peroxisome proliferator-activated receptor (PPAR) β/δ agonist GW501516 inhibits IL-6-induced signal transducer and activator of transcription 3 (STAT3) activation and insulin resistance in human liver cells
The aim of this study was to investigate whether the peroxisome proliferator-activated receptor (PPAR)β/δ agonist GW501516 could prevent IL-6-induced insulin resistance in human hepatic HepG2 cells. The results showed that GW501516 effectively prevented IL-6-induced reduction in insulin-stimulated AKT phosphorylation, IRS-1 and IRS-2 protein levels, and inhibited the IL-6-induced activation of STAT3. It also prevented the increase in SOCS3 caused by IL-6. Furthermore, GW501516 inhibited ERK1/2 phosphorylation, which is involved in serine STAT3 phosphorylation, and increased AMP-activated protein kinase (AMPK) phosphorylation, which can inhibit STAT3 phosphorylation. These findings suggest that GW501516 may be effective in preventing cytokine-induced insulin resistance in hepatic cells by modulating multiple signaling pathways.
You can read the full article at https://link.springer.com/article/10.1007/s00125-011-2401-4.
Yang X, Kume S, Tanaka Y, et al. GW501516, a PPARδ agonist, ameliorates tubulointerstitial inflammation in proteinuric kidney disease via inhibition of TAK1-NFκB pathway in mice. PLoS One. 2011;6(9):e25271. doi:10.1371/journal.pone.0025271.
GW501516, a PPARδ agonist, ameliorates tubulointerstitial inflammation in proteinuric kidney disease via inhibition of TAK1-NFκB pathway in mice
Peroxisome proliferator-activated receptors (PPARs) are ligand-inducible transcription factors with three isoforms: PPARα, δ, and γ. While PPARα and γ agonists have shown renoprotective effects in kidney diseases, the role of PPARδ agonists remains unclear. In a mouse nephropathy model with protein overload, GW501516, a PPARδ agonist, was found to protect against tubular damage, macrophage infiltration, and the upregulation of inflammatory genes. In cultured proximal tubular cells, GW501516 inhibited TNFα- and free fatty acid-induced MCP-1 expression by blocking the TAK1-NFκB pathway. These findings suggest that GW501516 may have therapeutic potential for mitigating tubulointerstitial lesions in proteinuric kidney diseases by exerting anti-inflammatory effects in renal tubular cells.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3178624/.
Magliano DC, Sarmento IB, de Souza Mello V, de Lacerda CAM, Aguila MB. GW501516, a PPAR-BETA/DELTA agonist, improves inflammatory pathways in the kidney of high-fructose fed mice. DiabetolMetabSyndr. 2015;7(Suppl 1):A121. Published 2015 Nov 11. doi:10.1186/1758-5996-7-S1-A121.
GW501516, a PPAR-BETA/DELTA agonist, improves inflammatory pathways in the kidney of high-fructose fed mice
The study aimed to assess the potential of GW501516 in alleviating kidney damage associated with high activation of the angiotensin-II type 1 receptor (AT1r) in mice fed a high-fructose diet. While GW501516 activated PPAR-beta/delta and related genes, it did not directly affect the ACE/AT1r axis or renin expression. However, GW501516 effectively reduced kidney inflammation by decreasing the expression of inflammatory genes like IL-1β, IL-6, MCP-1, and Cd68, independent of AT1 downregulation. Oxidative stress levels remained unchanged. In summary, GW501516, a PPAR-beta/delta agonist, appears to mitigate kidney inflammation downstream of AT1r activation in a high-fructose diet-induced model.
You can read the full article at https://dmsjournal.biomedcentral.com/articles/10.1186/1758-5996-7-S1-A121.
-
GW501516 Ameliorates A Fructose-Induced Inflammation Independent of AT1r Downregulation in Kidney
High activation of AT1r is associated with kidney damage, inflammation, and oxidative stress. This study aimed to assess the potential of GW501516 in mitigating kidney damage in mice with high AT1r activation induced by a high-fructose diet (HFru). While GW501516 improved blood pressure and urinary parameters in the HFru group, it did not directly affect ACE/AT1r axis or renin expression. However, it effectively reduced kidney inflammation by increasing IκB-α protein expression and decreasing ERK and JNK phosphorylation. Oxidative stress levels remained unchanged. In summary, GW501516 appears to alleviate kidney inflammation downstream of AT1r activation in an HFru-fed mouse model, independent of AT1r downregulation.
You can read the abstract of the article at https://www.researchgate.net/publication/311357375_GW501516_Ameliorates_A_Fructose-Induced_Inflammation_Independent_of_AT1r_Downregulation_in_Kidney.
Ruan X, Zheng F, Guan Y. PPARs and the kidney in metabolic syndrome. Am J Physiol Renal Physiol. 2008;294(5):F1032-47.
PPARs and the kidney in metabolic syndrome
Metabolic syndrome (MetS) encompasses several metabolic risk factors linked to conditions like type 2 diabetes and cardiovascular disease, with chronic renal disease often associated even without diabetes. Peroxisome proliferator-activated receptors (PPARs), a subset of ligand-activated transcription factors, are increasingly implicated in MetS pathogenesis. PPARs, including alpha, beta/delta, and gamma, regulate insulin sensitivity, adipogenesis, lipid metabolism, inflammation, and blood pressure, making them important in MetS and its renal complications. PPAR ligands, like PPARalpha activators and PPARgamma agonists, impact MetS components and renal disease progression. This review explores the role of PPARs in MetS and their potential as therapeutic targets for MetS-related kidney diseases.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajprenal.00152.2007?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Ruan X, Zheng F, Guan Y. PPARs and the kidney in metabolic syndrome. Am J Physiol Renal Physiol. 2008;294(5):F1032-47.
PPARs and the kidney in metabolic syndrome
Metabolic syndrome (MetS), characterized by metabolic risk factors like insulin resistance, central obesity, dyslipidemia, hyperglycemia, and hypertension, often leads to chronic renal disease, even without diabetes. Emerging evidence indicates that peroxisome proliferator-activated receptors (PPARs), a subgroup of ligand-activated transcription factors, may have a significant role in MetS development. PPARs, including alpha, beta/delta, and gamma, play crucial roles in regulating insulin sensitivity, adipogenesis, lipid metabolism, inflammation, and blood pressure. They are also implicated in renal conditions such as diabetic nephropathy. Ligands for PPARs, like hypolipidemic PPARalpha activators and antidiabetic PPARgamma agonists, impact various aspects of MetS and renal disease progression. This review explores the involvement of PPARs in MetS and discusses their potential as therapeutic targets for MetS-related kidney diseases.
You can read the full article at https://journals.physiology.org/doi/full/10.1152/ajprenal.00152.2007?rfr_dat=cr_pub++0pubmed&url_ver=Z39.88-2003&rfr_id=ori%3Arid%3Acrossref.org.
Lee HJ, Yeon JE, Ko EJ, et al. Peroxisome proliferator-activated receptor-delta agonist ameliorated inflammasome activation in nonalcoholic fatty liver disease. World J Gastroenterol. 2015;21(45):12787-99.
Peroxisome proliferator-activated receptor-delta agonist ameliorated inflammasome activation in nonalcoholic fatty liver disease
The study aimed to assess inflammasome activation and the impact of peroxisome proliferator-activated receptors (PPAR)-δ agonist treatment in nonalcoholic fatty liver disease (NAFLD) models. Male C57BL/6J mice were categorized into control or high-fat diet (HFD) groups, with or without PPAR-δ agonist (GW) treatment over 12 weeks (control, HFD, HFD + lipopolysaccharide (LPS), HFD + LPS + GW group). HepG2 cells were exposed to palmitic acid (PA) and/or LPS with or without GW. The HFD induced glucose intolerance and hepatic steatosis. In mice fed an HFD with LPS, liver caspase-1 and interleukin (IL)-1β levels significantly increased. GW treatment improved steatosis and reduced the overexpression of pro-inflammatory cytokines. In HepG2 cells, PA and LPS elevated mRNA levels of various nucleotide-binding and oligomerization domain-like receptor family members, caspase-1, and IL-1β, along with increased reactive oxygen species production. GW mitigated these effects and enhanced AMPK-α phosphorylation. In conclusion, the PPAR-δ agonist reduces inflammation and steatosis induced by fatty acids by suppressing inflammasome activation, suggesting therapeutic potential for NAFLD.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4671034/.
Magliano DC, Penna-de-carvalho A, Vazquez-carrera M, Mandarim-de-lacerda CA, Aguila MB. Short-term administration of GW501516 improves inflammatory state in white adipose tissue and liver damage in high-fructose-fed mice through modulation of the renin-angiotensin system. Endocrine. 2015;50(2):355-67.
Short-term administration of GW501516 improves inflammatory state in white adipose tissue and liver damage in high-fructose-fed mice through modulation of the renin-angiotensin system
This study aimed to assess the effects of short-term GW501516 administration on pro-inflammatory markers in white adipose tissue (WAT) and hepatic stellate cells (HSCs), as well as liver lipogenesis and insulin resistance in response to high-fructose diet (HFru)-induced ACE/AT1r axis activation. After 8 weeks of HFru diet, mice were randomly assigned to four groups and treated with GW501516 for 3 weeks. HFru diet activated the ACE/AT1r axis in WAT and the liver, leading to inflammation and liver damage. GW501516 mitigated ACE/AT1r axis activation in WAT, reduced inflammation in WAT, and diminished HSC activation in the liver. Moreover, GW501516 improved liver health by regulating genes related to beta-oxidation and decreasing genes and proteins associated with lipogenesis and gluconeogenesis. This suggests that GW501516 could be a therapeutic option for mitigating ACE/AT1r axis activation in WAT and the liver.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s12020-015-0590-1.
Nagasawa T, Inada Y, Nakano S, et al. Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet. Eur J Pharmacol. 2006;536(1-2):182-91.
Effects of bezafibrate, PPAR pan-agonist, and GW501516, PPARdelta agonist, on development of steatohepatitis in mice fed a methionine- and choline-deficient diet.
We assessed the effects of bezafibrate, a pan-agonist of peroxisome proliferator-activated receptors (PPARs), and GW501516, a PPARdelta agonist, on mice with methionine- and choline-deficient (MCD) diet-induced non-alcoholic steatohepatitis (NASH). The study aimed to determine the efficacy of bezafibrate in treating NASH and explore the role of PPARdelta in this context. Both bezafibrate (at doses of 50 or 100 mg/kg/day) and GW501516 (at 10 mg/kg/day) were administered daily for 5 weeks. These treatments effectively reduced hepatic triglyceride levels, oxidative stress, histopathological signs of liver damage, inflammation, and hepatic stellate cell activation induced by the MCD diet. Furthermore, both compounds increased the expression of genes associated with fatty acid beta-oxidation and decreased genes related to inflammatory cytokines. Bezafibrate uniquely lowered plasma alanine aminotransferase (ALT) levels, increased adiponectin levels, and upregulated the expression of adiponectin receptors. These findings suggest that bezafibrate and GW501516 can ameliorate hepatic steatosis by enhancing fatty acid beta-oxidation and directly suppressing inflammation. PPARdelta agonists, like GW501516, may have potential for treating NASH, and bezafibrate appears to improve NASH by activating both PPARalpha and PPARdelta, given its pan-agonistic properties.
You can read the abstract of the article at https://www.sciencedirect.com/science/article/abs/pii/S0014299906001993?via%3Dihub.
Tong L, Wang L, Yao S, et al. PPARδ attenuates hepatic steatosis through autophagy-mediated fatty acid oxidation. Cell Death Dis. 2019;10(3):197.
PPARδ attenuates hepatic steatosis through autophagy-mediated fatty acid oxidation
Peroxisome proliferator-activated receptor delta (PPARδ), a member of the nuclear receptor family with implications in metabolic diseases, is found to significantly enhance hepatic autophagic flux. Liver tissues in obese and aging mice display reduced levels of PPARδ and autophagy-related proteins. Through pharmacological and adenoviral interventions to increase PPARδ activity and expression in obese db/db mice and those on a high-fat diet, it was demonstrated that PPARδ reduces intrahepatic lipid accumulation and promotes hepatic beta-oxidation via an autophagy-lysosomal pathway mediated by AMPK/mTOR signaling. These findings shed new light on how PPARδ facilitates lipid breakdown through autophagy in the liver and suggest potential therapeutic benefits in non-alcoholic fatty liver disease (NAFLD).
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6393554/.
Li X, Li J, Lu X, et al. Treatment with PPARδ agonist alleviates non-alcoholic fatty liver disease by modulating glucose and fatty acid metabolic enzymes in a rat model. Int J Mol Med. 2015;36(3):767-75.
Treatment with PPARδ agonist alleviates non-alcoholic fatty liver disease by modulating glucose and fatty acid metabolic enzymes in a rat model
To investigate the role of PPARδ in non-alcoholic fatty liver disease (NAFLD), a rat model of NAFLD induced by a high-fat diet (HFD) was created and treated with GW501516, a PPARδ agonist. The treatment resulted in decreased lipid levels, reduced hepatocellular ballooning, and less inflammatory cell infiltration compared to untreated rats. GW501516 treatment also lowered insulin resistance (HOMA-IR) and LDL levels, while increasing insulin-like growth factor-1 (IGF-1) and high-density lipoprotein (HDL). Moreover, the elevated liver enzyme levels in the HFD group returned to normal levels with GW501516 treatment. Expression levels of sterol regulatory element binding protein-1c (SREBP-1c) and glucose transporter 2 (GLUT-2) were restored to normal, and enzymes related to lipid metabolism increased. In summary, GW501516 treatment alleviated NAFLD by modulating glucose and fatty acid metabolism.
You can read the full article at https://www.spandidos-publications.com/ijmm/36/3/767.
Barroso E, Rodríguez-calvo R, Serrano-marco L, et al. The PPARβ/δ activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1α-Lipin 1-PPARα pathway leading to increased fatty acid oxidation. Endocrinology. 2011;152(5):1848-59.
The PPARβ/δ activator GW501516 prevents the down-regulation of AMPK caused by a high-fat diet in liver and amplifies the PGC-1α-Lipin 1-PPARα pathway leading to increased fatty acid oxidation
We investigated the impact of the peroxisome proliferator-activated receptor (PPAR)-β/δ activator GW501516 on high-fat diet (HFD)-induced hypertriglyceridemia and hepatic fatty acid oxidation. HFD exposure led to hypertriglyceridemia and reduced hepatic mRNA levels of PPAR-γ coactivator 1 (PGC-1)-α and lipin 1, which were prevented by GW501516 treatment. GW501516 increased nuclear lipin 1 protein levels, enhancing the PGC-1α-PPARα signaling system, as evidenced by increased PPARα levels, PPARα-DNA binding activity, and the expression of PPARα-target genes involved in fatty acid oxidation. This resulted in elevated hepatic fatty acid oxidation, as indicated by increased plasma β-hydroxybutyrate levels. Furthermore, GW501516 enhanced the levels of the hepatic endogenous ligand for PPARα, 16:0/18:1-phosphatidylcholine, and markedly increased hepatic Vldl receptor expression. Notably, GW501516 also prevented the reduction in AMP-activated protein kinase (AMPK) phosphorylation and the increase in phosphorylated levels of ERK1/2 caused by HFD, possibly by increasing the AMP to ATP ratio in hepatocytes. These findings suggest that GW501516’s hypotriglyceridemic effect in HFD-fed mice is associated with increased phospho-AMPK levels and the amplification of the PGC-1α-lipin 1-PPARα pathway.
You can read the full article at https://academic.oup.com/endo/article/152/5/1848/2457097.
Liu HX, Fang Y, Hu Y, Gonzalez FJ, Fang J, Wan YJ. PPARβ Regulates Liver Regeneration by Modulating Akt and E2f Signaling. PLoS ONE. 2013;8(6):e65644.
PPARβ Regulates Liver Regeneration by Modulating Akt and E2f Signaling
This study investigated the role of peroxisome proliferator-activated receptor β (PPARβ) in liver regeneration, focusing on its influence on energy homeostasis and cell proliferation. Using a two-third partial hepatectomy (PH) model in both Wild-type (WT) and PPARβ-null (KO) mice, the researchers found that liver regeneration was delayed in KO mice, with the peak of Ki-67 positive cells occurring at 60 hours rather than the typical 36-48 hours seen in WT mice. RNA-sequencing revealed 1344 differentially expressed transcriptomes between regenerating WT and KO livers, primarily involving glycolysis and fatty acid synthesis pathways that failed to activate during liver regeneration due to PPARβ deficiency. Additionally, PPARβ deficiency resulted in the lack of activation of phosphoinositide-dependent kinase 1 (PDK1)/Akt and disrupted the expression of genes associated with E2f transcription factors, which have dual roles in regulating metabolism and proliferation. Transient steatosis was observed only in WT mice, not in KO mice, 36 hours after PH. These findings suggest that PPARβ plays a critical role in normal liver regeneration by regulating PDK1/Akt and E2f signaling, influencing both metabolism and proliferation.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3688817/.
Smith RW, Coleman JD, Thompson JT, Vanden Heuvel JP. Therapeutic potential of GW501516 and the role of Peroxisome proliferator-activated receptor β/δ and B-cell lymphoma 6 in inflammatory signaling in human pancreatic cancer cells. Biochem Biophys Rep. 2016;8:395-402. Published 2016 Nov 4. doi:10.1016/j.bbrep.2016.10.014.
Therapeutic potential of GW501516 and the role of Peroxisome proliferator-activated receptor β/δ and B-cell lymphoma 6 in inflammatory signaling in human pancreatic cancer cells
Peroxisome proliferator-activated receptor β/δ (PPARβ/δ) is a ligand-activated transcription factor involved in regulating the inflammatory response by activating anti-inflammatory genes and dissociating from the transcriptional repressor B-cell lymphoma 6 (BCL6). This study explores the role of PPARβ/δ and BCL6 in human pancreatic ductal cancer and the therapeutic potential of the PPARβ/δ agonist GW501516. Overexpression of PPARβ/δ reduces both basal and TNFα-induced Nfkb luciferase activity. Activation of PPARβ/δ by GW501516 suppresses TNFα-induced Nfkb reporter activity. Knockdown of Pparb attenuates GW501516’s effect on Nfkb luciferase, while Bcl6 knockdown enhances TNFα-induced Nfkb activity. PPARβ/δ activation dose-dependently induces the expression of anti-inflammatory genes and inhibits Mcp1 promoter-driven luciferase in a BCL6-dependent manner. Additionally, conditioned media from GW501516-treated pancreatic cancer cells reduces pro-inflammatory gene expression in macrophages and inhibits invasiveness across a basement membrane. These findings highlight the potential therapeutic role of PPARβ/δ activation in pancreatic cancer by regulating anti-inflammatory signaling and inhibiting pro-inflammatory gene expression.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5614479/.
Ji Y, Li H, Wang F, Gu L. PPARβ/δ Agonist GW501516 Inhibits Tumorigenicity of Undifferentiated Nasopharyngeal Carcinoma in C666-1 Cells by Promoting Apoptosis. Front Pharmacol. 2018;9:648. Published 2018 Jun 28. doi:10.3389/fphar.2018.00648.
PPARβ/δ Agonist GW501516 Inhibits Tumorigenicity of Undifferentiated Nasopharyngeal Carcinoma in C666-1 Cells by Promoting Apoptosis
The role of peroxisome proliferator-activated receptor β/δ (PPARβ/δ) in nasopharyngeal carcinoma (NPC) has been poorly understood. This study aimed to investigate PPARβ/δ’s impact on cell proliferation, clonogenicity, and xenograft growth in human NPC cell lines. PPARβ/δ gene and protein expression were notably reduced in undifferentiated NPC cells compared to control cells. Activation of PPARβ/δ by GW501516, a selective agonist, significantly inhibited cell proliferation, colony formation, induced G2/M phase arrest, and promoted apoptosis in undifferentiated NPC cells. Furthermore, GW501516 suppressed tumor growth in xenografts derived from these NPC cells in mice, which was associated with the inhibition of integrin-linked kinase (ILK) through AMPKα-dependent signaling pathways. These findings suggest that PPARβ/δ-targeting agents may hold promise for chemoprevention in poorly differentiated and undifferentiated NPC.
You can read the full article at https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6031703/.
Péchery A, Fauconnet S, Bittard H, Lascombe I. Apoptotic effect of the selective PPARβ/δ agonist GW501516 in invasive bladder cancer cells. Tumour Biol. 2016 Nov;37(11):14789-14802. doi: 10.1007/s13277-016-5305-6. Epub 2016 Sep 16. PMID: 27638828.
Apoptotic effect of the selective PPARβ/δ agonist GW501516 in invasive bladder cancer cells
GW501516, a potent synthetic agonist of peroxisome proliferator-activated receptor β/δ (PPARβ/δ), has shown antiproliferative and apoptotic effects in various cancer cell lines, but its potential in bladder tumor cells has not been explored. This study investigated the impact of GW501516 on RT4 and T24 urothelial cancer cells and elucidated the molecular mechanisms involved. In RT4 cells (low-grade papillary tumor), GW501516 did not induce cell death. However, in T24 cells (undifferentiated high-grade carcinoma), GW501516 exerted cytotoxic effects, including morphological changes, reduced cell viability, G2/M cell cycle arrest, and apoptosis through caspase-dependent pathways, involving both extrinsic and intrinsic apoptotic pathways. GW501516 also disrupted mitochondrial function, leading to cytochrome c release and reactive oxygen species (ROS) generation, although ROS was not responsible for cell death. These findings suggest GW501516’s potential as a therapeutic agent for high-grade urothelial cancers.
You can read the abstract of the article at https://link.springer.com/article/10.1007/s13277-016-5305-6.
Patient Success Stories

Before
After

At the age of 60, I look and feel better than I ever have in my entire life! Switching my health program and hormone replacement therapy regimen over to Genemedics was one of the best decisions I’ve ever made in my life! Genemedics and Dr George have significantly improved my quality of life and also dramatically improved my overall health.
Nick Cassavetes ,60 yrs old Movie Director (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer

Before
After

I am now in my mid-sixties and feel better than I did in my 20’s. Many people have commented that I actually look 20 years younger since I started the program at Genemedics. Calling Dr. George has proven to be one of the best decisions I have made in my life. Doctors and society convince us that developing various health issues and negative sy...
Pamela Hill ,66 yrs old Actress (“The Notebook”, “John Q”, “Alpha Dog”), Actor and Writer
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have
Free Consultation
Start Your Journey to a Younger, Healthier You!
Categories
Information
Free Consultation
STEPS AWAY FROM A YOUNGER. HEALTHIER YOU!
Call 800-277-4041 for a Free Consultation
What to expect during your consultation:
- Usually takes 15-30 minutes
- Completely confidential
- No obligation to purchase anything
- We will discuss your symptoms along with your health and fitness goals
- Free post-consult access for any additional questions you may have








